Abstract
Porous silica was synthesized from cornhusk using the sol–gel polymeric route and compared with ash obtained from the direct combustion process under laboratory conditions. The unmodified ash from the direct combustion process was dissolved in NaOH for 1 h to form sodium silicate, which was subsequently hydrolyzed with citric acid to yield a silica xerogel. The obtained xerogel was characterized using inductively coupled plasma–optical emission spectrometry (ICP-OES), Fourier transforms infrared (FTIR) spectroscopy, X-ray diffraction (XRD), simultaneous thermal analysis (STA), gas sorption techniques to determine their elemental constituents, functional groups, crystalline phases, thermal stability, and porosity, respectively. The results showed that the synthesized silica xerogel exhibited porous network structures with a high-specific surface area and mesopore volume of 384 m2/g and 0.35 cm3/g, respectively. The pore size distribution revealed a complete transformation of the pore network structures of the unmodified ash from a monomodal to a bimodal pore system, with micro- and mesopore peaks centered around 1.5 and 3.8 nm, respectively. The ICP-OES results showed that the silica content significantly increased from 52.93 to 91.96 wt.% db after the sol–gel treatment. XRD diffraction confirmed the amorphicity of the silica particles obtained from the sol–gel extraction method. In addition, the STA data showed that the silica xerogel has high thermal stability compared to the unmodified ash, as the latter exhibited poor thermal stability and low textural properties. The high surface area and narrow pore cavity size distribution of the porous silica xerogel make it an ideal substrate for catalysts and an excellent template for growing other nanoparticles within the pores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Silica products such as precipitated silica, fumed silica, arc silica, silica xerogels, and xerogels are industrially synthesized by (i) the hydrolysis of mineral precursors such as tetraethyl orthosilicate (TEOS), (ii) the acidification of sodium silicate solutions, and (iii) the smelting of quartz sand with sodium carbonate at elevated temperatures (> 1200 °C) [1,2,3]. Common to these synthesis routes are the energy-intensive attributes and the release of hazardous by-products along with CO2 emissions [4]. These downsides have rendered industrialized silica products less desirable from a cost and environmental considerations, limiting their wide applications in various industrial sectors. Besides, the intensification of global policies enacted by the EU [5] and the AU [6] against greenhouse gas emissions has spurred a renewed interest in finding a comprehensive, tailor-made solution to these exhausted synthesis routes by embracing a more sustainable approach.
As an alternative, biomass has been the subject of several investigations over the years due to its versatility, low cost, and biodegradability attribute [7]. The combustion of biomass fuels for bio-energy production could play a significant role in achieving a healthy ecosystem, as many perspectives perceive it as carbon–neutral [8]. Through photosynthesis, plants can capture the equivalent amount of CO2 released to the atmosphere during combustion. Thus, there is no net increase in greenhouse gas emissions based on life-cycle analysis [8, 9]. Presently, biomass plays a subordinate role to fossils in energy generation. Besides the cogeneration of heat, biomass can be considered for value-added material production, such as the extraction of biogenic silica [10]. High-quality biogenic silica has been extracted from agricultural residues such as rice husk, rice straw, oat and spelt processing wastes, miscanthus, and sugarcane bagasse with comparable properties to industrialized silica [11,12,13,14]. Consequently, the extracted silica has found applications in several industrial processes ranging from controlled porous glass production [15], catalyst support [16], optoelectronic application [17], ceramics, and in its finest form, as a filler for paints, plastics, and rubber [18]. Furthermore, recent developments in the field of biogenic silica have shown the synthesis of n-SiO2 and C-based nanomaterials as a result of the cost-effective fabrication of modern and smart sensor devices [19].
The main constituents of biomass are cellulose, hemicellulose, lignin and inorganic compounds [20]. Typically, the inorganic compounds consist of alkali and non-alkali metals (e.g., Na, K, Li), which, along with other mineral components (e.g., Si), result in a less active amorphous silica with low textural properties and purity [21]. The silica atoms are uniformly dispersed molecular units inter-twinned between the lignin and need to be liberated using pretreatment methods and thermochemical conversion processes such as combustion and gasification [11, 22]. The generation of high-quality biogenic silica from biomasses involves two main processes. First, the pretreatment of the biomass material is performed to remove the incorporated inorganic metals from the organic matrix, followed by combustion under controlled conditions to oxidize the organic components. The resulting ash is referred to as biogenic silica due to its high siliceous content [23]. During the pretreatment phase, an organic acid such as citric acid is often preferred due to its environmental friendliness compared to mineral acids, e.g., sulfuric acid [24].
Corn (Zea mays) is one of the staple food crops cultivated on the African continent, with global production figures of 1.16 billion tons in 2020 [25]. A known predominant by-product of corn production is the corn husk residues. Invariably, the intensification of production figures leads to a corresponding increase in the available corn husk residues [26]. From the perspective of waste management, the disposal of these vast quantities of residues presents a challenge; accordingly, they are disposed of in landfills or openly burned, causing pollution [27]. A major contributing factor to these preferred waste eradication options is the lack of value-addition pathways, especially for developing countries. In spite of this, recent reports have shown the high silica potential of corn husk residues, making it a cheap resource for amorphous silica extraction [28, 29]. However, one of the significant challenges associated with the biogenic silica from agricultural residues is the low-purity silica owing to the high impurities (e.g., concentrations of metal oxides) in the ashes after the direct combustion process, as shown in Table 1 for various agricultural residues [23, 28, 30]. These inorganic impurities hinder obtaining the required silica purity with less crystallinity and improved porosity needed for high-end applications such as catalyst support [31]. Thus, the low-purity silica requires additional post-treatment or wet chemical treatments, such as the hot hydrochloric leaching process associated with the solution-gelation (“sol–gel’’) polymeric method. The sol–gel polymeric method has been used to synthesize numerous nano-/micro structures, which have applications in the biomedical field as drug delivery vectors, optics, electronics, semi-/super-conductors, and biomaterials [32].
Reproduced with permission from Prempeh, C.O.; Formann, S.; Schliermann, T.; Dizaji, H.B.; Nelles, M. Extraction and Characterization of Biogenic Silica Obtained from Selected Agro-Waste in Africa. Applied Sciences 2021, 11, 10,363, Copyright (2021), and MDPI.
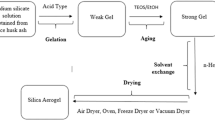
The chemistry of the sol–gel involves the isolation of the silica particles from the impure silica (usually, ash from the direct combustion process; herein, unmodified ash) in an alkaline medium to yield a sodium silicate solution, followed by a gelation period via a controlled pH process [33, 34]. The dissolution process is a low-temperature process usually performed in a boiling NaOH at 100 ± 10 °C for 1 h with continuous stirring, according to Eq. 1. The gelation starts during the acid precipitation process, and the pH is adjusted below 7 or as low as 4, according to eqs. 2 and 3. The solution is allowed to gel for 24 h, after which the gel is centrifuged to separate the silica from the mixture and thereafter dried [35,36,37].
One advantage of the sol–gel process is the ability to control the morphology, shape, mechanical, and pore structure of the synthesized silica xerogel, allowing an impressive range of applications [32]. Accordingly, the sol–gel method has been used to investigate the effects of nanostructuring on the crystal sizes of the silica obtained from corn cob, as well as the synthesis of nanosilica particles with average sizes from cassava periderm [4]. In addition, the development of sol‐gel and porous glass–based silica monoliths with hierarchical pore structures for solid‐liquid catalysis has been reported [38]. Previous studies have also shown the use of mineral acids, including HCl and H2SO4, in the neutralization reaction to promote gelation [39]. Nevertheless, these mineral acids are not only environmentally unfriendly but introduce into the structure of the synthesized nanosilica particles other ions (Cl− ions, as in the case of HCl as a gelation catalyst) (see Eq. 3) [24]. For further application of the extracted silica xerogels as a catalyst support system for encapsulating or anchoring noble metals such as Pd, Rh, and Pt, Cl− ions have been reported to have a poisoning effect on the catalyst operation [40]. Therefore, this study adopted citric acid (C6 H8O7) over the standard HCl usually adopted in the gelation step.
Against this background, this study explores the reuse of agricultural residue, with a particular focus on cornhusks, for synthesizing biogenic silica nanoparticles using the sol–gel polymeric method. The sol–gel product, the silica xerogel, was compared to biogenic silica produced from the direct combustion of cornhusk in the laboratory. The extracted silica particles were fully characterized using various techniques, including ICP-OES and gas sorption techniques, TGA/STA, XRD, and FTIR, to obtain information about their elemental compositions, micro-structural properties, and physicochemical characteristics. The results of this study would be relevant for effectively utilizing biomass resources and fabricating valuable nanosilica powder for essential applications.
2 Materials and method
2.1 Ash preparation
Cornhusks were purchased from farmers in the Ashanti region, Ghana, in the form of five round bales, each approximately 50 kg and a total of 250 kg. Upon collection, they were washed in an extractor (STAHL ATOLL 290 E, Gottlob Stahl Wäschereimaschinen GmbH, Sindelfingen, Germany) at 50 °C for 2 h to remove dirt and soil particles. The water-washing procedure has been reported to remove some water-soluble inorganic metals embedded in the biomass matrix [11]. The washed residues were dried overnight at 105 °C and stored in airtight plastic containers for further experimentation. 250 g of the biomass were directly combusted in a chamber furnace (Nabertherm 1185H66EA, Lilienthal, Germany) under an air atmosphere at 600 °C for 2 h using a heating rate of 10 K/min to obtain unmodified ash. The unmodified ash was then stored in airtight plastic containers until further use and characterization. The combustion temperature was chosen based on literature data [41].
2.2 Synthesis of silica xerogel
In a separate experiment, the sol–gel polymeric route was performed using the ashes from the direct combustion process following the experimental procedures of Falk et al. [39] and Pijarn et al. [42], with minor modifications. Twelve grams of the prepared unmodified ash was boiled at 100 °C in 350 ml of 1 M NaOH in a 500-ml Erlenmeyer flask with constant stirring to yield a sodium silicate solution. The sodium silicate solution was filtered using a vacuum-filtration unit containing a Unifil C41 filter paper. The filtrate was allowed to cool to room temperature before titrating with 2.5 w/v % citric acid solution (Sigma-Aldrich, Steinheim, Germany, purity of > 99.99%) until the pH reached 7. Gels started to form below a pH of 10. The solution was left to allow gel formation. After 24 h, distilled water was added to the formed gels and centrifuged (Eppendorf centrifuge 5430, Hamburg, German) at 4000 rpm for 5 min. This process was repeated several times to remove remnants of the citric acid solution and impurities. The obtained gels (silica xerogels) were dried at 80 °C for 12 h, after which they were crushed in an agate mortar and pestle. The dried xerogels were stored in desiccators and labelled as silica xerogel for further characterization. Figure 1 shows the schematic flow diagram for the sol–gel synthesis route of biogenic silica extracted from the cornhusk ash.
2.3 Analysis of physical and chemical properties
The elemental constituents of the prepared silica samples from the unmodified ash and silica xerogel were assessed by the inductively coupled plasma-optical emission spectrometry (ICP-OES, CETAC, ASX-520, Omaha, Nebraska, USA) using HF digestion and according to the DIN EN ISO 16967;2015–07, DIN EN ISO 1 standard method.
FTIR spectrometer (PerkinElmer, Solingen, Germany) was used to identify the types of functional groups present in the synthesized silica xerogel and unmodified ash from the direct combustion process. The spectrum scope was in the range of 400–4000 cm−1 with a resolution factor of 1 cm−1.
The textural properties were measured by gas sorption techniques using autosorb iQ-MP/XR apparatus, Quantachrome, USA. The samples were first degassed for 12 h at 250 °C under a vacuum to remove adsorbed water particles on the surface and within the pores. The specific surface area was determined by multipoint Brunauer–Emmett–Teller (BET) surface area analysis in the pressure range of p/p0 = 0.05–0.30 at 77 K and considering the cross-section area of N2 molecules of 16.2 Å [43]. The pore volume was determined using the nonlocal density functional theory (NDLFT) method as it is advantageous over the classical macroscopic method such as Barrett-Joyner-Halenda (BJH). NDLFT considers the adsorption mechanism over the complete micropore and mesopore range and can reliably estimate the pore size distribution from the adsorption and desorption branch of the isotherm data by considering the delay in condensation as a result of metastable adsorption films in mesopores [44].
The thermal stability of the silica xerogel was studied in a simultaneous thermal analysis (STA 449 F3 Jupiter®, NETZSCH, Selb, Germany) in a synthetic air flow atmosphere with a 100-mL/min flow rate at a heating rate of 10 K/min. The sample was first ground to a particle size of less than 0.5 mm using a cutting mill (IKATM MF 10 basic Mikrofeinmühle, Germany) and vigorously mixed in a plastic box to create homogenous starting material for the STA analysis. Ten milligrams of the samples were placed in alumina crucibles, and the samples were heated from room temperature to 1000 °C. The rate of mass loss was recorded simultaneously with the rise in temperature.
The amorphous phases in the silica particles were analyzed by X-ray diffraction (XRD, Malvern Panalytical GmbH, Kassel, Germany) equipped with Ni-filtered, Cu-Kα radiation (λ = 1.54 Å). The samples were first coated with a glass substrate and measured in an instrument (Rigaku XRD; Rigaku, Japan) operating at a voltage of 40 kV and a current of 30 mA and nickel monochromator filtering wave at a tube voltage of 40 kV and tube current of 30 mA.
3 Results and discussion
3.1 Characterizations of unmodified ash and silica aerogel
Table 2 shows the chemical compositions of the ashes produced from the various extraction methods and analyzed using ICP-OES. After the direct combustion process, the contents of principal ash-forming elements or impurities (CaO, MgO, SO3, and K2O) in the unmodified ash were predominant. However, CaO, MgO, and SO3 contents were effectively removed, whereas diminutive concentrations of K2O and Al2O3 remained (< 1 wt.% db) after the sol–gel treatment. Consequently, the silica content significantly increased from 52.93 to 91.96 wt.% db, with the content of Na2O also increasing to 7 wt.% db in the silica xerogel. The additional Na+ ions in the modified silica xerogel might have originated from the NaOH solution, as it was the principal surface modification agent. The addition of the citric acid (C6 H8O7) promoted the formation of high-purity silica due to the Na+ ions exchange from the structure of the sodium silicate to the acid. According to Umeda et al. [45], dilute acids are effective in accelerating the hydrolysis process of organics and, more importantly, the removal of metallic impurities. The enrichment in silica content demonstrates the effectiveness of the selected post-treatment protocol in obtaining high-purity silica. Similar improvements in silica purity have been reported in other studies after the sol–gel extraction process [29, 39]. The unmodified and synthesized silica xerogel moisture contents were 2.65 and 11.4 wt.% db (dry-basis), respectively. The high moisture content of the silica xerogel is probably caused by the presence of water-attractive structures such as phyllosilicate minerals which contains hydroxyl ion, OH− [46].
The visual images of the unmodified ash, wet and dried xerogels are shown in Fig. 2. As observed in the unmodified ash produced by direct combustion, there were few remnants of black particles due to high impurities, such as K+ ions, which could trap carbon within the organic matrix during the combustion process. The formation of black particles has been fully elucidated in the study of Krishnarao et al. [47]. In brief, black particle formation in the ashes affects the purity and the quality of the biogenic silica [47], and similar observations were also reported by Chandrasekhar et al. [48] during their investigations on rice husk ash.
With the sol–gel polymeric process, the silica in the unmodified ash is dissolved as sodium silicate according to Eq. (1), and ion pairing between alkali cations and anions (silicates or sodium silicates) decreases the repulsion between anions and accordingly enhances their condensation to form a gel [49]. Depending on the amount of hydrolysis catalyst (herein, C6 H8O7 or H2O), the hydrolysis process may proceed to the completion of silica xerogel, as shown in Eq. (4). Equally, there is a possibility of the formation of intermediate species such as [(OR)2 − Si − (OH)2] or [(OR)3 − Si − (OH)] in the formed silica xerogel as observed in Eq. (5), in which the functional groups were identified in the FTIR diagram [50, 51]. R represents an organic chain of the formula, CxH2x+1.
The Fourier transform infrared spectroscopy (FTIR) spectra of the major functional groups in the unmodified ash from direct combustion (unmodified ash), and the modified silica xerogel are shown in Fig. 3. The broad peak at 3500–3300 cm−1 corresponds to asymmetric stretching and bending of the O − H bond from the silanol group (Si − OH) as a result of adsorbed water molecules on the surface of the silica xerogel or from the intermediate species [(OR)3 − Si − (OH)] formed during the hydrolysis process [52]. The infrared spectra at 1626–1573 cm−1 are attributed to Si-H2O flexion as well as the bending of the H − O − H [53]. The bands at approximately 1095–1100 and 750–800 cm−1 were assigned to the asymmetric and symmetric vibration of Si–O–Si bonds, respectively [54].
The apparent differences between the spectra of the samples were the intensity of the absorbance peaks at 1095–1100 cm−1 attributed to the asymmetrical vibration of Si–O–Si, which was intensive in the silica xerogels compared to the unmodified ashes from the direct combustion. The sol–gel polymeric route has been reported to cause a higher polymerization degree of the Si − O group, thus enabling the formation of a stronger band between the silicon and the oxygen with higher silica concentration and the polymerization of silicate to form a more robust silica network [55]. This confirms that high-quality silica could be obtained by altering the skeleton network of the gel via the sol–gel polymeric route. Additional characteristic peaks of asymmetrical vibration of O − Si − O were also visible at absorption peaks 456–500 cm−1 in both samples, as shown in the FTIR spectra [56]. The observed infrared spectra peaks are comparable to similar studies reported in the literature [56,57,58,59].
3.2 Influence of the sol–gel polymeric route on the textural properties
The porosity of the unmodified ash and the silica xerogel is shown in Table 3. The results showed improvements in the specific surface area and pore volume of the unmodified ash after the sol–gel transformation process. The surface area largely increased from 88 m2/g in the unmodified ash to 384 m2/g in the silica xerogel. This increase in the surface area could be ascribed to the effective removal of the principal remaining impurities from the unmodified ash without destroying the structural integrity of the biogenic silica network. During combustion, the silica could interact with the inorganic impurities forming silicate melts, reducing the surface area, as evidenced in the unmodified ash and reported in the literature [31]. In addition, the pore volume was higher in the silica xerogel (0.35 cm3/g) than in the ashes from direct combustion (0.25 m3/g), which affirms the advantage of the proposed method on the porosity of the biogenic silica. Two accounts could explain the differences between the reported pore volumes: (i) cavitation effects that result from the differences in the pore structures of the two silica products and (ii) the formation of inaccessible porosity in the unmodified ash [44].
The N2 adsorption–desorption isotherms of unmodified ash and the modified silica xerogel in Fig. 4(a) are classified according to the IUPAC classification. For both samples, type IV(a) with hysteresis was observed. This type of isotherm is generally observed for mesoporous materials where the monolayer-multilayer formation is followed by pore condensations [44]. In Fig. 4(a), the shapes of the hysteresis loops, H3 in the unmodified ash, and H2(a) in the silica xerogels, indicate different pore structures in the two silica products, in accordance with the pore size distribution (PSD) diagram shown in Fig. 4(b). The hysteresis loop type H3 indicates slit-shaped mesopores or plate-like particles and is mostly observed in materials comprising aggregates (loose assemblages) [60, 61]. The H2 hysteresis loop is exhibited by numerous porous adsorbents (e.g., inorganic oxide gels and porous vycor glasses). It is mainly formed due to delayed condensation during adsorption. A shift in the liquid–vapor phase transition during desorption results in a more complex pore network, often referred to as “ink bottle’’ pores [44, 60]. According to Cychosz et al. [62], the pore-blocking phenomenon also leads to the formation of the H2(a) hysteresis loop.
N2 adsorption–desorption isotherms and pore size distribution of unmodified ash and modified silica xerogel. (a) Nitrogen sorption isotherms of the biogenic silica extracted from corn husk using sol–gel polymeric and direct combustion routes; silica xerogel and unmodified ash; (b) pore size distribution of silica xerogel and unmodified ash determined by applying a dedicated NLDFT adsorption branch kernel on the adsorption branch of the N2 (77 K) isotherm
In Fig. 4(a) of the isotherm of the silica xerogel, the existence of a horizontal plateau in the isotherm near the bulk saturation pressure indicates the complete filling of all pores with the adsorbate (N2). Conversely, the isotherm of the unmodified ash does not reach a plateau near p/po = 1, indicating the presence of larger meso- and macropores, which cannot be completely filled with nitrogen. In addition, the shape of the isotherm shows a broad distribution of pore width in the mesoporous range. In contrast, the H2(a) hysteresis is typical for materials that show a narrow pore cavity size distribution [62] and useful as a template or as a constrainer when growing nanoparticles inside the pores [61].
The NLDFT pore size distributions obtained for the nitrogen (77 K) isotherm data for the silica xerogels and unmodified ash are shown in Fig. 4(b). The PSD revealed a complete transformation of the pore network structure of the unmodified ash from a monomodal to a bimodal pore system under the sol–gel polymeric route. For all samples, the shape of the isotherms closer to the regions of lower pressures indicated that little or no micropores were present. However, the silica xerogel exhibited a bimodal pore system with micro- and mesopore peaks centered around 1.5 nm and 3.8 nm, respectively. Further analysis by t-plot showed that the silica xerogel exhibited a micropore volume Vmicro = 0.12 cm3/g and micropore surface area, Smicro = 211 m2/g, which explains the high total surface area of 384 m2/g [Smicro = 211 m2/g + external surface area (Sext) = 173 m2/g = 384 m2/g] as shown in Table 3. Conversely, the t-plot kernel showed a non-existence micropore volume and surface area (Vmicro and Smicro = 0) within the pore structure of the unmodified ash, although a small indication of closed pores or inaccessible micropores was found. Thus, conclusions about the existence of mesopores can be made on the silica xerogel. The previous report of biogenic silica derived from horse-tail has also shown the presence of micropores [63]. Similar studies into the transformations of silica materials, such as porous glass materials with a monomodal pore system, have been altered into a hierarchical pore system by methods such as sol–gel and pseudomorphic transformation reported in a previous study [64]. More importantly, this study affirms a complete transformation of the pore structure network and the presence of both open micro- and mesopores within the silica xerogel’s structure. The pore size mode (Dp) of the modified silica xerogel was slightly higher than the unmodified particles, which is acceptable within the deviation of the method used.
The thermal stability of the synthesized silica xerogels was evaluated in STA by measuring the weight loss caused by changing temperature, and the results are shown in Fig. 5. The thermogravimetric curves of the silica produced by direct combustion (unmodified ash) showed a total weight loss of 17.74%. In the early stages, there was a sharp decrease in weight at approximately 200 °C, mainly attributed to the dehydration of physically adsorbed water molecules on the surfaces or in the pores. Above 200 to 800 °C, there was an extra weight loss most likely caused by the volatilization of residual carbon in unmodified ash samples. Conversely, as shown in the curve for the modified silica xerogel, the total mass loss above 200 °C was 7.17%, attributed to the evaporation of chemically bound water and surface dehydroxylation of silica [65]. This demonstrates a greater hydrophobicity of the modified silica compared to the unmodified ash and, thus, could influence its capabilities as a potential catalyst or adsorbent. It is probable that due to the surface dehydroxylation of the silica xerogel at high temperatures (above 300 °C), hydrophobic surfaces in the pores were created [61]. Consequently, a strong negative capillary pressure was generated that prevented the entrance of water into the pores. On the other hand, the unmodified ash might contain hydrophilic surfaces that generate positive pressure and direct water into the pores [66]. Above 600 °C, no further weight loss was observed, an explicit confirmation/depiction of the modified xerogel’s thermal stability compared to the silica produced from direct combustion. The results of the TGA are entirely in tandem with similar results obtained by Feng et al. [55] and Pouretedal et al. [57] during the synthesis of high specific surface area silica xerogel from rice husk ash and sodium silicate, respectively.
The amorphicity of the extracted silica xerogel was determined by XRD measurement, as shown in Fig. 6. The broad hump-shaped diffraction peaks between 2θ = 15 and 35° are the characteristics of an amorphous substance [67]. The absence of sharp, defined peaks in the XRD pattern of the silica xerogel shows that the sol–gel polymeric method did not result in the crystallization of the silica particles and was predominantly amorphous. These observations are entirely in tandem with other studies showing peaks at 2θ = 15 and 35°, indicating the amorphicity of the extracted samples [68, 69]. However, there were weaker crystalline phases at peaks near 2θ = 30° and 38° in the XRD of the unmodified ash, as observed in Fig. 6. These weaker crystalline phases were identified in our earlier study [28] as quartz (SiO2) and anhydrite [Ca(SO4)] respectively, rendering the unmodified ash less amorphous compared to the silica xerogel. Consequently, it is anticipated that the amorphous property can be utilized in a vast array of applications.
4 Conclusions
This study investigated the extraction of high-quality biogenic silica from cornhusk using the sol–gel polymeric compared to the direct combustion routes. The study has shown that variations in the silica particles, such as different pore structure networks, physiochemical characteristics, and purity, can be achieved by altering the properties of the starting material. Consequently, the extracted silica xerogel exhibited an amorphous attribute with high thermal stability based on the XRD and STA results. A remarkable transformation of the textural properties of the ashes obtained from direct combustion was achieved as the sol–gel polymeric silica particles exhibited improved textural parameters with an opened surface area and pore volume close to 400 m2/g and 0.35 cm3/g, respectively. The disproportionate increase in the specific area and the mesopore volume of the sol–gel silica particles were attributed to additional micro- and mesopores formed during the sol–gel process, which led to the opening up of the pore structure by the elimination of inorganic impurities. Accordingly, there was a change in the pore size distribution system from a monomodal in the unmodified ash to a bimodal pore system in the silica xerogels. The presented sol–gel polymeric route offers a significantly low-cost approach to preparing high-quality biogenic silica particles with improved properties. Future research works should consider the potential application of the extracted sol–gel polymeric silica particles into catalysis or adsorption operations due to the high surface and pore volume. These value-addition pathways are expected to intensify the reuse of underutilized biomass and minimize the waste disposal problems in the agricultural or food production sector.
Data availability
On request from the authors.
References
Sudirman AM, Budianto E et al (2012) Synthesis and characterization of polyester-based nanocomposite. Procedia Chemistry 4(11):107–113. https://doi.org/10.1016/j.proche.2012.06.016
He ZW, Liu XQ, Su Q et al (2006) Improvement of electrical properties of low dielectric constant nanoporous silica films prepared using sol–gel method with catalyst HF. Appl Phys A 82(2):349–355. https://doi.org/10.1007/s00339-005-3376-0
Soltani N, Bahrami A, Pech-Canul MI et al (2015) Review on the physicochemical treatments of rice husk for production of advanced materials. Chem Eng J 264:899–935. https://doi.org/10.1016/j.cej.2014.11.056
Adebisi JA, Agunsoye JO, Bello SA et al (2017) Potential of producing solar grade silicon nanoparticles from selected agro-wastes: a review. Sol Energy 142(6):68–86. https://doi.org/10.1016/j.solener.2016.12.001
Adoption of the Paris agreement (2015) Proposal by the President. In: United Nations climate change. Available online: https://unfccc.int/documents/9064. Accessed 22 Nov 2022
African Climate Policy Centre (ACPC) (2010) Overview of the ClimDev Africa Programme. Available online: https://www.uneca.org/acpc. Accessed 22 Nov 2022
Shah MA, Khan MNS, Kumar V (2018) Biomass residue characterization for their potential application as biofuels. J Therm Anal Calorim 134(3):2137–2145. https://doi.org/10.1007/s10973-018-7560-9
Jiang L, Hu S, Sun L-S et al (2013) Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour Technol 146:254–260. https://doi.org/10.1016/j.biortech.2013.07.063
Marland G (2010) Accounting for carbon dioxide emissions from bioenergy systems. J Ind Ecol 14(6):866–869. https://doi.org/10.1111/j.1530-9290.2010.00303.x
Pode R (2016) Potential applications of rice husk ash waste from rice husk biomass power plant. Renew Sustain Energy Rev 53(3):1468–1485. https://doi.org/10.1016/j.rser.2015.09.051
BeidaghyDizaji H, Zeng T, Hartmann I et al (2019) Generation of high-quality biogenic silica by combustion of rice husk and rice straw combined with pre- and post-treatment strategies—a review. Appl Sci 9(6):1083. https://doi.org/10.3390/app9061083
Quispe I, Navia R, Kahhat R (2017) Energy potential from rice husk through direct combustion and fast pyrolysis: a review. Waste Manag 59:200–210. https://doi.org/10.1016/j.wasman.2016.10.001
Zemnukhova LA, Egorov AG, Fedorishcheva GA et al (2006) Properties of amorphous silica produced from rice and oat processing waste. Inorg Mater 42(1):24–29. https://doi.org/10.1134/S0020168506010067
Biswas B, Pandey N, Bisht Y et al (2017) Pyrolysis of agricultural biomass residues: comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour Technol 237:57–63. https://doi.org/10.1016/j.biortech.2017.02.046
Andreola F, Martín MI, Ferrari AM et al (2013) Technological properties of glass-ceramic tiles obtained using rice husk ash as silica precursor. Ceram Int 39(5):5427–5435. https://doi.org/10.1016/j.ceramint.2012.12.050
Liu D, Seeburg D, Kreft S et al (2019) Rice husk derived porous silica as support for Pd and CeO2 for low temperature catalytic methane combustion. Catalysts 9(1):26. https://doi.org/10.3390/catal9010026
Ebnalwaled AA, Sadek AH, Ismail SH et al (2022) Structural, optical, dielectric, and surface properties of polyimide hybrid nanocomposites films embedded mesoporous silica nanoparticles synthesized from rice husk ash for optoelectronic applications. Opt Quant Electron 54(11):45502. https://doi.org/10.1007/s11082-022-03976-2
Sae-Oui P, Rakdee C, Thanmathorn P (2002) Use of rice husk ash as filler in natural rubber vulcanizates: in comparison with other commercial fillers. J Appl Polym Sci 83(11):2485–2493. https://doi.org/10.1002/app.10249
Sharma SK, Sharma G, Sharma A et al (2022) Synthesis of silica and carbon-based nanomaterials from rice husk ash by ambient fiery and furnace sweltering using a chemical method. Applied Surface Science Advances 8:100225. https://doi.org/10.1016/j.apsadv.2022.100225
Rose M (2013) Catalysis for the conversion of biomass and its derivatives. (Max Planck Research Library for the history and development of knowledge.) edited by Malte Behrens and Abhaya K. Datye. Angewandte Chemie International Edition 52(37):9613–9613. https://doi.org/10.1002/anie.201305619
Long J, Song H, Jun X et al (2012) Release characteristics of alkali and alkaline earth metallic species during biomass pyrolysis and steam gasification process. Bioresour Technol 116:278–284. https://doi.org/10.1016/j.biortech.2012.03.051
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11(8):392–397. https://doi.org/10.1016/j.tplants.2006.06.007
Schneider D, Wassersleben S, Weiß M et al (2020) A generalized procedure for the production of high-grade, porous biogenic silica. Waste Biomass Valor 11(1):1–15. https://doi.org/10.1007/s12649-018-0415-6
Umeda J, Kondoh K (2008) High-purity amorphous silica originated in rice husks via carboxylic acid leaching process. J Mater Sci 43(22):7084–7090. https://doi.org/10.1007/s10853-008-3060-9
The State of Food and Agriculture (2022) In: FAO. https://www.fao.org/publications/sofa/2022/en/. Accessed 22 Nov 2022
Kemausuor F, Kamp A, Thomsen ST et al (2014) Assessment of biomass residue availability and bioenergy yields in Ghana. Resour Conserv Recycl 86(3):28–37. https://doi.org/10.1016/j.resconrec.2014.01.007
Lim JS, Abdul Manan Z, Wan Alwi SR et al (2012) A review on utilisation of biomass from rice industry as a source of renewable energy. Renew Sustain Energy Rev 16(5):3084–3094. https://doi.org/10.1016/j.rser.2012.02.051
Prempeh CO, Formann S, Schliermann T et al (2021) Extraction and characterization of biogenic silica obtained from selected agro-waste in Africa. Appl Sci 11(21):10363. https://doi.org/10.3390/app112110363
Velmurugan P, Shim J, Lee K-J et al (2015) Extraction, characterization, and catalytic potential of amorphous silica from corn cobs by sol-gel method. J Ind Eng Chem 29:298–303. https://doi.org/10.1016/j.jiec.2015.04.009
Lee JH, Kwon JH, Lee J-W et al (2017) Preparation of high purity silica originated from rice husks by chemically removing metallic impurities. J Ind Eng Chem 50(2):79–85. https://doi.org/10.1016/j.jiec.2017.01.033
BeidaghyDizaji H, Zeng T, Hölzig H et al (2022) Ash transformation mechanism during combustion of rice husk and rice straw. Fuel 307(1):121768. https://doi.org/10.1016/j.fuel.2021.121768
Owens GJ, Singh RK, Foroutan F et al (2016) Sol–gel based materials for biomedical applications. Prog Mater Sci 77(Suppl. 2):1–79. https://doi.org/10.1016/j.pmatsci.2015.12.001
Liou T-H, Yang C-C (2011) Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Mater Sci Eng, B 176(7):521–529. https://doi.org/10.1016/j.mseb.2011.01.007
Affandi S, Setyawan H, Winardi S et al (2009) A facile method for production of high-purity silica xerogels from bagasse ash. Adv Powder Technol 20(5):468–472. https://doi.org/10.1016/j.apt.2009.03.008
Saravanan K, Yuvakkumar R, Rajendran V et al (2012) Influence of sintering temperature and pH on the phase transformation, particle size and anti-reflective properties of RHA nano silica powders. Phase Transitions 85(12):1109–1124. https://doi.org/10.1080/01411594.2012.671322
Yuvakkumar R, Elango V, Rajendran V et al (2012) High-purity nano silica powder from rice husk using a simple chemical method. J Exp Nanosci 9(3):272–281. https://doi.org/10.1080/17458080.2012.656709
Anuar MF, Fen YW, Zaid MHM et al (2018) Synthesis and structural properties of coconut husk as potential silica source. Results in Physics 11:1–4. https://doi.org/10.1016/j.rinp.2018.08.018
Enke D, Gläser R, Tallarek U (2016) Sol-gel and porous glass-based silica monoliths with hierarchical pore structure for solid-liquid catalysis. Chem Ing Tec 88(11):1561–1585. https://doi.org/10.1002/cite.201600049
Falk G, Shinhe GP, Teixeira LB et al (2019) Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silicon via magnesiothermic reactions. Ceram Int 45(17):21618–21624. https://doi.org/10.1016/j.ceramint.2019.07.157
Chai H, Han W, Zhu H, Liu H (2006) The effect of chloride ion on ruthenium supported catalyst for ammonia synthesis. Progress in Chemistry 18(10):1262–1269
Titiloye JO, Abu Bakar MS, Odetoye TE (2013) Thermochemical characterisation of agricultural wastes from West Africa. Ind Crops Prod 47:199–203. https://doi.org/10.1016/j.indcrop.2013.03.011
Pijarn N, Galajak P (2014) New insight technique for synthesis of silica gel from rice husk ash by using microwave radiation. AMR 1025–1026:574–579. https://doi.org/10.4028/www.scientific.net/AMR.1025-1026.574
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–319. https://doi.org/10.1021/ja01269a023
Schlumberger C, Thommes M (2021) Characterization of hierarchically ordered porous materials by physisorption and mercury porosimetry—a tutorial review. Adv Mater Interfaces 8(4):2002181. https://doi.org/10.1002/admi.202002181
Umeda J, Kondoh K, Michiura Y (2007) Process parameters optimization in preparing high-purity amorphous silica originated from rice husks. Mater Trans 48(12):3095–3100. https://doi.org/10.2320/matertrans.mk200715
Dziewonski AM, Romanowicz BA (2015) Deep earth seismology: an introduction and overview. Treatise on Geophysics 1–28. https://doi.org/10.1016/b978-0-444-53802-4.00001-4
Krishnarao RV, Subrahmanyam J, Jagadish Kumar T (2001) Studies on the formation of black particles in rice husk silica ash. J Eur Ceram Soc 21(1):99–104. https://doi.org/10.1016/S0955-2219(00)00170-9
Chandrasekhar S, Pramada PN, Majeed J (2006) Effect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash. J Mater Sci 41(23):7926–7933. https://doi.org/10.1007/s10853-006-0859-0
Chen X, Mondal P (2020) Effects of NaOH amount on condensation mechanism to form aluminosilicate, case study of geopolymer gel synthesized via sol–gel method. J Sol-Gel Sci Technol 96(3):589–603. https://doi.org/10.1007/s10971-020-05360-6
Hanaor DAH, Chironi I, Karatchevtseva I et al (2013) Single and mixed phase TiO 2 powders prepared by excess hydrolysis of titanium alkoxide. Adv Appl Ceram 111(3):149–158. https://doi.org/10.1179/1743676111Y.0000000059
Singh LP, Bhattacharyya SK, Kumar R et al (2014) Sol-gel processing of silica nanoparticles and their applications. Adv Colloid Interface Sci 214:17–37. https://doi.org/10.1016/j.cis.2014.10.007
Moncada E, Quijada R, Retuert J (2007) Nanoparticles prepared by the sol–gel method and their use in the formation of nanocomposites with polypropylene. Nanotechnology 18(33):335606. https://doi.org/10.1088/0957-4484/18/33/335606
El Rassy H, Pierre AC (2005) NMR and IR spectroscopy of silica aerogels with different hydrophobic characteristics. J Non-Cryst Solids 351(19–20):1603–1610. https://doi.org/10.1016/j.jnoncrysol.2005.03.048
Frías M, Villar E, Savastano H (2011) Brazilian sugar cane bagasse ashes from the cogeneration industry as active pozzolans for cement manufacture. Cement Concr Compos 33(4):490–496. https://doi.org/10.1016/j.cemconcomp.2011.02.003
Feng Q, Chen K, Ma D et al (2018) Synthesis of high specific surface area silica aerogel from rice husk ash via ambient pressure drying. Colloids Surf, A 539:399–406. https://doi.org/10.1016/j.colsurfa.2017.12.025
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86(12–13):1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Pouretedal HR, Kazemi M (2012) Characterization of modified silica aerogel using sodium silicate precursor and its application as adsorbent of Cu2+, CD2+, and PB2+ ions. Int J Ind Chem 3(1):20. https://doi.org/10.1186/2228-5547-3-20
Reynolds JG, Coronado PR, Hrubesh LW (2001) Hydrophobic aerogels for oil-spill cleanup – synthesis and characterization. J Non-Cryst Solids 292(1–3):127–137. https://doi.org/10.1016/S0022-3093(01)00882-1
VenkateswaraRao A, Kulkarni MM, Amalnerkar DP et al (2003) Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J Non-Cryst Solids 330(1–3):187–195. https://doi.org/10.1016/j.jnoncrysol.2003.08.048
Sapei L (2007) Characterisation of silica in Equisetum hyemale and its transformation into biomorphous ceramics. In: KOBV. http://opus.kobv.de/ubp/volltexte/2007/1588/. Accessed 22 Nov 2022
Johansson EM (2010) Controlling the pore size and morphology of mesoporous silica (Licentiate dissertation, Linköping University Electronic Press). Retrieved from http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-70405. Accessed 22 Nov 2022
Cychosz KA, Guillet-Nicolas R, García-Martínez J et al (2017) Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem Soc Rev 46(2):389–414. https://doi.org/10.1039/C6CS00391E
Sapei L, Nöske R, Strauch P et al (2008) Isolation of mesoporous biogenic silica from the perennial plant equisetum hyemale. Chem Mater 20(5):2020–2025. https://doi.org/10.1021/cm702991f
Uhlig H, Muenster T, Kloess G et al (2018) Synthesis of MCM-48 granules with bimodal pore systems via pseudomorphic transformation of porous glass. Microporous Mesoporous Mater 257:185–192. https://doi.org/10.1016/j.micromeso.2017.08.033
Dubey RS, Rajesh YBRD, More MA (2015) Synthesis and characterization of SiO2 nanoparticles via sol-gel method for industrial applications. Materials Today: Proceedings 2(4–5):3575–3579. https://doi.org/10.1016/j.matpr.2015.07.098
Romero E, Quirantes M, Nogales R (2017) Characterization of biomass ashes produced at different temperatures from olive-oil-industry and greenhouse vegetable wastes. Fuel 208:1–9. https://doi.org/10.1016/j.fuel.2017.06.133
Proctor A (1990) X-ray diffraction and scanning electron microscope studies of processed rice hull silica. J Am Oil Chem Soc 67(9):576–584. https://doi.org/10.1007/BF02540770
Kalapathy U (2000) A simple method for production of pure silica from rice hull ash. Bioresour Technol 73(3):257–262. https://doi.org/10.1016/S0960-8524(99)00127-3
Kamath SR, Proctor A (1998) Silica gel from rice hull ash: preparation and characterization. Cereal Chem J 75(4):484–487. https://doi.org/10.1094/CCHEM.1998.75.4.484
Acknowledgements
The authors thank Mr. Bamgboye Kehinde and Mr. Manfred Eiden for their technical assistance during the laboratory experiments.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors are grateful for the financial support from the German Federal Ministry of Food and Agriculture (BLE/BMEL) Grant number: 2819DOKA05.
Author information
Authors and Affiliations
Contributions
Conceptualization, Clement Owusu Prempeh (C.O.P.), Steffi Formann (S.F.), and Ingo Hartmann (I. H.); methodology, C.O.P.; formal analysis, C.O.P.; investigation, C.O.P.; resources, C.O.P., S.F., I.H., and Michael Nelles (M.N.); data curation, C.O.P.; writing—original draft preparation, C.O.P.; writing—review and editing, C.O.P., S.F., and I.H.; visualization, C.O.P., S.F., and I. H.; supervision, S.F., I.H., and M.N.; project administration, S.F., I.H., and M.N. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prempeh, C.O., Formann, S., Hartmann, I. et al. An improved method for the production of biogenic silica from cornhusk using sol–gel polymeric route. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03615-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03615-6