Abstract
This study compared microbiological and chemical methods used in astaxanthin extraction from the exoskeleton of the shrimp species Penaeus japonicus and Penaeus semisulcatus. The microbiological method was performed using Saccharomyces cerevisiae (bakery yeast) or Lactobacillus acidophilus (from yogurt), followed by solvent extraction with hexane and acetone at different ratios (1:1, 1:2, and 1:3). The chemical method was performed traditionally using hexane. The highest astaxanthin yield from P. japonicus exoskeleton was obtained using either S. cerevisiae or L. acidophilus followed by solvent extraction with hexane and acetone at a ratio of 1:1 (8.5 and 8.1 mg/g waste, respectively) as well as by the chemical method (8.4 mg/g waste). Likewise, the highest astaxanthin yield from P. semisulcatus exoskeleton was obtained using either S. cerevisiae or L. acidophilus followed by solvent extraction with hexane and acetone at a ratio of 1:1 (3.0 and 4.1 mg/g waste, respectively) as well as by the chemical method (3.2 mg/g waste). The values obtained from P. semisulcatus exoskeleton were considerably lower than those attained from P. japonicus exoskeleton. In addition, the nuclear magnetic resonance (C-NMR) analysis confirmed that astaxanthin was the main carotenoid present in the extract. In conclusion, the pretreatment of exoskeleton wastes of P. japonicus using S. cerevisiae followed by solvent extraction with hexane and acetone at a ratio of 1:1 as well as the classical chemical treatment led to the highest astaxanthin content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Crustaceans are one of the oldest and the most diverse group of arthropods. They are also considered one of the most successful groups of invertebrates on Earth [1]. Crustaceans are regarded as an enriched source of many bioactive substances, such as carbohydrates, proteins, amino acids, fatty acids, vitamins, and minerals, especially the Decapoda order within the Malacostraca class. This order includes many familiar groups such as shrimps, prawns, crabs, lobsters, and crayfish, which have well-known nutritional function and importance. They are also delicious and easily digestible. In addition, their nutritional function depends on the biochemical composition of their bodies, which consists of high protein, low fat, and carbohydrate contents similar to those in fish flesh. Moreover, crustaceans are considered a source of omega-3 fatty acids, and vitamins, including A, B, especially B3 (niacin) and B12 (cobalamin). Hence, they are among the most valuable components of the human diet [2].
One of the major problems in modern food production is the generation of large quantities of underused by-products. These food wastes may contain substances of high value and important health benefits. Thus, the seafood processing industry, for example, produces a tremendous quantity of by-products and wastes, such as heads, tails, skins, scales, viscera, backbones, and shells that may be an amazing source of proteins, lipids, and pigments [3]. In addition, shells may be a source of chitinous materials and carotenoids. Therefore, the waste generated during food processing should be utilized as it is considered a wasted fortune [4]. This would also alleviate the problems generated by their disposal [5].
Carotenoids such as astaxanthin, beta-carotene, lutein, and others are extracted from crustacean exoskeletons. They are responsible for the pigmentation of most aquatic organisms. Some carotenoids are precursors of vitamin A. They can also act as antioxidants in the biological systems [6], and exhibit protective action against cancer [7]. In the carapace of crustaceans, carotenoids exist as free and esterified forms. Many crustaceans can produce astaxanthin from beta-carotene ingested from dietary algae via echinenone 3-hydroxyechinenone, canthaxanthin, and adonirubin, as shown in Fig. 1 [8]. Thus, astaxanthin has been reported to be 10 times larger than that of any other carotenoids such as zeaxanthin, lutein, and canthaxanthin [9].
Currently, astaxanthin is a renowned compound for its commercial application in various industries, comprising aquaculture, food, cosmetics, nutraceutical, and pharmaceutical [10]. Moreover, astaxanthin effectively suppresses cell damage caused by free radicals, and induction of matrix metalloproteinases (MMPs) in skin after UV irradiation [11]. It repairs DNA damage caused by skin exposure to UV radiation, which can lead to oncogenic mutations. Studies showed that astaxanthin inhibited the UV-induced DNA damage and increased the expression of oxidative stress-responsive enzymes [12]. Astaxanthin is also reported to be an inhibitor of matrix metalloproteinases (MMPs) in different cells, including macrophages and chondrocytes [13]. It has anti-inflammatory properties as it inhibits the gene expression of several proinflammatory biomarkers, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in Alzheimer disease (AD), animal model [14]. Furthermore, astaxanthin has anti-aging effects such as hyper-pigmentation suppression, melanin synthesis, photoaging inhibition, and wrinkle formation reduction [15]. Astaxanthin has antioxidant activity since it inhibits reactive oxygen species (ROS) formation and modulates the expression of oxidative stress-responsive enzymes such as heme oxygenase-1 (HO-1) [12, 16]. Astaxanthin has immune-enhancing effects as it increases natural killer (NK) cell cytotoxic activity [17], suggesting that it may regulate NK cells that serve as an immunosurveillance system against tumors and virus-infected cells [18]. In addition, astaxanthin supplementation was proven to be useful to heart failure patients with left ventricular systolic dysfunction (LVSD). It was found that after 3 months of astaxanthin supplementation in patients with LVSD, the levels of the oxidative stress markers decreased, and both cardiac contractility and exercise tolerance improved [19]. The recommended dose of astaxanthin for adult patients is 2–4 mg/day. In addition, astaxanthin is safe and has no side effects when consumed with food [20].
Khanafari et al. [20] used the Lactobacillus species Lactobacillus plantarum and Lactobacillus acidophilus for the extraction of astaxanthin from wastes of the shrimp species Penaeus semisulcatus. Also, Hamdi et al. [21] used Lactobacillus and Saccharomyces for the extraction of astaxanthin from byproducts of the crayfish Procambarus clarkia. Consequently, in the present research, Lactobacillus and Saccharomyces species were assessed as pretreatment for the extraction of astaxanthin from shrimp wastes as an ecofriendly alternative to the classical chemical method. The shrimp species selected, namely, Penaeus japonicus and Penaeus semisulcatus, are two commercially used shrimps in Egypt.
2 Materials and methods
2.1 Sample preparation
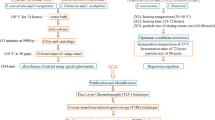
Fresh shrimps of two different species, Penaeus japonicus and Penaeus semisulcatus, were purchased from a local fish market. The shrimps were peeled, and all the internal organs were removed in order to obtain the exoskeleton (waste). Shrimp waste was then dried in an oven for 8–10 h at 55–60 °C [22]. The dried waste was ground to obtain a fine powder (1–3 mm particle size). The process was performed separately for each species. The powder samples were collected in sterilized containers, labeled, and stored in a fridge at 4 °C.
2.2 Microorganisms and culture media
Lactobacillus acidophilus was obtained from a commercial yogurt and identified biochemically according to Pyar and Peh [23]. Saccharomyces cerevisiae was obtained from a commercial bakery yeast in Egypt and identified biochemically and microbiologically. Czapek-Dox medium consisted of sucrose 20.0 g/L, sodium nitrate 2.0 g/L, dipotassium phosphate 1.0 g/L, magnesium sulfate 0.50 g/L, potassium chloride 0.50 g/L, ferrous sulfate 0.01 g/L, and agar 15.0 g/L. De Man, Rogosa, and Sharpe (MRS) broth medium was composed of peptone from casein 10.0 g/L, yeast extract 4.0 g/L, meat extract 8.0 g/L, D ( +) glucose 20.0 g/L, Tween 80 1.0 g/L, di-ammonium hydrogen citrate 2.0 g/L, sodium acetate 5.0 g/L, magnesium sulfate 0.2 g/L, manganese sulfate 0.04 g/L, and agar 15 g/L. The media were sterilized by autoclaving at 121 °C and 1.5 bars for 15 min. Streptomycin (30 mg/mL) that was previously sterilized by filtration (0.2 µm) was added after cooling down in order to prevent bacterial contamination. All the used flasks were cleaned by soaking them overnight in diluted H2SO4, followed by ethanol, and distilled water washing. Flasks (250 mL) containing 100 mL of Czapek-Dox medium were inoculated with 5 g of instant dry yeast and incubated for 5 days at 35 °C [24]. Flasks (250 mL) containing 100 mL of MRS medium were inoculated with 1 mL of L. acidophilus and incubated for 3 days at 30 °C [22].
2.3 Shrimp waste bioprocess
5 mL of previously cultured MRS broth containing L. acidophilus, and 5 mL of Czapek-Dox medium containing S. cerevisiae were added to a fermentative medium (100 mL distilled water + 10 g of the ground exoskeletons) and incubated for 5 days at 30 °C in the presence of 5% CO2 [25]. The fermentative medium was filtered with Whatman filter paper no. 41 and centrifuged at 3075 g for 5 min. 25 mL of the filtered fermentation medium was added to 25 mL of a mixture of hexane and acetone at different ratios (1:1, 1:2, and 1:3) to determine the one leading to the highest astaxanthin yield. The entire extraction process was performed separately for each species of shrimp. The obtained extracts were used for astaxanthin determination.
2.4 Chemical extraction
Astaxanthin was extracted by mixing 10 g of the ground exoskeletons of each shrimp species with 50 mL of hexane in a 100-mL flask, vortexed for 30 s, and placed in a 50 °C water bath for 10 min. Aqueous and organic layers were separated by centrifugation at 1008 g for 5 min. In the final step, 6 mL of dimethyl sulfoxide (DMSO) were added to the tube, vortexed vigorously, placed in a water bath for 10 min, and then vortexed again. The obtained extracts from each shrimp species were used for astaxanthin determination.
2.5 Determination of astaxanthin concentration
The astaxanthin contained in the extracts from each shrimp species obtained via all microbial and chemical methods was determined by UV–VIS spectrometry (Jenway 6300 spectrophotometer) at a wavelength of 476 nm [26]. In the blank, the extract was replaced by distilled water [27].
2.6 HPLC analysis
The astaxanthin content of the extracts was determined by high-performance liquid chromatography (HPLC) (Agilent 1260 series) equipped with a diode array detector (DAD) and an Eclipse C18 column (250 mm × 4.6 mm, 5 µm, Eclipse). The mobile phase consisted in water (A) and 0.05% of trifluoroacetic acid in acetonitrile (B), and the samples were eluted at a flow rate of 1 mL/min for 8 min with an isocratic gradient. The injection volume was 5 μL for both samples and standard. The column temperature was maintained at 40 °C. The UV detection of the elute was performed at 480 nm. Astaxanthin was qualitatively analyzed by comparing the retention time of the standard, and its quantification was done by using a calibration curve. This work was conducted at the National Research Centre, Cairo, Egypt.
2.7 C-NMR studies
The extracts from shrimp exoskeletons via the microbial and chemical methods were subjected to C-NMR analysis. The results were compared to the standard astaxanthin C-NMR analysis [28]. Dimethyl sulfoxide (DMSO) was used as a solvent. All NMR spectra were recorded on a Bruker Advance III 400 MHz. Chemical shifts were reported relative to TMS and referenced via residual carbon resonances of the appropriate deuterated solvent.
2.8 Statistical analysis
The matrix of the factorial experimental design is presented in Supplementary Table 2. The statistical analysis and the ANOVA of the experimental design were performed using the SPSS statistics software version 25 (Supplementary Tables 3 and 4). All determinations were carried out in triplicate. All data were expressed as mean ± SD. at (p < 0.001). The standard deviation of the samples was calculated using IBM SPSS statistics software version 25.
3 Results
3.1 Astaxanthin concentration in the extracts
3.1.1 UV–VIS spectroscopy
Astaxanthin concentrations in the extracts of P. japonicus exoskeleton were higher than 8.0 mg/g waste using either S. cerevisiae or L. acidophilus followed by solvent extraction with hexane and acetone at a ratio of 1:1 as well as by the chemical method (Fig. 2A). However, astaxanthin from the extracts of P. semisulcatus exoskeleton led to much lower values (3.0 mg/g waste with S. cerevisiae followed by solvent extraction with hexane and acetone at a ratio of 1:1, 4.1 mg/g waste with L. acidophilus followed by solvent extraction with hexane and acetone at a ratio of 1:1 and 3.2 mg/g waste with the chemical method) (Fig. 2B). It is noteworthy that the microbiological method followed by solvent extraction with hexane and acetone at a ratio of 1:2, and especially at 1:3, acutely decreased the extracted astaxanthin (Fig. 2).
3.1.2 HPLC
HPLC chromatographs of the extracts are presented in Supplementary Fig. 3. The obtained results (Table 1) were much lower than those determined by UV–VIS spectroscopy shown in Fig. 2. The reason for this is that the UV–Vis method does not enable the distinction between carotenoids in a given mixture and the most common protocols use a mean absorption coefficient and a mean absorption wavelength. Therefore, more accurate results are attained by the HPLC method for specific carotenoids.
3.1.3 C-NMR studies
The C-NMR analysis (Supplementary Fig. 4) of astaxanthin extracted from P. japonicus and P. semisulcatus by chemical and microbiological methods confirmed that astaxanthin was the main carotenoid present in the samples. The two peaks, between 7.004 and 8.557 ppm, symbolized the presence of protons of methine on the astaxanthin main chain [29]. These peaks proved the presence of astaxanthin as two sets in the monoesterified compounds due to the loss of symmetry. At 2.505 ppm, four protons corresponded to the methylene protons, and α to the carbonyl. Signals at 2.022 and 1.83 ppm corresponded to the methyl moieties [28]. A signal that represented the methylene protons on the astaxanthin fatty acid moiety was shown in the middle of 1.307 and 1.593 ppm signals. Overlapping peaks presented around 1.830 and 2.022 ppm referred to protons of the methylene moiety. A broad signal found at 3.892 ppm corresponded to the OH moiety on the astaxanthin molecule [29].
4 Discussion
In the marine crustaceans, astaxanthin is considered as the main carotenoid [30]. Traditionally, chemical extraction of astaxanthin was being used in the industry. In this study, the astaxanthin extracted from P. japonicus and P. semiculcatus via the chemical method were 8.4 and 3.2 mg/g waste, respectively. These values are comparable to those attained using the microbiological method, followed by solvent extraction with hexane and acetone (1:1). However, Khanafari et al. [20] found that the microbiological method led to higher astaxanthin yields than the chemical method for shrimp waste of P. semisulcatus.
The results obtained indicated that astaxanthin extraction via the microbiological method depended on the microbiological strain used, as well as the solvent system used afterwards. Thus, in the present study, P. japonicus led to a markedly higher astaxanthin yield than P. semisulcatus. Similarly, Lim et al. [31] elucidated that P. japonicus was a good source of astaxanthin. As for the solvent system used, increasing the amount of acetone (polar solvent) in the hexane/acetone solvent mixture decreased astaxanthin extraction. This might be due to an increase in the polar solvent, which favored the extraction of other components, thus hampering astaxanthin extraction.
According to the sustainable development goals (SDGs), combining microbial with chemical extraction is better for the environment since it reduces the use of chemicals [32]. In this sense, green technologies, including fermentation via probiotic bacteria, have recently been developed [33]. Microbial extraction is a humble and eco-friendly method for the extraction of extremely unstable pigments such as carotenoids [25] due to the action of the extracellular proteolytic enzymes secreted by the microorganisms [34]. In addition, shrimp waste is extremely perishable. The carotenoid-rich broth obtained by the microbiological process can be stored for a long-time span under normal storage conditions, which is not possible with other extraction methods [33].
The presence of astaxanthin was confirmed by NMR analysis. In the same way, Azizan et al. [28] confirmed the presence of astaxanthin in the hexane extract of Chaetoceros calcitrans at 1.34 ppm, which represented the methylene protons on the astaxanthin fatty acid moiety, shown in the middle of 1.307 and 1.593 ppm in the NMR chart of the present study (Supplementary Fig. 4).
5 Conclusion
According to the obtained results, P. japonicus led to a significantly higher astaxanthin yield than P. semisulcatus, which means that the former was a better source of astaxanthin. In addition, the extraction of astaxanthin from shrimp wastes via the microbial method led to equal or slightly higher yields than those extracted via the conventional chemical method. Therefore, the usage of chemicals in extraction can be reduced and replaced by edible microorganisms. This paves the way for the exchange of some chemicals with useful microorganisms commonly found in nature.
References
De Grave S, Pentcheff N, Ahyong S, Chan T, Crandall K, Dworschak P, Felder D, Feldmann R, Fransen C, Goulding L, Lemaitre R, Low M, Martin J, Ng P, Schweitzer C, Tan S, Tshudy D, Wetzer R (2009) A classification of living and fossil genera of decapod crustaceans. Raffles Bull Zool Suppl 21:1–109
Szaniawska A (2018) Function and importance of crustaceans. In: Baltic crustaceans. Springer, Cham, pp 185–188
Wang H, He W, Dansou D, Zhang H, Dwi Nugroho R, Tang C, Guo X, Yu Y, Zhao Q, Qin Y, Zhang J (2022) Astaxanthin improved the storage stability of docosahexaenoic acid-enriched eggs by inhibiting oxidation of non-esterified poly-unsaturated fatty acids. Food Chem 381:132256
Hamed I, Özogul F, Regenstein J (2016) Industrial applications of crustacean byproducts (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci Technol 48:40–50
Yan N, Chen X (2015) Sustainability: don’t waste seafood waste. Nature 524:155–157
Mezzomo N, Ferreira SR (2016) Carotenoids functionality, sources, and processing by supercritical technology: a review. J Chem 7:1–16
Guerin M, Huntley Mark E, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biochem 21:210–216
Maoka T (2011) Carotenoids in marine animals. Mar Drugs 9:278–293
Naguib Y (2000) Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem 48:1150–1154
Davinelli S, Nielsen ME, Scapagnini G (2018) Astaxanthin in skin health, repair, and disease: a comprehensive review. Nutrients 10:522
Suganuma K, Nakajima H, Ohtsuki M, Imokawa G (2010) Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J Dermatol Sci 58:136–142
Camera E, Mastrofrancesco A, Fabbri C, Daubrawa F, Picardo M, Sies H, Stahl W (2009) Astaxanthin, canthaxanthin and β-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp Dermatol 18:222–231
Kishimoto Y, Tani M, Uto-Kondo H, Iizuka M, Saita E, Sone H, Kondo K (2010) Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur J Nutr 49:119–126
Park J, Yeo I, Han J, Suh J, Lee H, Hong J (2018) Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp Dermatol 27:378–385
Tominaga K, Hongo N, Karato M, Yamashita E (2012) Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim Pol 59:43–47
Kim M, Min E, Kim J, Koo J, Kang J (2015) Growth performance and immunological and antioxidant status of Chinese shrimp, Fennerpenaeus chinensis reared in biofloc culture system using probiotics. Fish Shellfish Immunol 47:141–146
Jyonouchi H, Zang L, Gross M, Tomita Y (1994) Immunomodulating actions of carotenoids: enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr Cancer 21:47–58
Chew B, Mathison B, Hayek M, Massimino S, Reinhart G, Park J (2011) Dietary astaxanthin enhances immune response in dogs. Vet Immunol Immunopathol 140:199–206
Kato T, Kasai T, Sato A, Ishiwata S, Yatsu S, Matsumoto H, Shitara J, Murata A, Shimizu M, Suda S, Hiki M, Naito R, Daida H (2020) Effects of 3-month astaxanthin supplementation on cardiac function in heart failure patients with left ventricular systolic dysfunction-a pilot study. Nutrients 12:1896, https://doi.org/10.3390/nu12061896.
Khanafari A, Saberi A, Azar M, Vosooghi G, Jamili S, Sabbaghzadeh B (2007) Extraction of astaxanthin esters from shrimp waste by chemical and microbial methods. J Environ Health Sci Eng 4:93–98
Hamdi S, Elsayed N, Algayar M, Ishak V, Ahmed M, Ahmed S, Kamal M, Abd E-G (2022) Eco-friendly methods for recycling of crayfish “Procambarus clarkii” by-product for astaxanthin extraction and quantification. Egyptian Journal of Aquatic Biology & Fisheries 26(2):239–251
Ranga Rao A, Baskaran V, Sarada R, Ravishankar G (2013) In vivo bioavailability and antioxidant activity of carotenoids from micro algal biomass—a repeated dose study. Food Res Int 54:711–717
Pyar H, Peh K (2014) Characterization and identification of Lactobacillus acidophilus using biolog rapid identification system. Int J Pharm Pharm Sci 6(1):189–193
Masters J (2010) American type culture collection standards development organization workgroup ASN-0002. Cell line misidentification: the beginning of the end. Nat Rev Cancer 10:441–448
Armenta-López R, Guerrero I, Huerta S (2002) Astaxanthin extraction from shrimp waste by lactic fermentation and enzymatic hydrolysis of the carotenoprotein complex. J Food Sci 67:1002–1006
Asker D, Awad TS, Beppu T, Ueda K (2018) Rapid and selective screening method for isolation and identification of carotenoid- producing bacteria. Methods Mol Biol 1852:143–170
Subramanium B, Thibaulta M, Djaoued Y, Pelletier C, Touaibiac M, Tchoukanovaa N (2015) Chromatographic, NMR and vibrational spectroscopic investigations of astaxanthin esters. R Soc Chem 245–250.
Azizan A, Ahamad Bustamam MS, Maulidiani M, Shaari K, Ismail IS, Nagao N, Abas F (2018) Metabolite profiling of the microalgal diatom Chaetoceros calcitrans and correlation with antioxidant and nitric oxide inhibitory activities via 1H NMR-based metabolomics. Mar Drugs 16:154
Di Pietro ME, Mannu A, Mele A (2020) NMR determination of free fatty acids in vegetable oils. Processes 8:410
Pitacco W, Samorì C, Pezzolesi L, Gori V, Grillo A, Tiecco M, Vagnoni M, Galletti P (2022) Extraction of astaxanthin from Haematococcus pluvialis with hydrophobic deep eutectic solvents based on oleic acid. Food Chem 379:132–156
Lim K, Yusoff F, Shariff M, Kamarudin M (2018) Astaxanthin as feed supplement in aquatic animals. Rev Aquac 10:738–773
WHO (2006) Strategic approach to international chemicals management.
Routray W, Dave D, Cheema SK, Ramakrishnan VV, Pohling J (2019) Biorefinery approach and environment-friendly extraction for sustainable production of astaxanthin from marine wastes. Crit Rev Biotechnol 39:469–488
Gimeno M, Ramírez-Hernández JY, Mártinez-Ibarra C, Pacheco N, García-Arrazola R, Bárzana E, Shirai K (2007) One-solvent extraction of astaxanthin from lactic acid fermented shrimp wastes. J Agric Food Chem 55:10345–10350
Funding
Open Access funding provided by LUT University (previously Lappeenranta University of Technology (LUT)).
Author information
Authors and Affiliations
Contributions
Salwa A. H. Hamdi: conceptualization, result analysis, methodology, project administration, writing the original draft, and supervision; Ghadeer M Ghonaim: methodology, visualization, result analysis, and writing the original draft; Rana R. El Sayed: visualization, methodology, result analysis, and writing the original draft; Susana Rodríguez-Couto: reviewing and editing manuscript; Mohamed N. Abd El-Ghany: conceptualization, data curation, formal analysis, investigation, methodology, result analysis, project administration, writing the original draft, resources, software, supervision, validation, visualization, writing, review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamdi, S.A.H., Ghonaim, G.M., El Sayed, R.R. et al. Bioprocess of astaxanthin extraction from shrimp waste via the common microorganisms Saccharomyces cerevisiae and Lactobacillus acidophilus in comparison to the chemical method. Biomass Conv. Bioref. 14, 8333–8339 (2024). https://doi.org/10.1007/s13399-022-02984-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02984-2