Abstract
The aim of the present research was to investigate the influence of the application of a novel cold-pressing system in olive oil manufacturing on the characteristics of olive pomace (OP) and on its valorization by anaerobic digestion (AD). Green olives and olives in veraison, both from the Picual variety, were used with the objective of assessing the effect of ripening level on the performance of the AD processes. The AD processes of these OPs were assessed in biochemical methane potential (BMP) tests. The maximum methane yield (327 ± 5 mL CH4/g VS) and biodegradability value (90.8%) were found for the OP derived from green olives without cold-pressing, which showed the highest soluble COD (113 g O2/L) and the lowest total phenolic concentration (9 g gallic acid/L). The first-order and Transference Function (TF) kinetic models were employed to evaluate the variation in methane production with time and to obtain the kinetic parameters of the anaerobic processes of the four OPs tested. The kinetic constant from the first-order model, k, did not show significant differences for the four OPs tested and ranged between 0.23 and 0.27 day−1. The TF revealed that the values for the maximum methane production rate (Rmax) were slightly higher for the OPs derived from green olives compared to those obtained from olives in veraison. For the green olives, the cold-pressing system caused a decrease in the value of Rmax from 87 ± 7 to 73 ± 6 mL CH4/(g VS·d).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The quality of olive oil begins in the field and passes through the different stages of production and packaging. At all points in these stages, increasing attention is being paid not only to obtaining quality oil, but also to improving the management of the by-products and residues produced [1]. Nowadays, the concept of quality is also linked to the environmental quality of the production environment. In this way, increasingly sustainable processes are sought which also provide new tools for improving our health through food and the health of the environment. This is why there is an important trend in the use of by-products to improve the use of our natural resources. This is the case of olive oil by-products. Many studies have led the industry to change its mentality and initiate a transformation process based on the integral use through a biorefinery [2]. This is based on obtaining the components of interest followed by the application of bioprocesses aimed at obtaining energy, compost, or animal feed.
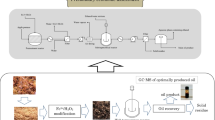
At the same time, the olive oil sector is also evolving to improve production systems by focusing the properties of extra virgin olive oil and of all the by-products which are generated [3]. One of the steps being taken to improve the organoleptic properties of the oil is to bring forward the harvesting of the olives and process them at an early stage of ripeness, i.e., green before they start to darken. This reduces extraction yield, but improves the fruity, bitter, and spicy attributes, increasing its sensory quality. Different systems have been proposed to improve oil extraction at the earliest stages of maturation, such as the use of vacuum during beating or the use of electric pulses after milling. These systems are still in a testing phase that can improve the fat yield from extraction. However, in the present work, we propose to go a step further and advance not only to increase the amount of oil that can be extracted from an under-ripe olive, but also to enhance the properties of the by-products generated, which would allow them to be better used. In this way, the use of green olives would be encouraged in order to improve both the quality of the oil and the quality of the by-products generated from it. This work is based on the study of the application of a novel cold-pressing system that improves the extractability of the oil and provides a residual solid (olive pomace, OP) enriched in phenolic components of greater value for its use, focusing the study on the integral use of this solid for the generation of energy after the application of anaerobic digestion (AD). Figure 1 shows a schematic diagram of the novel cold-pressing system for illustration. High-pressure systems are used industrially to improve food preservation. The cold-pressing system is based on the application of high pressure for short periods of time and at room temperature. In this process, the structure of the surface microorganisms is broken down, helping to reduce the microbial load of the food and thereby increasing its shelf-life [4]. The pressure system used in the present work is based on the same principle, but increasing the time to affect the structure of the plant wall and thus improving the subsequent beating and centrifugation step, increasing the recovery of oil and facilitating the valorization of the olive pomace by AD.
Therefore, the aim of this study was to investigate the methane production efficiency in batch mode of the olive pomace derived from a new olive oil manufacturing process in which the olives were previously subjected to a cold-pressing process at 7 kg/cm2 and 12 °C temperature. The effect of the olive ripening level was also assessed using green olives and olives in veraison. In both cases, the variety of the olives used was "Picual". Biochemical methane potential (BMP) tests were carried out in order to evaluate the anaerobic process. As far as we know, there is no previous literature focused on comparing the anaerobic digestion of the substrate obtained under the manufacturing conditions explained above for olive oil. This study also examines the potential of two mathematical models (i.e., first-order and Transference Function models) for the production of biomethane, the specific rate constant, the rate of maximum methane production, and the lag time of anaerobic processes using the experimental results of methane production and time.
2 Materials and methods
2.1 Cold-pressing reactor for olive processing
A pressurized reactor of 100-L capacity was used. The reactor prototype was designed by our research group at the Instituto de la Grasa (Seville, Spain). The reactor has a 100-L capacity stainless steel reservoir that can operate at a maximum pressure of 1.2 MPa. Olive samples of the Picual variety were introduced into the reactor at a rate of 5 to 10 kg of sample. The reactor was then closed and pressurized with air until a pressure of 7 kg/cm2 was reached. This pressure was maintained for 10 min. The initial temperature of the olives was 12 °C and the temperature after the pressure treatment was 12.2 °C. Finally, the reactor was depressurized and the olives were taken out. Oil was extracted using an Abencor system as a lab mill (MC2 Ingenierıa Sistemas, Seville, Spain). The olives were crushed with an Abencor hammer mill equipped with a 4-mm sieve and 600-g paste. The malaxation was carried out at 29 °C and was processed for 45 min. The amount of water added to the paste was 100 mL with the addition of talc. The non-fat liquid and solid fractions (olive pomaces) were stored under refrigeration at − 20 °C until use.
Green olives and olives in veraison, both from the Picual variety, were used in order to study the influence of the ripening level on the anaerobic digestion processes for the OPs obtained. The olives were cultivated and collected from “Valle de los Pedroches, Pozoblanco” (Cordoba Province, Spain). The date of harvest for green olives and olives in veraison was 5 December 2020.
Thus, four OPs were tested: OP from green olives subjected to the cold-pressing system, OP from these olives without being subjected to cold-pressing (control), OP from olives in veraison with cold-pressing, and OP from these last olives without pressing (control).
2.2 Biochemical methane potential tests
The anaerobic digestion experiments for the OPs tested were carried out in batch mode in 250-mL total volume and 210 ± 2 mL working volume reactors. The reactors were kept in thermostatic baths (35 ± 2 °C) under constant stirring at 400 rpm. The initial volatile solid concentration of the anaerobic inoculum was 19.9 g/L. In the reactors, an inoculum-to-substrate ratio of 2 (as volatile solids) was maintained. The anaerobic inoculum used was a mesophilic granular sludge from a full-scale UASB reactor treating wastewater from a brewery industry. The reactors were prepared with the addition of the inoculum and the substrate together with a micronutrient solution [5, 6]. The main characteristics of the inoculum were pH: 7.5 ± 0.2, total solids (TS): 25.0 ± 1.1 g/kg, volatile solids (VS): 19.9 ± 1.2 g/kg. In order to maintain anaerobic conditions, nitrogen gas was flushed to the reactors for 2 min at the beginning of the experiments (40 mL of headspace volume). The reactors were then sealed and placed in the thermostatic baths. The reactors were activated in three replicates per substrate. A triplicate was also placed in the reactor with only inoculum, without substrate, in order to subtract the inoculum’s endogenous methane production. The produced biogas was passed through a 2 N NaOH solution to retain the CO2. The displacement volume was assumed to be methane. The methane production was expressed under standard conditions of pressure and temperature (0 °C, 1 atm). The main characteristics of the different substrates used in the experiments are shown in Table 1.
2.3 Analytical methods
Prior to the anaerobic digestion process, the following parameters were analyzed in all the olive pomaces tested: total chemical oxygen demand (TCOD), soluble chemical oxygen demand (SCOD), total and volatile solids (TS and VS, respectively), as well as the total phenol concentration. After 26 days of operation period, the following parameters were determined in the resulting digestates or process effluents: SCOD, TS and VS, pH, total alkalinity, and volatile fatty acids (VFA). The methods used for the different analyses carried out were previously described by Fernández-Rodríguez et al. [5].
2.4 Kinetic models
2.4.1 First-order kinetic model
In order to study the process kinetics and estimate the process performance in the anaerobic digestion of the different substrates studied, the following first-order kinetic model was used:
where G is the cumulative specific methane production (mL CH4/g VSadded), Gm is the ultimate methane production (mL CH4/g VSadded), k is the specific rate constant (day−1), and t is the digestion time (day). This kinetic model is generally applied to evaluate the kinetics of the batch anaerobic digestion processes for different types of biodegradable substrates [7, 8]. This model is based on the premise that methane generation is proportional to the amount of substrate and not limited by microbial cell mass [9].
2.4.2 Transference Function (TF) model
The Transference Function (TF) model was also used to fit the experimental data of methane production during biochemical methane production (BMP) tests (Eq. (2)). The TF (reaction curve-type model) (RC), used mainly for control purposes, contemplates that any process might be analyzed as a system receiving inputs and producing outputs [10]. The TF model was successfully applied by several authors for the biomethanization of different organic wastes [7, 10, 11]. The TF model is given by the following expression:
where B (mL CH4/g VSadded) is the cumulative specific methane production, Bmax (mL CH4/g VSadded) is the ultimate methane production, Rmax is the maximum methane production rate (mL CH4/(g VSadded·d)), t (days) is the digestion time, and ℷ (days) is the lag time.
Determination coefficients (R2), errors (%), and standard errors of estimates (SEE) were calculated to evaluate the goodness-of-fit and the accuracy of the results for both models. The errors in percentage were calculated from the difference between the experimental and theoretical values (predicted by the model) of the ultimate methane production. The kinetic parameters for each experiment and mathematical adjustment were determined numerically from the experimental data obtained by non-linear regression using the software Sigma-Plot (version 11).
2.5 Energy output
The methane yield experimental data obtained in the BMP tests were used to determine the energy output by using Eq. (3) [12]:
where
- E0:
-
is the energy output in (kJ/g VSremoved),
- PCH4:
-
is the cumulative methane production after digestion time (m3),
- Ɛ:
-
is the lowest heating value for methane (35,800 kJ/m3 CH4),
- λm:
-
is the energy conversion factor of methane (0.9),
- VSremoved:
-
is the grams of VS removed at the end of the BMP test (g/L).
2.6 Statistical analysis
Triplicate experiments and analyses were carried out. The results were expressed as means ± standard deviation. The SigmaStat software for Windows (Palo Alto, CA94303, USA) was used for all the statistical analyses performed. Data were analyzed using a one-way analysis of variance (ANOVA) test, which was used to determine levels of significance among various results. The bio-kinetic parameters for each experiment and mathematical adjustments were determined numerically from the experimental data obtained by non-linear regression using the software Sigma-Plot (version 11).
3 Results and discussion
3.1 Biodegradability and methane yield coefficients
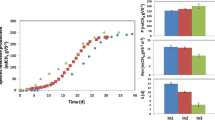
Figure 2 shows the accumulated methane yield coefficient throughout the experimental time for the four OPs assayed. As can be seen, the four substrates tested exhausted most of the biogas production after a 10-day period. The following ultimate methane yield values were obtained: 258 and 327 mL CH4/g VS for OP derived from green olives subjected to cold-pressing or without cold-pressing (control), respectively, and 249 and 276 mL CH4/g VS for OP derived from olives in veraison subjected to cold-pressing or without cold-pressing (control), respectively. The methane yield values obtained for OPs derived from green olives were higher than those achieved for OPs from olives in veraison. These results are in accordance with the higher biodegradability values reached for the OPs from green olives, i.e., 86.4 and 90.8% when cold-pressing is applied and for the control, without cold-pressing, respectively, compared to 74.5 and 84.2% for OPs from olives in veraison when cold-pressing is applied and for the control, respectively. Biodegradability was defined as the percentage of VS removed at the end of the BMP processes [23, 24].
Variation in methane production versus time for the different substrates tested. Values represent means ± standard deviation. OP green olives 7 kg/cm2: Olive pomace from green olives subjected to the cold-pressing system; OP green olives control: Olive pomace from green olives without being subjected to cold-pressing (control); OP olives in veraison 7 kg/cm.2: Olive pomace from olives in veraison subjected to the cold-pressing system; OP olives in veraison control: Olive pomace from olives in veraison without pressing (control)
Therefore, the maximum value for the ultimate methane yield was found for the OP derived from green olives without pressing (327 mL CH4/g VS), which is in accordance with the higher soluble COD concentration (113.0 ± 2.8 g O2/L) contained in this OP compared to the others, whose values ranged between 105.9 and 107.4 g O2/L. A high dissolved organic matter result in a nutrients and microorganisms’ synergism [13].
Serrano et al. [14] reported lower methane yield values (261 ± 2 mL CH4/g VS) for the biomethanization of untreated OP under mesophilic conditions. By subjecting the OP to a high-temperature thermal pre-treatment at 170 °C for 30 min, the AD of the pre-treated OP provided a similar methane yield (264 mL CH4/g VS) to untreated OP from the green olives tested in the present work. The co-digestion of the pre-treated mixture of the solid and liquid fractions obtained did not produce a synergy effect on the methane yield compared to the biomethanization of untreated OP. In this case, the increase in soluble compounds due to the thermal pre-treatment did not result in a significant enhancement in methane production. The fact that an improvement in methane production was not always produced by increasing soluble matter through thermal pre-treatment has also been reported by other authors [15]. In the study cited, the non-significant improvement in methane production subsequent to thermal pre-treatment can be due to non-degradable or toxic compounds which could partially inhibit the AD process [16].
Higher values for methane yield (461 mL CH4/g VS) were reached after co-digesting the OP with the microalga Scenedesmus quadricauda [5]. The co-digestion mixture contained an optimum C/N ratio [17]. The use of two co-substrates aided in the stability of the anaerobic system as well. The Chlorophyta was able to balance the C/N ratio and provide extra alkalinity. Co-digestion can increase the anaerobic digestibility of OP by improving the substrate composition, as has been determined from other investigations. A co-effect can take place in some substrates so that they stimulate enzymatic synthesis which can also improve anaerobic digestion yield [18].
3.2 Characterization of the anaerobic digestion effluents
The pH of the different anaerobic effluents (Table 2) ranged between 7.85 ± 0.02 (OP from green olives subjected to cold-pressing) and 8.0 ± 0.06 (OP from olives in veraison control). Very similar intermediate pH values were found for the effluents of the BMPs of OP from green olives control (7.98 ± 0.02) and OP from olives in veraison subjected to cold-pressing (7.92 ± 0.01). Once the batch experiments had ended, the pH values fell within the optimal pH range for the methanogenic phase [19]. Alkalinity is known to be one more important parameter involved in the AD process and also for the most pH-sensitive microorganisms [20]. After the experiments, the effluents had a good buffering capacity since values in the range of 6,414 and 6,684 mg CaCO3/L were obtained for all the substrates assayed (Table 2). The optimal alkalinity value was reported to range from 2,500 to 6,500 mg CaCO3/L, and that these values serve to buffer pH changes inside the anaerobic reactor [20].
In addition, at the end of the experiments, acetic and butyric acids alone were detected as volatile fatty acids. This is another parameter which points to the positive evolution and adequate performance of the substrates under study in anaerobic processes (Table 2). Values for acetic acid in the range of 78.4 to 100.3 mg/L and butyric acid concentration lower than 3.7 mg/L were achieved for all the OPs tested. These results indicated that no acidification processes occurred during the anaerobic processes, which proves high stability for the four processes investigated.
3.3 Estimation of the model parameters by kinetic modeling
3.3.1 First-order kinetic model
The kinetic parameters determined from Eq. (1) for the anaerobic digestion of the OPs coming from green and veraison olives after the prior cold-pressing of 7 kg/cm2 or without pressing (control) both at room temperature are summarized in Table 3. The low values for the standard deviations of the kinetic parameters and errors (differences between the ultimate methane productions and those which are theoretical or predicted by the model) (less than 3.6%) and the high determination coefficient values are indicative of the appropriate fit of the experimental results to the proposed model.
As can be observed in Table 3, there were no significant differences among the values for the specific rate constant of the four OPs tested, which ranged between 0.23 ± 0.01 (OP for olives in veraison control) and 0.27 ± 0.02 day−1 (OP from olives in veraison subjected to cold-pressing). The values for the kinetic constants were slightly higher when the olives were subjected to cold-pressing prior to the manufacturing process both for the green olives and olives in veraison. In any case, the values for the kinetic constants were much higher than those obtained from the AD of OP from olives subjected to the traditional two-phase system (0.013 ± 0.004 day−1 [6]. This fact can be attributed to the higher VS/TS ratios found in the OPs from olives subjected to pressing, whose values ranged between 94.8 and 96.6%, while the VS/TS ratio for the classical OP was only 81%.
By contrast, similar k values to those found in the present research were reported when the classical OP was previously pre-treated at 121 °C, for 30 min and at 1 bar of pressure and later subjected to AD (k = 0.19 day−1) and when the OP was co-digested with the microalga Dunaliella salina (k = 0.21 day−1) [21]. During the AD of OP, hydrolysis step was detected as the main drawback due to the low biodegradability of this substrate [22]. The soft hydrothermal pre-treatment aided in breaking down the lignocellulose fibers [23], but nevertheless, the bacteria involved in the first step are the cause of an imbalance in the AD process. During co-digestion, an improvement in the hydrolysis step was seen, with the co-substrate acting as a catalyst for hydrolytic enzymes [22].
In addition, the theoretical Gmax values obtained from the model were higher for the OPs derived from green olives than those olives in veraison in a similar way to the variation in the respective experimental values. On the other hand, for both types of olives processed, there were decreases in the Gmax values of 25.8 and 9.4% when the pressing process was not applied (control samples) for green and veraison olives, respectively. A similar decrease was observed in the values of the experimental ultimate methane yields when no pressing was used prior to the manufacturing process. The maximum Gmax value (321 ± 6 mL CH4/g VS) was found for the OP derived from green olives without cold-pressing (control), the sample that showed the higher biodegradability value (90.8%) compared to the other OPs tested. However, this highest Gmax value was lower than those recently reported for the anaerobic digestion of thermally pre-treated OP (395 mL CH4/g VS) and the co-digestion of OP and the microalga D. salina (451 mL CH4/g VS) [21]. Particularly, final methane production rates from the first-order kinetic model were increased by 8.5 and 24% when the OP was thermally pre-treated (121 °C at 1 bar for 30 min) and co-digested with D. salina (95% OMSW–5% D. salina) respectively, compared to the untreated OMSW [21]. During the co-digestion experiment, even though most of the organic matter originated from the OP, the contribution of the microalgae aided in the hydrolytic stage, making it more evenly spaced over time, which resulted in an overall improvement in the AD process [21].
3.3.2 Transference Function model
Table 4 summarizes the parameters determined from the application of the TF to the experimental data of methane production over time, as shown in Fig. 2, where the errors defined as the differences between measured and predicted methane yields were less than 3.9% for the four OPs tested. Again, the high values for the determination coefficients (R2) and the low values for the standard errors of estimates (Table 4) show the superb fit of the experimental results to the Transference Function model.
The values for maximum methane production rate (Rmax) were somewhat higher for the OP derived from green olives compared to those obtained from olives in veraison. In the case of the OPs derived from green olives, the pressing process caused a decrease in this parameter from 87 ± 7 to 73 ± 6 mL CH4/(g VS·d); while for the OPs from olives in veraison, there was no significant difference between the values (71 ± 5 and 64 ± 4 mL CH4/(g VS·d) for olives subjected to pressure and control, respectively). It was observed that the highest values in the Rmax coincided with the lowest values for the phenolic contents of the OPs, which were found to be 10.28 and 9.09 g gallic acid/L for OPs from green olives with pressing and control, respectively, and 9.86 and 10.50 g gallic acid/L for OPs from olives in veraison subjected to pressing and control, respectively. Therefore, only in the case of the OP derived from green olives, did the previous pressure process cause a decrease in the Rmax value. In addition, the maximum Rmax value achieved in the present research (87 ± 7 mL CH4/ (g VS·d)) for OP derived from green olives without pressing was very similar to that obtained during the anaerobic co-digestion of OP and the microalga S. quadricauda (89 mL CH4/(g VS·d), for which an optimum carbon to nitrogen ratio of 25.3 was determined [5].
On the other hand, the maximum value for ultimate methane production (Bmax) was also observed for the OP derived from green olives without cold-pressing (319 ± 6 mL CH4/g VS), which is similar to what occurred for Rmax. This result could be explained by the higher content in soluble matter (SCOD: 113 g/L) present in this OP compared to the others (SCOD ranging from 105 to 107 g/L). The highest Bmax achieved for this OP coincides with the maximum biodegradability value reached for this substrate (90.8%) compared to the other OPs, whose values ranged from 74.5 to 86.4%. It is also interesting to point out that the Bmax achieved for the OP from the green olive control (319 ± 6 mL CH4/g VS) was 9% higher than that obtained for an OP previously subjected to a steam explosion (200 °C for 5 min with rapid decompression) (294 mL CH4/g VS) [14]. This behavior is due to the fact that the steam explosion process generates some undesirable compounds that act as inhibitors in the AD process [24].
The lag periods found for all the substrates tested in the present work were very low (0.2–0.3 days), and the shape of the methane production curves with time underwent rapid growth for all the POs assayed. These low lag time values (ʎ) across the board indicate a rapid consumption of the most readily available biodegradable components of all the OPs assayed and in all the AD processes studied.
The highest values for ultimate methane yield (Bmax) and the maximum methane production rate (Rmax) achieved in the anaerobic digestion of OP from green olives processed at ambient temperature without pressing (control) revealed the robustness of OP AD compared to the one obtained after the cold-pressing of 7 kg/cm2 and for the OPs derived from olives in veraison, both with and without pressing.
3.4 Energy assessment
Although anaerobic digestion is a resource-efficient process because it reduces the organic content and considerably diminishes pollution coming from wastes, it is extraordinary that a high methane production and energy output are obtained during the process. The energy output was determined from experimental BMP data from these assays by applying Eq. (3) [12]. The energy yield or viability of the process is a factor of ultimate importance in scaling up the anaerobic digestion process. It should also be noted that the inoculum had not been acclimatized to the new substrates prior to the start of the anaerobic experiments and therefore, the methane yield was underestimated. The energy output values for OPs from green olives were 12.40 and 13.86 kJ/g VS removed in the case of olives subjected to cold-pressing and control, respectively; while for the OPs from olives in veraison, these values were 8.27 (with pressing) and 11.40 kJ/g VS removed (control), respectively.
As can be seen for the two olives selected, the energy output decreased when cold-pressing was applied to the fruits prior to the olive oil manufacturing process, which agrees with the low biodegradability values observed when pressing was used. More specifically, the energy yield decreased by 10.5 and 27.5% when pressing was applied for the OPs from green olives and olives in veraison, respectively.
At the same time, the energy output was higher in the case of OPs from green olives compared to the OPs from olives in veraison. The highest value (13.86 kJ/g VS removed) was achieved for the OP from green olives without pressing (control). This result is in line with the highest methane yield and maximum methane production rate values observed in this OP compared to the others. Pasalari et al. [12] reported energy output values from 9.43 to 25.5 kJ/g VS removed for the anaerobic digestion process of landfill leachate after being pre-treated with electrochemical oxidation. The values obtained in the present study were in line with the values reported previously. High net energy output values (76.25 kJ/g fed VS) were also reported for the anaerobic co-digestion of sludge (SS) and food waste (FW) when both substrates were subjected to microwave (MW) pre-treatment [25]. These results show that MW pre-treatment was effective in the dissolution of organic matter, conversion of protein to NH4+-N, and cumulative methane production and methane yield, as well as the reaction rate of methane production in the SS and FW anaerobic co-digestion systems [25].
4 Conclusions
The anaerobic digestion (AD) of olive pomaces (OPs) derived from a novel cold-pressing system applied both to green olives and olives in veraison could be a sustainable method for management of theses wastes. BMP tests on these OPs compared to others carried out without previous cold-pressing revealed that maximum values for methane yield (327 mL CH4/g VS) and biodegradability (90.8%) were achieved for the OP derived from green olives which were not subjected to cold-pressing, which showed the highest soluble COD and lowest phenolic compound concentration. The slight increase in the methane yield values obtained in the AD of OPs when the olives were not subjected to the cold-pressing process is attributed to the higher biodegradability values and lower phenolics content achieved in this case when compared to the values observed in the OPs when the pressure system is applied. The data from the AD experiments of these wastes were accurately described by the first-order and Transference Function (TF) models. The first-order kinetic parameters were similar for the four OPs tested and ranged from 0.23 to 0.27 day−1. The highest value for maximum methane production rate (Rmax) (TF model) was found for the OP from green olives which were not subjected to the cold-pressing system (87 ± 7 mL CH4/g VS·d). It can be concluded that the AD of these olive pomaces is a promising and effective alternative for the generation of energy. Future research is required in order to assess the influence of this new cold-pressing system on the characteristics and anaerobic biodegradability of OPs derived from other olives varieties with different maturation levels.
Code availability
Code used in this study can found in the Sigma-Plot (version 11) and The Sigma-Stat software for Windows (Palo Alto, CA94303, USA).
Change history
09 October 2022
Missing Open Access funding information has been added in the Funding Note.
References
Alamprese C, Caponio F, Chiavaro E (2021) Sustainability of the olive oil system. Foods 10(8):1730. https://doi.org/10.3390/foods10081730
Serrano A, Fermoso FG, Rodríguez-Gutiérrez G, Fernandez-Bolaños J, Borja R (2017) Biomethanization of olive mill solid waste after phenols recovery through low-temperature thermal pre-treatment. Waste Manage 61:229–235. https://doi.org/10.1016/j.wasman.2016.12.033
Pérez M, Lopez-Yerena A, Lozano-Castellon J, Olmo-Cunillera A, Lamuela-Raventos RM, Martin-Belloso O, Vallverdu-Queralt A (2020) Impact of emerging technologies on virgin olive oil processing, consumer acceptance, and the valorization of olive mill wastes. Antioxidants 10(3):417. https://doi.org/10.3390/antiox10030417
Houška M, Kubásek M, Strohalm J, Landfeld, A, Kamarád J (2006) Warming of olive oil processed by high hydrostatic pressure. High Pressure Res 303-308https://doi.org/10.1080/08957950410001721173
Fernández-Rodríguez MJ, De la Lama-Calvente D, Jiménez-Rodriguez A, Borja R, Rincón B (2019) Anaerobic co-digestion of olive mil solid Waste and microalga Scenedesmus quadricauda: effect of different carbon to nitrogen ratios on process performance and kinetics. J Appl Phycol 31:3583–3591. https://doi.org/10.1007/s10811-019-01858-x
De la Lama-Calvente D, Fernandez-Rodríguez MJ, Llanos J, Mancilla-Leyton JM, Borja R (2021) Enhancing methane production from the invasive macroalga Rugulopteryx okamurae through anaerobic co-digestion with olive mill solid waste: process performance and kinetic analysis. J Appl Phycol 33:4113–4124. https://doi.org/10.1007/s10811-021-02548-3
Li L, Kong X, Yang F, Li D, Yuan Z, Sun Y (2012) Biogas production potential and kinetics of microwave and conventional thermal pretreatment of grass. Appl Biochem Biotechnol 166:1183–1191. https://doi.org/10.1007/s12010-011-9503-9
Scarcelli PG, Serejo ML, Paulo PL, Boncz MÁ (2020) Evaluation of biomethanization during co-digestion of thermally pretreated microalgae and waste activated sludge, and estimation of its kinetic parameters. Sci Total Environ 706:135745. https://doi.org/10.1016/j.scitotenv.2019.135745
Wang M, Lee E, Dilbeck MP, Liebelt M, Zhang Q, Ergas SJ (2017) Thermal pre-treatment of microalgae for biomethane production: experimental studies, kinetics and energy analysis. J Chem Technol Biotechnol 92:399–407. https://doi.org/10.1002/jctb.5018
Donoso-Bravo A, Perez-Elvira SI, Fernández-Polanco F (2010) Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem Eng J 160:607–614. https://doi.org/10.1016/j.cej.2010.03.082
Pinto-Ibieta F, Serrano A, Jeison D, Borja R, Fermoso FG (2016) Effect of cobalt supplementation and fractionation on the biological response in the biomethanization of Olive Mill Solid Waste. Bioresour Technol 211:58–64. https://doi.org/10.1016/j.biortech.2016.03.031
Pasalari H, Esrafili A, Rezaee A, Gholami M, Farzadkia M (2021) Electrochemical oxidation pretreatment for enhanced methane potential from landfill leachate in anaerobic co-digestion process: Performance, Gompertz model, and energy assessment. Chem Eng J 422https://doi.org/10.1016/j.cej.2021.130046
Barua VB, Rathore V, Kalamdhad AS (2019) Anaerobic co-digestion of water hyacinth and banana peels with and without thermal pretreatment. Renew Energy 134:103–112. https://doi.org/10.1016/j.renene.2018.11.018
Serrano A, Fermoso FG, Alonso-Fariñas B, Rodríguez-Gutiérrez G, Fernandez-Bolaños J, Borja R (2017) Phenols recovery after steam explosion of olive mill solid waste and its influence on a subsequent biomethanization process. Bioresour Technol 243:169–178. https://doi.org/10.1016/j.biortech.2017.06.093
Ghasimi DSM, Aboudi K, De Kreuk M, Zandvoort MH, Van Lier JB (2016) Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem Eng J 295:181–191. https://doi.org/10.1016/j.cej.2016.03.045
Carrere H, Antonopoulou G, Affes R, Passos F, Battimelli A, Lyberatos G, Ferrer I (2016) Review of feedstock pretreatment strategies for improved anaerobic digestion: from lab-scale research to full-scale application. Bioresour Technol 199:386–397. https://doi.org/10.1016/j.biortech.2015.09.007
Habiba L, Hassib B, Moktar H (2009) Improvement of activated sludge stabilization and filterability during anaerobic digestion by fruit and vegetable waste addition. Bioresour Technol 100:1555–1560. https://doi.org/10.1016/j.biortech.2008.09.019
Ajeej A, Thanikal JV, Narayanan C, Kumar RS (2015) An overview of bio augmentation of methane by anaerobic co-digestion of municipal sludge along with microalgae and waste paper. Renew Sustain Energy Rev 50:270–276. https://doi.org/10.1016/j.rser.2015.04.121
Arif S, Liaquat R, Adil M (2018) Applications of materials as additives in anaerobic digestion technology. Renew Sustain Energy Rev 97:354–366. https://doi.org/10.1016/j.rser.2018.08.039
Fannin KF, Biljetina R (1987) Reactor design. In: Chynoweth DP, Isaacson R (eds) Anaerobic digestion of biomass. Elsevier Appl Sci, London, pp 109–128
Fernández-Rodríguez MJ, De la Lama-Calvente D, Jiménez-Rodriguez A, Borja R, Rincon B (2021) Evolution of control parameters in biochemical methane potential tests of olive mil solid waste (OMSW), thermal pre-treated OMSW, and its co-digestion with Dunaliella salina. J Appl Phycol 33:419–429. https://doi.org/10.1007/s10811-020-02297-9
Chiu SLH, Lo IMC (2016) Reviewing the anaerobic digestion and codigestion process of food waste from the perspectives on biogas production performance and environmental impacts. Environ Sci Pollut Res 23:24435–24450. https://doi.org/10.1007/s11356-016-7159-2
Fernández-Rodríguez MJ, de la Lama-Calvente D, Jiménez-Rodríguez A, Pino-Mejías R, Borja R, Rincón-Llorente B (2020) Impact of soft hydrothermal pre-treatments on the olive mill solid waste characteristics and its subsequent anaerobic digestion. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-020-00759-1
Rincón B, Rodríguez-Gutiérrez G, Bujalance L, Fernández-Bolaños J, Borja R (2016) Influence of a steam-explosion pre-treatment on the methane yield and kinetics of anaerobic digestion of two-phase olive mill solid waste or alperujo. Process Safety Environ Protect 102:361–369. https://doi.org/10.1016/j.psep.2016.04.010
Liu J, Zhao M, Lu C, Yue P (2020) The effect of microwave pretreatment on anaerobic co-digestion of sludge and food waste: performance, kinetics and energy recovery. Environ Res 189:109856. https://doi.org/10.1016/j.envres.2020.109856
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by Project PY18-616 funded by the regional government of Andalucía, Junta de Andalucía, Consejería de Economía, Conocimiento, Empresas y Universidad, Andalucía, Spain, and Project PID2020-114975RB-100/AEI/10.13039/501100011033 financed by the Spanish Ministry of Science and Innovation. The project FEDER UPO-380782 financed by the regional government of Andalucía, Junta de Andalucía, Consejería de Trasformación Económica, Industria, Conocimiento providing financial support to Dr. Fernández-Rodríguez.
Author information
Authors and Affiliations
Contributions
M.J. Fernández-Rodríguez: conceptualization, investigation, validation, writing—review and editing; J. Cubero-Cardoso: investigation conceptualization, validation; D. de la Lama-Calvente: investigation, conceptualization, validation; A. Fernández-Prior: investigation, conceptualization, validation; G. Rodríguez-Gutiérrez: supervision, resources, funding acquisition, writing—original draft; R. Borja: supervision, writing—original draft, writing—review and editing, resources, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Rodríguez, M.J., Cubero-Cardoso, J., de la Lama-Calvente, D. et al. Performance and kinetic evaluation of the anaerobic digestion of olive pomace derived from a novel manufacturing process based on an olive cold-pressing system: influence of the fruit ripening level. Biomass Conv. Bioref. 14, 10035–10043 (2024). https://doi.org/10.1007/s13399-022-03034-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03034-7