Abstract
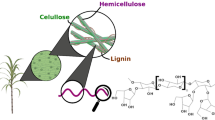
Xylooligosaccharides (XOS) are one of the emerging oligomers exhibiting prebiotic activity. They are mainly produced by the hydrolysis of cheap and abundant lignocellulosic feedstocks. It has been demonstrated that the oligomers with low degree of polymerization (2–4) are more selective for the microbiota. Therefore, several attempts have been made to develop efficient processes for the production of such types of XOS by employing different strategies. Microbial xylanases possess a crucial role in these processes. They are highly specific biocatalysts that do not produce any undesirable byproducts during oligomer production. This review provides detailed information about the xylanases belonging to different glycoside hydrolase families with respect to their ability to produce XOS. The enzyme integrated methodologies for XOS production are described from a commercial perspective. The studies focusing on XOS production by xylanase enzymes from different microbial sources were summarized in a strain specific manner for the first time. The physicochemical and technological properties of XOS, their current applications, and market potential were presented.


Similar content being viewed by others
Data availability
Not applicable.
References
Valdes AM, Walter J, Segal E, Spector TD (2018) Role of the gut microbiota in nutrition and health. BMJ 361:36–44. https://doi.org/10.1136/bmj.j2179
Carlson JL, Erickson JM, Lloyd BB, Slavin JL (2018) Health effects and sources of prebiotic dietary fiber. Curr Dev Nutr 2(3):1–4. https://doi.org/10.1093/cdn/nzy005
Farias DdP, de Araujo FF, Neri-Numa IA, Pastore GM (2019) Prebiotics: trends in food, health and technological applications. Trends Food Sci Technol 93:23–35. https://doi.org/10.1016/j.tifs.2019.09.004
Vazquez MJ, Alonso JL, Dominguez H, Parajo JC (2000) Xylooligosaccharides: manufacture and applications. Trends Food Sci Technol 11(11):387–393. https://doi.org/10.1016/S0924-2244(01)00031-0
Amorim C, Silverio SC, Prather KLJ, Rodrigues LR (2019) From lignocellulosic residues to market: production and commercial potential of xylooligosaccharides. Biotechnol Adv 37(7):107397. https://doi.org/10.1016/j.biotechadv.2019.05.003
Amorim C, Silverio SC, Silva SP, Coelho E, Coimbra MA, Prather KLJ, Rodrigues LR (2018) Single-step production of arabino-xylooligosaccharides by recombinant Bacillus subtilis 3610 cultivated in brewers’ spent grain. Carbohydr Polym 199:546–554. https://doi.org/10.1016/j.carbpol.2018.07.017
Van Craeyveld V, Swennen K, Dornez E, Van de Wiele T, Marzorati M, Verstraete W, Delaedt Y, Onagbesan O, Decuypere E, Buyse J, De Ketelaere B, Broekaert WF, Delcour JA, Courtin CM (2008) Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J Nutr 138:2348–2355. https://doi.org/10.3945/jn.108.094367
Ho AL, Kosik O, Lovegrove A, Charalampopoulos D, Rastall RA (2018) In vitro fermentability of xylo-oligosaccharide and xylo-polysaccharide fractions with different molecular weights by human faecal bacteria. Carbohydr Polym 179:50–58. https://doi.org/10.1016/j.carbpol.2017.08.077
Santibanez L, Henriquez C, Corro-Tejeda R, Bernal S, Armijo B, Salazar O (2021) Xylooligosaccharides from lignocellulosic biomass: a comprehensive review. Carbohydr Polym 251:117118. https://doi.org/10.1016/j.carbpol.2020.117118
Palaniappan A, Antony U, Emmambux MN (2021) Current status of xylooligosaccharides: production, characterization, health benefits and food application. Trends Food Sci Technol 111:506–519. https://doi.org/10.1016/j.tifs.2021.02.047
Carvalho AFA, Neto PO, da Silva DF, Pastore GM (2013) Xylooligosaccharides from lignocellulosic materials: chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res Int 51:75–85. https://doi.org/10.1016/j.foodres.2012.11.021
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23. https://doi.org/10.1016/j.femsre.2004.06.005
Kabel MA, Kortenoeven L, Schols HA, Voragen AGJ (2002) In vitro fermentability of differently substituted xylo-oligosaccharides. J Agric Food Chem 50(21):6205–6210. https://doi.org/10.1021/jf020220r
Karlsson EN, Schmitz E, Linares-Pasten JA, Adlercreutz P (2018) Endo-xylanases as tools for production of substituted xylooligosaccharides with prebiotic properties. Appl Microbiol Biotechnol 102:9081–9088. https://doi.org/10.1007/s00253-018-9343-4
Ohbuchi T, Takahashi T, Azumi N, Sakaino M (2009) Structual analysis of neutral and acidic xylooligosaccharides from hardwood kraft pulp, and their utilization by intestinal bacteria in vitro. Biosci Biotechnol Biochem 73(9):2070–2076. https://doi.org/10.1271/bbb.90260
Falck P, Precha-Atsawanan S, Grey C, Immerzeel P, Stalbrand H, Adlercreutz P, Karlsson EN (2013) Xylooligosaccharides from hardwood and cereal xylans produced by a thermostable xylanase as carbon sources for Lactobacillus brevis and Bifidobacterium adolescentis. J Agric Food Chem 61(30):7333–7340. https://doi.org/10.1021/jf401249g
Liu X, Yang S, Ma J, Yu J, Yan Q, Jiang Z (2020) Efficient production of acetylated xylooligosaccharides from Hawthorn kernels by a xylanase from Paecilomyces aerugineus. Ind Crop Prod 158:112962. https://doi.org/10.1016/j.indcrop.2020.112962
Puchart V, Biely P (2015) Redistribution of acetyl groups on the non-reducing end xylopyranosyl residues and their removal by xylan deacetylases. Appl Microbiol Biotechnol 99:3865–3873. https://doi.org/10.1007/s00253-014-6160-2
Puchart V, Suchova K, Biely P (2021) Xylanases of glycoside hydrolase family 30 – an overview. Biotechnol Adv 47:107704. https://doi.org/10.1016/j.biotechadv.2021.107704
http://www.cazy.org/Glycoside-Hydrolases.html (Accession: January 2022).
Hurlbert JC, Preston JF (2001) Functional characterization of a novel xylanase from a corn strain of Erwinia chrysanthemi. J Bacteriol 183(6):2093–2100. https://doi.org/10.1128/JB.183.6.2093-2100.2001
St John FJ, Rice JD, Preston JF (2006) Characterization of XynC from Bacillus subtilis subsp. subtilis strain 168 and analysis of ıts role in depolymerization of glucuronoxylan. J Bacteriol 188(24):8617–8626. https://doi.org/10.1128/JB.01283-06
Gallardo O, Fernandez-Fernandez M, Valls C, Valenzuela SV, Roncero MB, Vidal T, Diaz P, Pastor FIJ (2010) Characterization of a family GH5 xylanase with activity on neutral oligosaccharides and evaluation as a pulp bleaching aid. Appl Environ Microbiol 76(18):6290–6294. https://doi.org/10.1128/AEM.00871-10
St John FJ, Gonzalez JM, Pozharski E (2010) Consolidation of glycosyl hydrolase family 30: a dual domain 4/7 hydrolase family consisting of two structurally distinct groups. FEBS Lett 584:4435–4441. https://doi.org/10.1016/j.febslet.2010.09.051
Suzuki T, Ibata K, Hatsu M, Takamizawa K, Keiichi K (1997) Cloning and expression of a 58-kDa xylanase VI gene (xynD) of Aeromonas caviae ME-1 in Escherichia coli which is not categorized as a family F or family G xylanase. J Ferment Bioeng 84:86–89. https://doi.org/10.1016/S0922-338X(97)82792-4
Kleywegt GJ, Zou JY, Divne C, Davies GJ, Sinning I, Stahlberg J, Reinikainen T, Srisodsuk M, Teeri TT, Jones TA (1997) The crystal structure of the catalytic core domain of endoglucanase I from Trichoderma reesei at 3.6 A resolution, and a comparison with related enzymes. J Mol Biol 272:383–397. https://doi.org/10.1006/jmbi.1997.1243
Gosalbes MJ, Perez-Gonzalez JA, Gonzalez R, Navarro A (1991) Two beta-glycanase genes are clustered in Bacillus polymyxa: molecular cloning, expression, and sequence analysis of genes encoding a xylanase and an endo-beta- (1,3)-(1,4)-glucanase. J Bacteriol 173:7705–7710. https://doi.org/10.1128/jb.173.23.7705-7710.1991
Collins T, Meuwis MA, Stals I, Claeyssens M, Feller G, Gerday C (2002) A novel family 8 xylanase, functional and physicochemical characterization. J Biol Chem 277:35133–35139. https://doi.org/10.1074/jbc.M204517200
Berrin JG, Juge N (2008) Factors affecting xylanase functionality in the degradation of arabinoxylan. Biotechnol Lett 30:1139–1150. https://doi.org/10.1007/s10529-008-9669-6
Motta FL, Andrade CCP, Santana MHA (2013) A review of xylanase production by the fermentation of xylan: classification, characterization and applications. In: Chandel AK, da Silva SS (eds.), Sustainable degradation of lignocellulosic biomass - techniques, applications and commercialization, IntechOpen, pp 251–275. https://www.intechopen.com/chapters/44332
Pollet A, Delcour JA, Courtin CM (2010) Structural determinants of the substrate specificities of xylanases from different glycoside hydrolase families. Crit Rev Biotechnol 30(3):176–191. https://doi.org/10.3109/07388551003645599
Biely P, Vrsanska M, Tenkanen M, Kluepfe ID (1997) Endo-β-1,4-xylanase families: differences in catalytic properties. J Biotechnol 57:151–166. https://doi.org/10.1016/S0168-1656(97)00096-5
Vardakou M, Flint J, Christakopoulos P, Lewis RJ, Gilbert HJ, Murray JW (2005) A family 10 Thermoascus aurantiacus xylanase utilizes arabinose decorations of xylan as significant substrate specificity determinants. J Mol Biol 352:1060–1067. https://doi.org/10.1016/j.jmb.2005.07.051
Derewenda U, Swenson L, Green R, Wei Y, Morosoli R, Shareck F, Kluepfel D, Derewenda ZS (1994) Crystal structure, at 2.6-A resolution, of the Streptomyces lividans xylanase A, a member of the F family of beta-1,4-d-glycanases. J Biol Chem 269:20811–20814. https://doi.org/10.1016/S0021-9258(17)31892-6
Solomon V, Teplitsky A, Shulami S, Zolotnitsky G, Shoham Y, Shoham G (2007) Structure–specificity relationships of an intracellular xylanase from Geobacillus stearothermophilu. Acta Crystallogr D Struc Biol 63:845–859. https://doi.org/10.1107/S0907444907024845
Pell G, Szabo L, Charnock SJ, Xie H, Gloster TM, Davies GJ, Gilbert HJ (2004) Structural and biochemical analysis of Cellvibrio japonicus xylanase 10C: how variation in substrate binding cleft influences the catalytic profile of family GH-10 xylanases. J Biol Chem 279:11777–11788. https://doi.org/10.1074/jbc.M311947200
http://www.cazy.org/GH11.html (Accession: January 2022).
Bray MR, Clarke AJ (1992) Action pattern of xylo-oligosaccharide hydrolysis by Schizophyllum commune xylanase A. Euro J Biochem 204:191–196. https://doi.org/10.1111/j.1432-1033.1992.tb16623.x
Berrin JG, Ajandouz EH, Georis J, Arnaut F, Juge N (2007) Substrate and product hydrolysis specificity in family 11 glycoside hydrolases: an analysis of Penicillium funiculosum and Penicillium griseofulvum xylanases. Appl Microbiol Biotechnol 74:1001–1010. https://doi.org/10.1007/s00253-006-0764-0
Cervera Tison M, Andre-Leroux G, Lafond M, Georis J, Juge N, Berrin JG (2009) Molecular determinants of substrate and inhibitor specificities of the Penicillium griseofulvum family 11 xylanases. Biochim Biophys Acta 1794:438–445. https://doi.org/10.1016/j.bbapap.2008.11.024
Fushinobu S, Ito K, Konno M, Wakagi T, Matsuzawa H (1998) Crystallographic and mutational analyses of an extremely acidophilic and acid-stable xylanase: biased distribution of acidic residues and importance of Asp37 for catalysis at low pH. Protein Eng 11:1121–1128. https://doi.org/10.1093/protein/11.12.1121
Oakley AJ, Heinrich T, Thompson CA, Wilce MCJ (2003) Characterization of a family 11 xylanase from Bacillus subtilis B230 used for paper bleaching. Acta Crystallogr D Biol Crystallogr 59:627–636. https://doi.org/10.1107/S0907444903001227
Payan F, Leone P, Porciero S, Furniss C, Tahir TA, Williamson G, Durand A, Manzanares P, Gilbert HJ, Juge N, Roussel A (2004) The dual nature of the wheat xylanase protein inhibitor XIP-1 — structural basis for the inhibition of family 10 and family 11 xylanases. J Biol Chem 279:36029–36037. https://doi.org/10.1074/jbc.M404225200
Wouters J, Georis J, Engher D, Vandenhaute J, Dusart J, Frere JM, Depiereux E, Charlier P (2001) Crystallographic analysis of family 11 endo-β-1,4-xylanase Xyl1 from Streptomyces sp. S38. Acta Crystallogr D Biol Crystallogr 57:1813–1819. https://doi.org/10.1107/S0907444901015153
Gruber K, Klintschar G, Hayn M, Schlacher A, Steiner W, Kratky C (1998) Thermophilic xylanase from Thermomyces lanuginosus: high-resolution X-ray structure and modeling studies. Biochem 37:13475–13485. https://doi.org/10.1021/bi980864l
Yegin S (2017) Single-step purification and characterization of an extreme halophilic, ethanol tolerant and acidophilic xylanase from Aureobasidium pullulans NRRL Y-2311-1 with application potential in the food industry. Food Chem 221:67–75. https://doi.org/10.1016/j.foodchem.2016.10.003
Paes G, Berrin JG, Beaugrand J (2012) GH11 xylanases: structure/function/properties relationships and applications. Biotechnol Adv 30:564–592. https://doi.org/10.1016/j.biotechadv.2011.10.003
Katsimpouras C, Dedes G, Thomaidis NS, Topakas E (2019) A novel fungal GH30 xylanase with xylobiohydrolase auxiliary activity. Biotechnol Biofuels 12:120. https://doi.org/10.1186/s13068-019-1455-2
Valenzuela SV, Diaz P, Pastor FIJ (2012) Modular glucuronoxylan-specific xylanase with a family CBM35 carbohydrate-binding module. Appl Environ Microbiol 78:3923–3931. https://doi.org/10.1128/AEM.07932-11
Maehara T, Yagi H, Sato T, Ohnishi-Kameyama M, Fujimoto Z, Kamino K, Kitamura Y, St JohnYaoiKaneko FKS (2018) GH30 glucuronoxylan-specific xylanase from Streptomyces turgidiscabies C56. Appl Environ Microbiol 84(4):e01850-e1917. https://doi.org/10.1128/AEM.01850-17
St John FJ, Dietrich D, Crooks C, Balogun P, de Serrano V, Pozharski E, Smith JK, Bales E, Hurlbert J (2018) A plasmid borne, functionally novel glycoside hydrolase family 30 subfamily 8 endoxylanase from solventogenic Clostridium. Biochem J 475:1533–1551. https://doi.org/10.1042/BCJ20180050
Biely P, Puchart V, Stringer MA, Mørkeberg Krogh KBR (2014) Trichoderma reesei XYN VI – a novel appendage dependent eukaryotic glucuronoxylan hydrolase. FEBS J 281:3894–3903. https://doi.org/10.1111/febs.12925
Nakamichi Y, Fouquet T, Ito S, Watanabe M, Matsushika A, Inoue H (2019) Structural and functional characterization of a GH30-7 xylanase B from the filamentous fungus Talaromyces cellulolyticus. J Biol Chem 294:4065–4078. https://doi.org/10.1074/jbc.RA118.007207
Surek E, Buyukkileci AO, Yegin S (2021) Processing of hazelnut (Corylus avellana L.) shell autohydrolysis liquor for production of low molecular weight xylooligosaccharides by Aureobasidium pullulans NRRL Y–2311–1 xylanase. Ind Crop Prod 161:113212. https://doi.org/10.1016/j.indcrop.2020.113212
Garrote G, Dominguez H, Parajo J (2002) Autohydrolysis of corncob: study of non-isothermal operation for xylooligosaccharide production. J Food Eng 52(3):211–218. https://doi.org/10.1016/S0260-8774(01)00108-X
Akpinar O, Erdogan K, Bostanci S (2009) Enzymatic production of xylooligosaccharide from selected agricultural wastes. Food Bioprod Process 87(2):145–151. https://doi.org/10.1016/j.fbp.2008.09.002
de Freitas C, Carmona E, Brienzo M (2019) Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact Carbohydr Diet Fibre 18:100184. https://doi.org/10.1016/j.bcdf.2019.100184
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Gowdhaman D, Ponnusami V (2015) Production and optimization of xylooligosaccharides from corncob by Bacillus aerophilus KGJ2 xylanase and its antioxidant potential. Int J Biol Macromol 79:595–600. https://doi.org/10.1016/j.ijbiomac.2015.05.046
Zhu Y, Kim TH, Lee YY, Chen R, Elander RT (2006) Enzymatic production of xylooligosaccharides from corn stover and corn cobs treated with aqueous ammonia. Appl Biochem Biotechnol 129–132:586–598. https://doi.org/10.1385/ABAB:130:1:586
Wan Azelee NI, Jahim JM, Ismail AF, Fuzi SFZM, Rahman RA, Illias RM (2016) High xylooligosaccharides (XOS) production from pretreated kenaf stem by enzyme mixture hydrolysis. Ind Crop Prod 81:11–19. https://doi.org/10.1016/j.indcrop.2015.11.038
Poletto P, Pereira GN, Monteiro CRM, Pereira MAF, Bordignon SE, de Oliveira D (2020) Xylooligosaccharides: transforming the lignocellulosic biomasses into valuable 5-carbon sugar prebiotics. Process Biochem 91:352–363. https://doi.org/10.1016/j.procbio.2020.01.005
Chen H, Liu J, Chang X, Chen D, Xue Y, Liu P, Lin H, Han S (2017) A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol 160:196–206. https://doi.org/10.1016/j.fuproc.2016.12.007
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18. https://doi.org/10.1016/j.biortech.2008.05.027
Mazlan NA, Samad KA, Wan Yussof H, Saufi SM, Jahim J (2019) Xylooligosaccharides from potential agricultural waste: characterization andscreening on the enzymatic hydrolysis factors. Ind Crop Prod 129:575–584. https://doi.org/10.1016/j.indcrop.2018.12.042
Ying W, Fang X, Xu Y, Zhang J (2022) Combined acetic acid and enzymatic hydrolysis for xylooligosaccharides and monosaccharides production from poplar. Biomass Bioenergy 158:106377. https://doi.org/10.1016/j.biombioe.2022.106377
Kumar V, Satyanarayana T (2015) Generation of xylooligosaccharides from microwave irradiated agroresidues using recombinant thermo-alkali-stable endoxylanase of the polyextremophilic bacterium Bacillus halodurans expressed in Pichia pastoris. Bioresour Technol 179:382–389. https://doi.org/10.1016/j.biortech.2014.12.049
Antov MG, Dordevic TR (2017) Environmental-friendly technologies for the production of antioxidant xylooligosaccharides from wheat chaff. Food Chem 235:175–180. https://doi.org/10.1016/j.foodchem.2017.05.058
Jagtap S, Deshmukh RA, Menon S, Das S (2017) Xylooligosaccharides production by crude microbial enzyme from agricultural waste without prior treatment and their potential applications as nutraceuticals. Bioresour Technol 245:283–288. https://doi.org/10.1016/j.biortech.2017.08.174
Purohit A, Rai SK, Chownk M, Sangwan RS, Yadav SK (2017) Xylanase from Acinetobacter pittii MASK 25 and developed magnetic crosslinked xylanase aggregate produce predominantly xylopentose and xylohexose from agro biomass. Bioresour Technol 244:793–799. https://doi.org/10.1016/j.biortech.2017.08.034
Pereira GF, Bastiani D, Gabardo S, Squina F, Ayub MAZ (2018) Solid-state cultivation of recombinant Aspergillus nidulans to co-produce xylanase, arabinofuranosidase, and xylooligosaccharides from soybean fibre. Biocatal Agric Biotechnol 15:78–85. https://doi.org/10.1016/j.bcab.2018.05.012
da Silva MB, Rossi DM, Squina F, Ayub MAZ (2018) Xylooligosaccharides production by fungi cultivations in rice husk and their application as substrate for lactic acid bacteria growth. Bioresour Technol Rep 2:100–106. https://doi.org/10.1016/j.biteb.2018.05.004
Zheng H, Sun M, Meng L, Pei H, Zhang X, Yan Z, Zeng W, Zhang J, Hu J, Lu F, Sun J (2014) Purification and characterization of a thermostable xylanase from Paenibacillus sp. NF1 and its application in xylooligosaccharides production. J Ind Microbiol Biotechnol 24:489–496. https://doi.org/10.4014/jmb.1312.12072
Heinen PR, Bauermeister A, Ribeiro LF, Messias JM, Almeida PZ, Moraes LAB, Vargas-Rechia CG, de Oliveira AHC, Ward RJ, Filho EXF, Kadowaki MK, Jorge JA, Polizeli MLTM (2018) GH11 xylanase from Aspergillus tamarii Kita: purification by one-step chromatography and xylooligosaccharides hydrolysis monitored in real-time by mass spectrometry. Int J Biol Macromol 108:291–299. https://doi.org/10.1016/j.ijbiomac.2017.11.150
Amorim C, Silverio SC, Rodrigues LR (2019) One-step process for producing prebiotic arabino-xylooligosaccharides from brewer’s spent grain employing Trichoderma species. Food Chem 270:86–94. https://doi.org/10.1016/j.foodchem.2018.07.080
Reque PM, Pinilla CMB, Gauterio GV, Kalil SJ, Brandelli A (2019) Xylooligosaccharides production from wheat middlings bioprocessed with Bacillus subtilis. Food Res Int 126:108673. https://doi.org/10.1016/j.foodres.2019.108673
Wang J, Zhang S, Li C, Liu X, Xu Z, Wang T (2022) Efficient secretion of xylanase in Escherichia coli for production of prebiotic xylooligosaccharides. LWT 162:113481. https://doi.org/10.1016/j.lwt.2022.113481
de Figueiredo FC, Carvalho AFA, Brienzo M, Campioni TS, de Oliva-Neto P (2017) Chemical input reduction in the arabinoxylan and lignocellulose alkaline extraction and xylooligosaccharides production. Bioresour Technol 228:164–170. https://doi.org/10.1016/j.biortech.2016.12.097
Cologna NMD, Gómez-Mendoza DP, Zanoelo FF, Giannesi GC, Guimarães NCA, Moreira LRS, Filho EXF, Ricart CAO (2018) Exploring Trichoderma and Aspergillus secretomes: proteomics approaches for the identification of enzymes of biotechnological interest. Enzyme Microb Technol 109:1–10. https://doi.org/10.1016/j.enzmictec.2017.08.007
Aachary AA, Prapulla SG (2008) Corncob-induced endo-1,4-β-D-xylanase of Aspergillus oryzae MTCC 5154: production and characterization of xylobiose from glucuronoxylan. J Agric Food Chem 56(11):3981–4398. https://doi.org/10.1021/jf073430i
Akpinar O, Ak O, Kavas A, Bakir U, Yilmaz L (2007) Enzymatic production of xylooligosaccharides from cotton stalks. J Agric Food Chem 55(14):5544–5551. https://doi.org/10.1021/jf063580d
Chapla D, Pandit P, Shah A (2012) Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour Technol 115:215–221. https://doi.org/10.1016/j.biortech.2011.10.083
de Freitas C, Terrone CC, Masarin F, Carmona EC, Brienzo M (2021) In vitro study of the effect of xylooligosaccharides obtained from banana pseudostem xylan by enzymatic hydrolysis on probiotic bacteria. Biocatal Agric Biotechnol 33:101973. https://doi.org/10.1016/j.bcab.2021.101973
Aachary AA, Prapulla SG (2009) Value addition to corncob: Production and characterization of xylooligosaccharides from alkali pretreated lignin-saccharide complex using Aspergillus oryzae MTCC 5154. Bioresour Technol 100(2):991–995. https://doi.org/10.1016/j.biortech.2008.06.050
Yang CY, Sheih IC, Fang TJ (2012) Fermentation of rice hull by Aspergillus japonicus under ultrasonic pretreatment. Ultrason Sonochem 19:687–691
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463. https://doi.org/10.1016/j.enzmictec.2007.01.018
Aragon CC, Santos AF, Ruiz-Matute AI, Corzo N, Guisan JM, Monti R, Mateo C (2013) Continuous production of xylooligosaccharides with immobilized-stabilized biocatalysts of xylanase from Aspergillus versicolor. J Mol Catal B Enzym 98:8–14. https://doi.org/10.1016/j.molcatb.2013.09.017
Heinen PR, Pereira MG, Rechia CGV, Almeida PZ, Monteiro LMO, Pasin TM, Messias JM, Cereia M, Kadowaki MK, Jorge JA, Polizeli MLTM (2017) Immobilized endo-xylanase of Aspergillus tamarii Kita: an interesting biological tool for production of xylooligosaccharides at high temperatures. Process Biochem 53:145–152. https://doi.org/10.1016/j.procbio.2016.11.021
Felipuci JP, Schmatz AA, de Angelis DA, Brienzo M (2021) Biological pretreatment improved subsequent xylan chemical solubilization. Biomass Convers Biorefin.https://doi.org/10.1007/s13399-021-01506-w
Yamamoto Y, Kishimura H, Kinoshita Y, Saburi W, Kumagai Y, Yasui H, Ojima T (2019) Enzymatic production of xylooligosaccharides from red alga dulse (Palmaria sp.) wasted in Japan. Process Biochem 82:117–122. https://doi.org/10.1016/j.procbio.2019.03.030
Chen Z, Zaky AA, Liu YL, Chen YY, Liu L, Li ST, Jia YM (2019) Purification and characterization of a new xylanase with excellent stability from Aspergillus flavus and its application in hydrolyzing pretreated corncobs. Protein Expr Purif 154:91–97. https://doi.org/10.1016/j.pep.2018.10.006
Ismail SA, Nour SA, Hassan AA (2022) Valorization of corn cobs for xylanase production by Aspergillus flavus AW1 and its application in the production of antioxidant oligosaccharides and removal of food stain. Biocatal Agric Biotechnol 41:102311. https://doi.org/10.1016/j.bcab.2022.102311
Vieira TF, Corrêa RCG, de Fatima Peralta Muniz Moreira R, Peralta RA, de Lima EA, Helm CV, Garcia JAA, Bracht A, Peralta RM (2021) Valorization of peach palm (Bactris gasipaes Kunth) waste: production of antioxidant xylooligosaccharides. Waste Biomass Valori 12:6727–6740. https://doi.org/10.1007/s12649-021-01457-3
Aiewviriyasakul K, Bunterngsook B, Lekakarn H, Sritusnee W, Kanokratana P, Champreda V (2021) Biochemical characterization of xylanase GH11 isolated from Aspergillus niger BCC14405 (XylB) and its application in xylooligosaccharide production. Biotechnol Lett 43:2299–2310. https://doi.org/10.1007/s10529-021-03202-1
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87(3):787–799. https://doi.org/10.1007/s00253-010-2632-1
Sabiha-Hanim S, Noor MA, Rosma A (2011) Effect of autohydrolysis and enzymatic treatment on oil palm (Elaeis guineensis Jacq.) frond fibres for xylose and xylooligosaccharides production. Bioresour Technol 102(2):1234–1239. https://doi.org/10.1016/j.biortech.2010.08.017
Jayapal N, Samanta AK, Kolte AP, Senani S, Sridhar M, Suresh KP, Sam-path KT (2013) Value addition to sugarcane bagasse: xylan extraction and its process optimization for xylooligosaccharides production. Ind Crops Prod 42:14–24. https://doi.org/10.1016/j.indcrop.2012.05.019
Qian S, Zhou J, Chen X, Ji W, Zhang L, Hu W, Lu Z (2020) Evaluation of an efficient fed-batch enzymatic hydrolysis strategy to improve production of functional xylooligosaccharides from maize straws. Ind Crop Prod 157:112920. https://doi.org/10.1016/j.indcrop.2020.112920
de Oliveira SM, Moreno-Perez S, Terrasan CRF, Romero-Fernandez M, Vieira MF, Guisan JM, Rocha-Martin J (2018) Covalent immobilization-stabilization of β-1,4-endoxylanases from Trichoderma reesei: production of xylooligosaccharides. Process Biochem 64:170–176. https://doi.org/10.1016/j.procbio.2017.09.018
Su Y, Fang L, Wang P, Lai C, Huang C, Ling Z, Sun S, Yong Q (2021) Efficient production of xylooligosaccharides rich in xylobiose and xylotriose from poplar by hydrothermal pretreatment coupled with post-enzymatic hydrolysis. Bioresour Technol 342:125955. https://doi.org/10.1016/j.biortech.2021.125955
Rathamat Z, Choorit W, Chsti Y, Prasertsan P (2021) Two-step isolation of hemicellulose from oil palm empty fruit bunch fibers and its use in production of xylooligosaccharide prebiotic. Ind. Crop Prod. 160:113124. https://doi.org/10.1016/j.indcrop.2020.113124
Houbraken J, de Vries RP, Samson RA (2014) Chapter four- modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Adv Appl Microbiol 86:199–249. https://doi.org/10.1016/B978-0-12-800262-9.00004-4
Arumugam N, Biely P, Puchart V, Singha S, Pillai S (2018) Structure of peanut shell xylan and its conversion to oligosaccharides. Process Biochem 72:124–129. https://doi.org/10.1016/j.procbio.2018.06.024
Singh R, Nadar C, Muir J, Arora A (2019) Green and clean process to obtain low degree of polymerisation xylooligosaccharides from almond shell. J Clean Prod 241:118237. https://doi.org/10.1016/j.jclepro.2019.118237
Katapodis P, Kavarnou A, Kintzios S, Pistola E, Kekos D, Macris BJ, Christakopoulos P (2002) Production of acidic xylo-oligosaccharides by a family 10 endoxylanase from Thermoascus aurantiacus and use as plant growth regulators. Biotechnol Lett 24:1413–1416. https://doi.org/10.1023/A:1019898414801
Garbin AP, Garcia NFL, Cavalheiro GF, Silvestre MA, Rodrigues A, Paz MF, Fonseca GG, Leite RSR (2021) β-glucosidase from thermophilic fungus Thermoascus crustaceus: production and industrial potential. An Acad Bras Cienc 93(1):e20191349. https://doi.org/10.1590/0001-3765202120191349
Brienzo M, Carvalho W, Milagres AMF (2010) Xylooligosaccharides production from alkali pretreated sugarcane bagasse using xylanase from Thermoascus aurantiacus. Appl Biochem Biotechnol 162:1195–1205. https://doi.org/10.1007/s12010-009-8892-5
Kalogeris E, Christakopoulos P, Kekos D, Macris BJ (1998) Studies on the solid-state production of thermostable endoxylanases from Thermoascus aurantiacus: characterization of two isozymes. J Biotechnol 60(3):155–163. https://doi.org/10.1016/S0168-1656(97)00186-7
Nacos M, Katapodis P, Pappas C, Daferera D, Tarantilis PA, Christakopoulos P, Polissiou M (2006) Kenaf xylan — a source of biologically active acidic oligosaccharides. Carbohydr Polym 66(1):126–134. https://doi.org/10.1016/j.carbpol.2006.02.032
Nascimento CEO, Simoes LCO, Pereira JC, Silva RR, Lima EA, Almeida GC, Penna ALB, Boscolo M, Gomes E, Silva R (2022) Application of a recombinant GH10 endoxylanase from Thermoascus aurantiacus for xylooligosaccharide production from sugarcane bagasse and probiotic bacterial growth. J Biotechnol 347:1–8. https://doi.org/10.1016/j.jbiotec.2022.02.003
Gostincar C, Ohm RA, Kogej T, Sonjak S, Turk M, Zajc J, Zalar P, Grube M, Sun H, Han J, Sharma A, Chiniquy J, Ngan CY, Lipzen A, Barry K, Grigoriev IV, Gunde-Cimerman N (2014) Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genom 15:549. https://doi.org/10.1186/1471-2164-15-549
Gauterio GV, Vieira MC, Goncalves LGG, Hubner T, Sanzo AVL, Kalil SJ (2018) Production of xylanolitic enzymes and xylooligosaccharides by Aureobasidium pullulans CCT 1261 in submerged cultivation. Ind Crop Prod 125:335–345. https://doi.org/10.1016/j.indcrop.2018.09.011
Gauterio GV, da Silva LGG, Hubner T, Ribeiro TDR, Kalil SJ (2021) Xylooligosaccharides production by crude and partially purified xylanase from Aureobasidium pullulans: biochemical and thermodynamic properties of the enzymes and their application in xylan hydrolysis. Process Biochem 104:161–170. https://doi.org/10.1016/j.procbio.2021.03.009
Gauterio GV, Hübner T, Ribeiro TDR, Ziotti APM, Kalil SJ (2022) Xylooligosaccharide production with low xylose release using crude Xylanase from Aureobasidium pullulans: effect of the enzymatic hydrolysis parameters. Appl Biochem Biotechnol 194:862–881. https://doi.org/10.1007/s12010-021-03658-x
Yegin S, Buyukkileci AO, Sargin S, Goksungur Y (2017) Exploitation of agricultural wastes and by-products for production of Aureobasidium pullulans Y-2311-1 xylanase: screening, bioprocess optimization and scale up. Waste Biomass Valori 8(3):999–1010. https://doi.org/10.1007/s12649-016-9646-6
Dong Q, Wang H, Xing X, Ji S (2012) Identification and characterization of a special species of Paecilomyces. Ann Microbiol 62:1587–1592. https://doi.org/10.1007/s13213-011-0414-3
Teng C, Yan Q, Jiang Z, Fan G, Shi B (2010) Production of xylooligosaccharides from the steam explosion liquor of corn cobs coupled with enzymatic hydrolysis using a thermostable xylanase. Bioresour Technol 101:7679–7682. https://doi.org/10.1016/j.biortech.2010.05.004
Abdella A, Ramadan S, Hamouda RA, Saddiq AA, Alhazmi NM, Al-Saman MA (2021) Paecilomyces variotii xylanase production, purification and characterization with antioxidant xylo-oligosaccharides production. Sci Rep 11:16468. https://doi.org/10.1038/s41598-021-95965-w
Jaichakan P, Thongsook T, Nakphaichit M, Wattanasiritham LS, Phongthai S, Pattarapisitporn A, Utama-ang N, Laokuldilok T, Klangpetch W (2022) Xylobiose and xylotriose production from alkali soluble defatted rice bran arabinoxylan using endoxylanase from Neocallimastix partriciarum. Starch-Stärke 74(3–4):2100177. https://doi.org/10.1002/star.202100177
Schallmey M, Singh A, Ward OP (2004) Developments in the use of Bacillus species in industrial production. Can J Microbiol 50:1–17. https://doi.org/10.1139/w03-076
Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J (2019) Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front Microbiol 10:302. https://doi.org/10.3389/fmicb.2019.00302
Qeshmi FI, Homaei A, Fernandes P, Hemmati R, Dijkstra BW, Khajeh K (2020) Xylanases from marine microorganisms: a brief overview on scope, sources, features and potential applications. Biochim Biophys Acta Proteins Proteom 1868(2):140312. https://doi.org/10.1016/j.bbapap.2019.140312
Ghosh A, Chandra A, Dhar A, Shukla P, Baishya D (2021) Multi-efficient thermostable endoxylanase from Bacillus velezensis AG20 and its production of xylooligosaccharides as efficient prebiotics with anticancer activity. Process Biochem 109:59–71. https://doi.org/10.1016/j.procbio.2021.06.011
Bragatto J, Segato F, Squina FM (2013) Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinant xylanase from Bacillus subtilis. Ind Crop Prod 51:123–129. https://doi.org/10.1016/j.indcrop.2013.08.062
Kallel F, Driss D, Bouaziz F, Neifer M, Ghorbel R, Chaabouni SE (2015) Production of xylooligosaccharides from garlic straw xylan by purified xylanase from Bacillus mojavensis UEB-FK and their in vitro evaluation as prebiotics. Food Bioprod Process 94:536–546. https://doi.org/10.1016/j.fbp.2014.07.012
Tseng YH, Lee WC, Krisomdee K, Natesuntorn W, Chatsurachai S, Sriroth K (2022) Xylooligosaccharide production from sugarcane bagasse using recombinant endoxylanase of Bacillus halodurans. Sugar Tech 24(4):1029–1036. https://doi.org/10.1007/s12355-021-01096-x
Ratnadewi AAI, Santoso AB, Sulistyaningsih E, Handayani W (2016) Application of cassava peel and waste as raw materials for xylooligosaccharide production using endoxylanase from Bacillus subtilis of soil termite abdomen. Procedia Chem 18:31–38. https://doi.org/10.1016/j.proche.2016.01.007
Reddy SS, Krishnan C (2016) Production of xylooligosaccharides in SSF by Bacillus subtilis KCX006 producing β-xylosidase-free endo-xylanase and multiple xylan debranching enzymes. Prep Biochem Biotechnol 46:49–55. https://doi.org/10.1080/10826068.2014.970694
Battan B, Sharma J, Kuhad RC (2006) High-level xylanase production by alkaliphilic Bacillus pumilus ASH under solid state fermentation. World J Microbiol Biotechnol 22:1281–1287. https://doi.org/10.1007/s11274-006-9173-x
Sanghi A, Garg N, Sharma J, Kuhar K, Kuhad RC, Gupta VK (2008) Optimization of xylanase production using inexpensive agro-residues by alkalophilic Bacillus subtilis ASH in solid-state fermentation. World J Microbiol Biotechnol 24:633–640. https://doi.org/10.1007/s11274-007-9521-5
Reddy SS, Krishnan C (2016) Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT Food Sci Technol 65:237–245. https://doi.org/10.1016/j.lwt.2015.08.013
Chang S, Guo Y, Wu B, He B (2017) Extracellular expression of alkali tolerant xylanase from Bacillus subtilis Lucky9 in E. coli and application for xylooligosaccharides production from agro-industrial waste. Int J Biol Macromol 96:249–256. https://doi.org/10.1016/j.ijbiomac.2016.11.032
Chang S, Chu J, Guo Y, Li H, Wu B, He B (2017) An efficient production of high-pure xylooligosaccharides from corncob with affinity adsorption-enzymatic reaction integrated approach. Bioresour Technol 241:1043–1049. https://doi.org/10.1016/j.biortech.2017.06.002
Khandeparker R, Parab P, Amberkar U (2017) Recombinant xylanase from Bacillus tequilensis BT21: biochemical characterization and its application in the production of xylobiose from agricultural residues. Food Technol Biotechnol 55(2):164–172. https://doi.org/10.17113/ftb.55.02.17.4896
Liu MQ, Huo WK, Xu X, Weng XY (2017) Recombinant Bacillus amyloliquefaciens xylanase A expressed in Pichia pastoris and generation of xylooligosaccharides from xylans and wheat bran. Int J Biol Macromol 105:656–663. https://doi.org/10.1016/j.ijbiomac.2017.07.073
Hero JS, Pisa JH, Raimondo EE, Martínez MA (2021) Proteomic analysis of secretomes from Bacillus sp. AR03: characterization of enzymatic cocktails active on complex carbohydrates for xylooligosaccharides production. Prep Biochem Biotechnol 51(9):871–880. https://doi.org/10.1080/10826068.2020.1870136
Khushboo KP, Dubey KK, Usmani Z, Sharma M, Gupta VK (2021) Biotechnological and industrial applications of Streptomyces metabolites. Biofuel Bioprod Biorefin 16:244–264. https://doi.org/10.1002/bbb.2294
Procopio RE, Silva IR, Martins MK, Azevedo JL, Araujo JM (2012) Antibiotics produced by Streptomyces. Braz J Infect Dis 16(5):466–471. https://doi.org/10.1016/j.bjid.2012.08.014
Ai Z, Jiang Z, Li L, Deng W, Kusakabe I, Li H (2005) Immobilization of Streptomyces olivaceoviridis E-86 xylanase on Eudragit S-100 for xylooligosaccharide production. Process Biochem 40:2707–2714. https://doi.org/10.1016/j.procbio.2004.12.006
Li XT, Li E, Zhu Y, Ten C, Sun BG, Song H, Yang R (2012) A typical endoxylanasefrom Streptomyces rameus L2001 and its unique characteristics in xylooligosaccharide production. Carbohydr Res 359:30–36. https://doi.org/10.1016/j.carres.2012.05.005
Boonchuay P, Techapun C, Seesuriyachan P, Chaiyaso T (2014) Production of xylooligosaccharides from corncob using a crude thermostable endo-xylanase from Streptomyces thermovulgaris TISTR1948 and prebiotic properties. Food Sci Biotechnol 23:1515–1523. https://doi.org/10.1007/s10068-014-0207-0
Seesuriyachan P, Kawee-ai A, Chaiyaso T (2017) Green and chemical-free process of enzymatic xylooligosaccharide production from corncob: enhancement of the yields using a strategy of lignocellulosic destructuration by ultra-high pressure pretreatment. Bioresour Technol 241:537–544. https://doi.org/10.1016/j.biortech.2017.05.193
Liu L, Xu M, Cao Y, Wang H, Shao J, Xu M, Zhang Y, Wang Y, Zhang W, Meng X, Liu W (2020) Biochemical characterization of xylanases from Streptomyces sp. B6 and their application in the xylooligosaccharide production from viscose fiber production waste. J Agric Food Chem 68(10):3184–3194. https://doi.org/10.1021/acs.jafc.9b06704
Liberato V, Benevenuti C, Coelho F, Botelho A, Amaral P, Pereira NJ, Ferreira T (2019) Clostridium sp. as bio-catalyst for fuels and chemicals production in a biorefinery context. Catalysts 9:962. https://doi.org/10.3390/catal9110962
Rajagopalan G, Yew KW, He J, Yang KL (2013) Production, purification, and characterization of a xylooligosaccharides-forming xylanase from high-butanol-producing strain Clostridium sp. BOH3. Bioenerg Res 6(2):448–457. https://doi.org/10.1007/s12155-012-9259-2
Rajagopalan G, Shanmugavelu K, Yang KL (2017) Production of prebiotic-xylooligosaccharides from alkali pretreated mahogany and mango wood sawdust by using purified xylanase of Clostridium strain BOH3. Carbohydr Polym 167:158–166. https://doi.org/10.1016/j.carbpol.2017.03.021
Mandelli F, Brenelli LB, Almeida RF, Goldbeck R, Wolf LD, Hoffmam ZB, Ruller R, Rocha GJM, Mercadante AZ, Squina FM (2014) Simultaneous production of xylooligosaccharides and antioxidant compounds from sugarcane bagasse via enzymatic hydrolysis. Ind Crop Prod 52:770–775. https://doi.org/10.1016/j.indcrop.2013.12.005
Daud NS, Mohd Din ARJ, Rosli MA, Azam ZM, Othman NZ, Sarmidi MR (2019) Paenibacillus polymyxa bioactive compounds for agricultural and biotechnological applications. Biocatal Agric Biotechnol 18:101092. https://doi.org/10.1016/j.bcab.2019.101092
Xu Z, Zhang S, Mu Y, Kong J (2018) Paenibacillus panacisoli enhances growth of Lactobacillus spp. by producing xylooligosaccharides in corn stover ensilages. Carbohydr Polym 184:435–444. https://doi.org/10.1016/j.carbpol.2017.12.044
Liu X, Liu Y, Jiang Z, Liu H, Yang S, Yan Q (2018) Biochemical characterization of a novel xylanase from Paenibacillus barengoltzii and its application in xylooligosaccharides production from corncobs. Food Chem 264:310–318. https://doi.org/10.1016/j.foodchem.2018.05.023
Thakur V, Kumar V, Kumar V, Singh D (2022) Xylooligosaccharides production using multi-substrate specific xylanases secreted by a psychrotolerant Paenibacillus sp. PCH8. Carbohyd Polym Technol App 3:100215. https://doi.org/10.1016/j.carpta.2022.100215
Liolios K, Sikorski J, Lu M, Nolan M, Lapidus A, Lucas S, Hammon N, Deshpande S, Cheng JF, Tapia R, Han C, Goodwin L, Pitluck S, Huntemann M, Ivanova N, Pagani I, Mavromatis K, Ovchinikova G, Pati A, Chen A, Palaniappan K, Land M, Hauser L, Brambilla EM, Kotsyurbenko O, Rohde M, Tindall BJ, Abt B, Göker M, Detter JC, Woyke T, Bristow J, Eisen JA, Markowitz V, Hugenholtz P, Klenk HP, Kyrpides NC (2011) Complete genome sequence of the gliding, heparinolytic Pedobacter saltans type strain (113). Stand Genom Sci 5(1):30–40. https://doi.org/10.4056/sigs.2154937
Sharma K, Antunes IL, Rajulapati V, Goyal A (2018) Molecular characterization of a first endo-acting beta-1,4-xylanase of family 10 glycoside hydrolase (PsGH10A) from Pseudopedobacter saltans comb. nov. Process Biochem 70:79–89. https://doi.org/10.1016/j.procbio.2018.03.025
Sharma K, Morla S, Khaire KC, Thakur A, Moholkar VS, Kumar S, Goyal A (2020) Extraction, characterization of xylan from Azadirachta indica (neem) sawdust and production of antiproliferative xylooligosaccharides. Int J Biol Macromol 163:1897–1907. https://doi.org/10.1016/j.ijbiomac.2020.09.086
Yang H, Wang K, Song X, Xu F (2011) Production of xylooligosaccharides by xylanase from Pichia stipitis based on xylan preparation from triploid Populas tomentosa. Bioresour Technol 102:7171–7176. https://doi.org/10.1016/j.biortech.2011.03.110
Bian J, Peng F, Peng XP, Peng P, Xu F, Sun RC (2013) Structural features and antioxidant activity of xylooligosaccharides enzymatically produced from sugarcane bagasse. Bioresour Technol 127:236–241. https://doi.org/10.1016/j.biortech.2012.09.112
https://www.marketwatch.com (Accession: May 2022).
Ibrahim O (2018) Functional oligo-saccharides: chemicals structure, manufacturing, health benefits, applications and regulations. Food Chem Nanotechnol 4(4):65–76. https://doi.org/10.17756/jfcn.2018-060
Kumar V, Bahuguna A, Ramalingam S, Kim M (2021) Developing a sustainable bioprocess for the cleaner production of xylooligosaccharides: an approach towards lignocellulosic waste management. J Clean Prod 316:128332. https://doi.org/10.1016/j.jclepro.2021.128332
Zhang B, Hao G, Cao H, Tang H, Zhang Y, Deng S (2018) The cryoprotectant effect of xylooligosaccharides on denaturation of peeled shrimp (Litopenaeus vannamei) protein during frozen storage. Food Hydrocoll 77:228–237. https://doi.org/10.1016/j.foodhyd.2017.09.038
Ayyappan P, Abirami A, Anbuvahini NA, Tamil Kumaran PS, Naresh M, Malathi D, Antony U (2016) Physicochemical properties of cookies enriched with xylooligosaccharides. Food Sci Technol Int 22(5):420–428. https://doi.org/10.1177/1082013215617567
Ferrao LL, Ferreira MVS, Cavalcanti RN, Carvalho AFA, Pimentel TC, Silva HLA, Silva R, Esmerino EA, Neto RPC, Tavares MIB, Freitas MQ, Menezes JCV, Cabral LM, Moraes J, Silva MC, Mathias SP, Raices RSL, Pastore GM, Cruz AG (2018) The xylooligosaccharide addition and sodium reduction in requeijão cremoso processed cheese. Food Res Int 107:137–147. https://doi.org/10.1016/j.foodres.2018.02.018
Souza FP, Balthazar CF, Guimaraes JT, Pimentel TC, Esmerino EA, Freitas MQ, Raices RS, Silva MC, Cruz AG (2019) The addition of xyloligoosaccharide in strawberry-flavored whey beverage. LWT - Food Sci Technol (Lebensmittel- Wissenschaft -Technol) 109:118–122. https://doi.org/10.1016/j.lwt.2019.03.093
Wu YB, Lin KW (2011) Influences of xylooligosaccharides on the quality of Chinese-style meatball (Kung-Wan). Meat Sci 88:575–579. https://doi.org/10.1016/j.meatsci.2011.02.018
Wu YB, Lin KW (2014) Influences of xylooligosaccharides and saccharides on the properties of meat batter during frozen storage. J Food Process Preserv 38:1439–1446. https://doi.org/10.1111/jfpp.12103
Liu H, Li Y, Tang B, Peng Y, Wu X, Che L, Quek SY, He N (2021) Effects of xylooligosaccharide on angiotensin I-converting enzyme inhibitory activity of fish actomyosin and quality of snakehead balls with or without high hydrostatic pressure treatment. LWT - Food Sci Technol 140:110803. https://doi.org/10.1016/j.lwt.2020.110803
Ribeiro T, Cardoso V, Ferreira LMA, Lordelo MMS, Coelho E, Moreira ASP, Domingues MRM, Coimbra MA, Bedford MR, Fontes CMGA (2018) Xylooligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poult Sci 97:4330–4341. https://doi.org/10.3382/ps/pey336
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yegin, S. Microbial xylanases in xylooligosaccharide production from lignocellulosic feedstocks. Biomass Conv. Bioref. 13, 3619–3658 (2023). https://doi.org/10.1007/s13399-022-03190-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03190-w