Abstract
Biochars are biosorbents made from a wide range of agricultural or animal residues that involve functional groups to enable binding with different species. In the current work, pyrolysis of artichoke leaves was done to generate nanobiochar (NanoBch) and enriched with ethylenediamine (EDA) as nitrogen-rich derivative to yield (NanoBch-EDA). This was characterized by different techniques and compared with NanoBch. The evaluated NanoBch-EDA showed promising removal performance under optimized conditions of contact time, nanosorbent dosage, initial concentration of Pn and Cr(VI), pH, temperature, and interfering ions. The elimination processes of Pn and Cr(VI) onto (NanoBch-EDA) by four isotherm models (Langmuir, Dubinin-Radushkevitch, Temkin, and Freundlich) were discussed. In Pn elimination process, the Langmuir model achieved (R2 = 0.99) as the highest correlation coefficient. Otherwise, the Freundlich (R2 = 0.98) was the applicable model in Cr(VI) elimination. The values of maximum capacity qmax (mg g−1) for adsorption of Pn and Cr(VI) onto NanoBch-EDA were 21.93 and 79.37 mg g−1, respectively. Based on the obtained R2 (0.999), the pseudo-second-order model was shown to be the superlative match for describing the elimination of Pn and Cr(VI) by NanoBch-EDA. The reaction was stated as spontaneous and endothermic based on parameters signs and values obtained from thermodynamic studies. NanoBch-EDA (60 mg) was found highly effective in extracting 95.0%, 90.0%, and 92.0% of Pn (25 mg L−1) from tap, waste, and sea water, respectively, while 20 mg of NanoBch-EDA removed 96.0%, 94.6%, and 97.3% of Cr(VI) 10 mg L−1 from tap, waste, and sea water, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water is among the globe’s utmost natural sources as required for the permanence of all life forms as well as human outgrowth. However, the issue of water shortage has emerged as a major serious obstacle to economic growth [1]. Pollution is a source of major concern based on the direct influence on both human and aquatic life [2]. In the meantime, water contamination, especially with heavy metals pollution has become a global environmental issue. Certain origins are the prime reasons for existence of heavy metals in water, for example, electroplating, chemical plants, mining, metallurgy, and municipal and agricultural wastewater [3]. Current researches have been recently focused on the detection and disposal of some chemicals and pollutants as heavy metals and drugs from aqueous matrices due to the harmful effects of these substances on human health [4,5,6,7,8,9,10,11,12,13,14]. Pharmaceuticals long-term exposure in ecosystems exhibits harmful health and environmental impacts, including infectious diseases, mutagenesis, drug resistance, allergic reactions, and carcinogenic behaviors [15,16,17,18]. These pollutants are well known for their bioaccumulation and thus are presumed to pose serious global threats, with harmful implications for non-target and aquatic organisms [19].
Glucocorticoids are classified in a category of drugs that are extensively used throughout the world and therefore, they have become a topic of recent scientific studies as environmental contaminants due to their potential threats to aquatic systems and organisms even at lower concentrations ng L−1 [20]. The synthetic corticosteroid compound prednisolone (Pn) has the chemical formula C21H26O5 and several reports confirmed that prednisolone can assist in treatment of inflammatory disorders, extreme allergies, hypertension, asthma, obesity, cancers, and adrenal disturbances [21]. There are several side effects, such as toxicity, which are caused by the drug’s prolonged absorption from the intestine as a result of high and regular doses of Pn over time [21]. Prednisolone is primarily liberated into the aquatic medium by mammals [22]. In addition, the amounts of prednisolone in polluted medical center sewage water varied from 315 to 1918 ng L−1, and it was also found in the ecosystem bases around the world, with quantities varies from (ng L−1– µg L−1) [23].
A number of research papers have been concerned with removing prednisolone from aqueous solutions, including grapheme graphene-oxide (GO) nanoplatelets as a possible and effective adsorbent for removing 92.68% prednisolone and 90.04% ranitidine from their various aqueous samples [24]. Other researches were also concentrated on the determination and removal of glucocorticoids (GCs) in (STPs) sewage treatment plants. Six types of GCs as prednisone (PRE), cortisone (COR), dexamethasone (DEX), cortisol (CRL), triamcinolone a cetonide (TLA), and methylprednisolone (MPREL) were removed by STPs in the range 85–100%. Otherwise, elimination of prednisolone (PREL) and flumethasone (FM) mainly have low degradation potencies, in aerobic and anoxic tanks as they showed low efficiencies as 66% and 50%, respectively [25, 26]. Nine glucocorticoids like prednisolone (PRNL), cortisone (CORT), hydrocortisone (CORS), beclomethasone dipropionate (BD), hydrocortisone butyrate (HB), dexamethasone DEXA, prednisone (PRD), budesonide (BUD), and cortisone acetate (CA) were also removed from aqueous matrices by using sodium dodecyl sulfate-multi-walled carbon nanotubes and the devised method proved to be effective and accurate [27].
Water contamination with heavy metals has adverse impacts on biological environments because of nonbiodegradability with the reason for causing a variety of diseases and disturbances even at relatively low concentrations. Heavy metals can bind to some necessary cellular elements including structural proteins, nucleic acids, and enzymes, preventing them from functioning normally. Over the whole, long-term excessive exposure to heavy metals may be harmful [28]. Chromium is an example of the very toxic transition metal with various oxidation states as mainly exists in the most popular valence states, trivalent and hexavalent chromium [29]. Environmental concerns about chromium are focused on the potentially harmful health effects of Cr(VI)–polluted soils, drinking water, and groundwater. Hexavalent chromium is the most dangerous, carcinogenic, and mutagenic, as well as the most water soluble to enhance its access to the living cells easily [30]. There are three major ways human exposure to heavy metals and these are based on adsorption by the skin, digestion by eating or drinking and inhalation by breathing of substance containing Cr(VI) [29]. In addition, hexavalent chromium is classified as a category “A” health hazard due to its potential carcinogenic characteristics and therefore, it was pointed out in the category grade of harmful compounds. Several reports confirmed that hexavalent chromium threatens both humans and aquatic mammals, and it has been also reported that excessive intake of hexavalent chromium by plants severely affects the mitotic operation and decline seed germination in extensively cultivated pulse crops [31]. Because of its detrimental effects on biological life and human health, chromium contamination in the ecosystem is a significant concern [32,33,34,35]. As a result, removing of toxic Cr(VI) from water supplies will certainly enhance the water quality. Therefore, Cr(VI) elimination techniques and mechanisms are available today to help minimize the negative impact on human health. Several processes such as solvent extraction, precipitation, evaporation, flotation, ion exchange, and reverse osmosis have been developed for extracting and removal of Cr(VI) from industrial effluents and wastewater [36,37,38]. Owing to the higher operating costs and energy demands, these traditional approaches have been documented as complex and costly processes [39,40,41]. On the other hands, adsorption technique is much more efficient than other traditional operations for removing inorganic pollutants as chromium (VI) from wastewater [42, 43]. It is currently considered as one of the supreme effective methods due to its ease of use, adaptability, and cost-effective advantages [44,45,46,47]. Furthermore, adsorbents resulting from agricultural waste have attracted a lot of awareness because of high environmental friendly characteristics [48,49,50]. In prior researches, various merchant sorbents and agricultural waste stocks, for example, tea residues, turmeric waste, coconut husk, bamboo, wheat straw, citrus, and water melon peel were utilized as adsorbents for extracting Cr(VI) and other heavy metals from polluted water [51,52,53]. Biochars, which are mainly produced from the thermal degradation of agricultural or animal residues, were utilized as a low-cost adsorbent [18]. As a result of their outstanding physicochemical characteristics, such as surface electronegativity and superior aromatization, they were lately attracted great interests [54]. Recently, biochar activation through using physical and chemical processes has gotten more interest to develop porous structures with high improvement in adsorption capacity [55, 56].
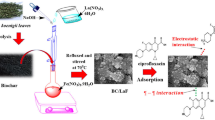
In this work, a prepared nanobiochar from artichoke leaves (NanoBch) was modified with nitrogen-rich ethylenediamine (EDA) to yield (NanoBch-EDA) as a pH sensitive task specific nanosorbent for removing Pn as an antihistamine drug and Cr(VI) as a highly toxic heavy metal from their aqueous solutions. For describing and verifying the surface of NanoBch and NanoBch-EDA, a variety of characterization techniques were used, including (SEM) scanning electron microscopy, (FT-IR) Fourier transform infrared spectroscopy, (XRD) X-ray diffraction, (TGA) thermal gravimetric analysis, and (BET) Brunauer–Emmett–Teller. The major purpose of the current study is devoted to estimate the potency and eligibility of (NanoBch-EDA) on the elimination operation of prednisolone and hexavalent chromium from real water samples. Several factors were examined and evaluated in the elimination processes of Pn and Cr(VI) by NanoBch-EDA, for example, pH solution of Pn and Cr(VI), vibration time, NanoBch-EDA amount, initial Pn, and Cr(VI) concentration and the involvement of interfering salts.
2 Experimentations experimental
2.1 Chemicals and solutions
The artichoke leaves were purchased from a nearby marketplace. Sodium hydroxide (NaOH, FW: 40 g mol−1, 98%) and hydrochloric acid (HCl, FW: 36.46 g mol−1, 37%) were provided from (SD fine Chem. Limited, India). Magnesium sulfate heptahydrate (MgSO4.7H2O, FW: 246.48 g mol−1, 98%) and calcium sulfate (CaSO4, FW: 136.14 g mol−1, 97%) were gotten from Adwic company, in Egypt. Sodium chloride (NaCl, FW: 58.44 g mol−1, 99.0%), potassium chloride (KCl, FW: 74.55 g mol−1, 99.5%), and ammonium chloride (NH4Cl, FW: 53.49 g mol−1, 99.0%) were obtained from Riedel-de-Haen, Germany. Ethylenediamine (CF: NH2CH2CH2NH2, FW: 60.10 g mol−1, 99.5%), potassium dichromate (CF: K2Cr2O7, FW: 294.18 g mol−1, ≥ 99.0%), 1,5-diphenylcarbohydrazide (CF: C13H14N4O, FW: 242.28 g mol−1, ≥ 98.0%), and sulfuric acid (CF: H2SO4, FW: 98.08 g mol−1, 95.0–97.0%,) were obtained from Sigma-Aldrich company, in USA. Methyl prednisolone sodium succinate (CF: C26H33NaO8, FW: 496.5 g mol−1) was purchased from Egyptian-European-Pharmaceutical-manufacture.
2.2 Preparation of Pn and Cr(VI) solution
A 2.829 g of potassium dichromate was dissolved in 1000 mL of deionized water yielded a stock solution of 1000 mg L−1 Cr(VI). To obtain an optimum test solution concentration, the stock solution was diluted with distilled water [31]. Furthermore, 100 mg of Pn was dissolved in 1000 mL deionized water to make a 100 mg L−1 stock solution. The 25 mg L−1 Pn concentration used in this work were accomplished by diluting of the stock solution. Pn absorbance was spectrophotometrically characterized at λmax = 247 nm. A 1.0 g of 1, 5-diphenylcarbazide was dissolved in 100 mL acetone in a 0.2 N sulfuric acid and used to detect the absorbance of residual Cr(VI) via a UV–visible Visible spectrometer at (λmax = 540 nm) [57].
2.3 Preparation of NanoBch and NanoBch-EDA adsorbents
To produce the aimed nanobiochar, plucked artichoke leaves was washed with deionized water for heating at 350 °C in a muffle oven for 2 h (NanoBch). Then, 5.0 g of NanoBch was mixed with 10 mL EDA and stirred for 1 h on a hotplate. The functionalized nitrogen-rich nanobiochar (NanoBch-EDA) was dried for 24 h at 70 °C in an oven.
2.4 NanoBch and NanoBch-EDA characterization
The instruments used to characterize the surface of NanoBch and NanoBch-EDA, include FT-IR, SEM, TGA, XRD, and BET-surface area. To show the variance among NanoBch and NanoBch-EDA wise in size and morphology, a BRUKER Vertex 70 and JEOL Ltd, JSM-6360LA and JSM-5300 were used for recording the FT-IR and SEM, respectively. TGA patterns and XRD analysis were obtained using the Perkin-ElmerTGA7 and Shimadzu lab X6100 model, respectively. In addition, BET was used for measuring the surface area of NanoBch and NanoBch-EDA. The absorbance of Pn and Cr(VI) concentrations were measured by instrument Model: Jasco v 530 and determined through using a UV–Vis spectrophotometer.
2.5 Adsorption tests
Batch tests were accomplished to establish the removal operation of Pn and Cr(VI) onto NanoBch-EDA. Various effective factors and environments were examined for example the mass of NanoBch-EDA, contact time between adsorbate solution and NanoBch-EDA, initial concentration of adsorbate (Pn and Cr(VI)), pH of Pn and Cr(VI), reaction temperature, presence of interfering salts, as well as the application by using water samples from various sources.
To investigate the impact of different NanoBch-EDA masses on Pn extraction, 20 mL of Pn (25 mg L−1) was applied to 20 mL of NanoBch-EDA mass (5–80 mg). All mixtures were shaken by automatic shaker for 30 min. After the filtration stage, the remaining concentrations of Pn in these mixtures were identified via UV–Vis spectrophotometer (at λmax = 247 nm), then Eq. (1) used for determining the elimination percentage of Pn by NanoBch-EDA as in Table 1. Also, the same selected masses from NanoBch-EDA were mixed with 20 mL Cr(VI) (10 mg L−1) and shaken for 30 min. The remaining concentrations of Cr(VI) were configured out at (λmax = 540 nm) to know how the NanoBch-EDA dose affected on the percentage removal of Cr(VI).
In the pH range of (2–12), the influence of pH on the elimination of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) by NanoBch-EDA was examined, where 20 mL Pn (25 mg L−1) was contacted with 60 mg NanoBch-EDA and the pH of the mixtures was changed with HCl or NaOH. And for 30 min, all adjusted mixtures were shaken and then were filtered for determining the residual concentrations of Pn in the filtrate by a UV–Vis spectrophotometer (λmax = 247 nm). The percentage removal of Pn (25 mg L− 1) by NanoBch-EDA was calculated from Eq. (1). The same experiment and steps were repeated to calculate the percentage elimination of Cr(VI) (10 mg L−1) by using 20 mg NanoBch-EDA in the pH range of (2–12).
A 40 mL of 0.01 M KCl and 100 mg NanoBch-EDA was added together for identifying NanoBch-EDA point of zero charge (PZC). HCl or NaOH solution was utilized to adjust the mixtures at pH range (2.0–12.0), and then shaken for 24 h. After that, pH of each suspension was measured with a pH meter, and the PZC of NanoBch-EDA was obtained from the plot between pH (final–initial) and pHi (initial).
Additionally, kinetic studies and contact time impact were examined in which 20 mL Pn (25 mg L−1) was added to 60 mg NanoBch-EDA to contact at dissimilar time periods as (1, 5, 10, 15, 20, 25, 30, 40, 45, 60, 90, and 120 min) to estimate the ability of NanoBch-EDA towards Pn removal.
The previous procedures and studies were examined by using 20 mg NanoBch-EDA with 20 mL Cr(VI) at different time intervals as listed above. The influence of the variation in the concentration of Pn on the removal process and isotherm models was evaluated.
A 60 mg NanoBch-EDA was added to various Pn (5–100 mg L−1) concentrations adjusted at pH = 2.0 for examining the influence of initial Pn concentration on elimination process of Pn by NanoBch-EDA. The solutions were shaken for 30 min, and then filtrated for identifying the removal (%) of Pn from Eq. (1). Similarly, the impact of Cr(VI) concentration on the elimination process of Cr(VI) by NanoBch-EDA was deliberated at similar condition as above but 20 mg NanoBch-EDA was utilized in this case.
The temperature effect and the thermodynamic tests were evaluated where 20 mL Pn (25 mg L−1) was added to 60 mg NanoBch-EDA at various temperatures (288, 293, 298, 303, 313, 323, and 333 K) for 30 min in a thermostatic shaker. All mixtures were filtrated after shaking and through a UV–Vis spectrophotometer the absorbance of Pn was detected at (λmax = 247 nm), then from Eq. (1), Pn extraction was calculated. Similar trials were utilized to determine the impact of temperature on the elimination of Cr(VI) (10 mg L−1) by NanoBch-EDA at (λmax = 540 nm).
The interference study by the presence of 100 mg of NaCl, KCl, NH4Cl, CaSO4, and MgSO4 towards removal of Pn (25 mg L−1) onto NanoBch-EDA was performed to figure out the ability of NanoBch-EDA. These salts were separately mixed with 60 mg NanoBch-EDA and 20 mL Pn (25 mg L−1) and shaken for 30 min, then filtrated for determining at (λmax = 247 nm) the absorbance of Pn (25 mg L−1). Equation (1) was employed to calculate the removal (%) of Pn (25 mg L−1) by NanoBch-EDA in the attendance and deficiency of these salts. Furthermore, the impact of these salts on the exclusion of Cr(VI) (10 mg L−1) by 20 mg NanoBch-EDA was considered. The absorbance and the elimination percentage of Cr(VI) (10 mg L−1) was identified by a UV–Vis spectrophotometer, at (λmax = 540 nm) and calculated from Eq. (1), respectively.
The reusability test and the appropriateness of recycling of NanoBch-EDA-bounded-Pn and NanoBch-EDA-bounded-Cr(VI) were accomplished by using HCl for elimination of surface loaded Pn and Cr(VI), respectively. A 100 mL Pn (25 mg L−1) was mixed with 250 mg NanoBch-EDA, then shaken for 30 min and filtrated. NanoBch-EDA-bounded-Pn was dried at 70 °C and the recycled NanoBch-EDA was added to 100 mL HCl (0.1 mol L−1), shaking 30 min, then filtrated and dried at 70 °C. After drying, the recycled NanoBch-EDA-bounded-Pn was added to 100 mL of Pn (25 mg L−1) for shaking 30 min to apply in another adsorption cycle. The mixture was filtrated to determine the absorbance of the Pn concentration. The previous regeneration test was repetitive five times and the reagent HCl (0.1 mol L−1) was added in every cycle. Similar trials were utilized to determine the reusability of NanoBch-EDA-bounded-Cr(VI) on the elimination of Cr(VI) (10 mg L−1) at (λmax = 540 nm).
As a final factor, 50-mL samples from (tap, waste, and sea water) were spiked with Pn (25 mg L−1), then adjusted at pH 2 and mixed well with 60 mg NanoBch-EDA and shaken for 30 min. The mixtures solutions were filtrated for detecting at (λmax = 247 nm) the Pn absorbance, and the elimination (%) of Pn (25 mgL−1) by NanoBch-EDA was calculated from Eq. (1). The prior procedure was reiterated as application on exclusion of Cr(VI) (10 mg L−1) by NanoBch-EDA at pH 2 from various water samples, and the absorbance of (10 mg L−1) Cr(VI) was identified at (λmax = 540 nm).
3 Results and discussion
3.1 Characterization
3.1.1 FT-IR examination
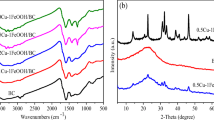
The FT-IR analysis of NanoBch and NanoBch-EDA are demonstrated in Fig. 1(a–b), which reveals the appearance of a number of peaks. The two materials have signified different peaks where most peaks in NanoBch-EDA were appeared more sharply than in NanoBch. The carboxylic –OH groups were found responsible for the wide band at 3214 cm−1 in NanoBch. On the other hand, a sharp peak at 3161.99 cm−1 may be responsible to the N–H stretching in NanoBch-EDA. The specific peak at 2924 cm−1 in NanoBch is due to aliphatic CH-stretching, which was sharpened at 2914 cm−1 as a result of loading EDA in NanoBch-EDA [18]. The detected peak at 1567 cm−1 is mostly recognized owing to the existence of covalent bond (C = C) in NanoBch structure. The noticed band at 1571.5 cm−1 in NanoBch-EDA is ascribed to the manifestation of N–H (amine group) in ethylenediamine [58]. In addition, the peaks at 1038 and 1376 cm−1 are respectively corresponding to the C–O and C–OH stretching bands. The extra peaks at 558–1114 cm−1 range may be classified as the = C–H unit, which is close to alkenes structure.
3.1.2 XRD analysis
The XRD of NanoBch and NanoBch-EDA are presented in Fig. 2(a–b). NanoBch (Fig. 2a) was displayed by three strongest peaks appearing at 2θ = 28.4°, 40.6° and 50.3° with counts intensity 108, 62, and 17, respectively. Figure 2b represents NanoBch-EDA with the same above three peaks, but with small changes in their intensity as well as two other additional peaks at 2θ = 31.5°, 39.3° with low intensity 45 and 39, respectively. Moreover, NanoBch-EDA (Fig. 2b) was also distinguished by the appearance of a three high intensity new peaks that are related to EDA at 2θ = 10.9°, 18.4°, and 22.7° with 275, 130, and 232, respectively. The attendance of these new peaks is a proven indicator for the modification of NanoBch with EDA to form NanoBch-EDA.
3.1.3 TGA analysis
Figure 3 displays the thermal gravimetric analysis of NanoBch and NanoBch-EDA at (25–800 °C). Decomposition of both NanoBch and NanoBch-EDA were evident by the appearance of five degradation steps as illustrated in Fig. 3a–b with total weight losses = 81.8% and 96.4%, respectively. Firstly, NanoBch and NanoBch-EDA were characterized to desorb water molecules from their surface at (25–110 °C) and (26–124 °C) with weight loss = 7.625% and 5.11%, respectively. It is evident that the fourth degradation steps in both NanoBch and NanoBch-EDA exhibited the highest weight losses 50.408% at (417.97–546.15 °C) and 44.25% at (428.96–529.93 °C), respectively.
3.1.4 SEM and BET
As displayed in Fig. 4(a–b), SEM examination was technicality utilized to identifying and explaining the surface characteristics and morphologies of NanoBch and NanoBch-EDA, respectively. The images of these two materials show the formation of homogenous particles. NanoBch shows more regularity in particle size distribution in the range 26.75–47.21 nm (Fig. 4a). Consequently, EDA has shown its contribution as an active surface loading moiety on the formation of NanoBch-EDA based on the evident changes in shape and particle size distribution in the range 20.98–26.3 nm (Fig. 4b). The BET technicality was dissected for estimating the surface area, pore volume and diameter of NanoBch and NanoBch-EDA as shown in Fig. 5(a–b). The pore diameter, pore volume and the specific surface area (BET) of NanoBch were characterized as (7.9890 nm, 0.02119 cm3 g−1 and 10.607 m2 g−1), respectively. Otherwise, the BET of NanoBch-EDA is 6.9187 m2 g−1, and the pore diameter and volume were corresponding to 10.914 nm to 0.01888 cm3 g−1, respectively.
3.2 Adsorption experiments
3.2.1 NanoBch-EDA dose
Impacts of increasing in the NanoBch-EDA mass dose (5–80 mg) on the recovery of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) were discussed. The results are displayed in Fig. 6 to validate that 60 mg NanoBch-EDA was characterized to removed 90.6% from Pn (25 mg L−1). As a result of increasing in NanoBch-EDA dose from 5 to 80 mg, the improvement in the recovery percentage of Pn (25 mg L−1) from 25.9 to 90.85% was characterized. Similarly, an enhancement in the percentage of Cr(VI)) (10 mg L−1) elimination from 64.9 to 96.25% was obtained owing to the increase in NanoBch-EDA dose from 5 to 80 mg. The adsorption efficiency of NanoBch-EDA to eliminate Pn and Cr(VI) is mostly explained by the possibility and the availability of active functional groups on NanoBch-EDA surface which are freely interacted with the Pn and Cr(VI) [18].
3.2.2 pH impact
Identification of the (pHpzc) is very issue from the practical point of view for any adsorbent in order to identify the surface charge either positive, neutral, or negative. This helps in describing the possible binding mechanism between the adsorbate as prednisolone drug or hexavalent chromium and the adsorbent as NanoBch-EDA. It is well known that the pHpzc corresponds to the pH with the charge on the adsorbent surface is zero. At this pH, both charge of the positive and negative active sites are equalized. In addition, identifying the pHpzc helps to make accurate predictions on how functional groups on adsorbent surface ionize and associate with the adsorbate [59]. The function of NanoBch-EDA characterization as porosity, surface area, and morphology was approved using point of zero charge tests (PZC) at various pH levels of Pn solution and Cr(VI). The pHpzc of NanoBch-EDA was examined at various pH ranges from (2.0–12.0) where the result indicated that NanoBch-EDA had a positive surface charge at pH range (2.0–6.0), as seen in Fig. 7a. The surface charge of NanoBch-EDA is mostly positive when the pH of the solution was less than 6.0. As a result, the positively charged surface of NanoBch-EDA will electrostatically capture the negatively charged Cr(VI) anions from solution. Where in a solution with a range of pH (1.0–6.0), Cr(VI) ions are existing in the hydrogen chromate (HCrO4−) anion as the dominant species over the others [60]. Beside, at low pH the positively charged on NanoBch-EDA surface may interact to negatively charged particles on drug surface (Pn).
The pH impact of Pn and Cr(VI) solutions on the adsorption operation of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) by NanoBch-EDA is an important factor to consider and investigate because the solution pH has a remarkable impact on the functional groups and their charge distribution on the adsorbent. The pH of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) were varied at the range (pH 2–12) through adding (0.1 mol L−1) of HCl or NaOH. Metal ion adsorption Cr(VI) is strongly affected by the medium pH [61]. The pH of the medium determines whether or not the metal is in its ionic form, as well as the functional groups related to it [62]. Additionally, as the pH changes from acidic to basic, the adsorption of Cr(VI) decreases, to refer that the acidic conditions are the best adsorption condition.
Figure 7b shows the highest adsorption of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) onto NanoBch-EDA at pH 2.0 which found to decline at pH > 2.0. Therefore, pH 2 could be listed as the appropriate condition for elimination of Pn and Cr(VI) by NanoBch-EDA. At this pH, the surface of NanoBch-EDA is highly positively charged due to protonation of nitrogen-rich EDA molecules and under this condition the negatively charges on Pn surface and Cr(VI) ions would strongly bound to the positively charged nitrogen functional group for the formation of ion-pairs interaction. As a confirmation of this proposed mechanism, one can conclude that as the pH increases, the surface of NanoBch-EDA is turned into neutral or negatively charged and thus resulting in the adsorption behavior decrease of Pn molecules and Cr(VI) ions [61, 63].
Several important adsorption isotherms models were significantly considered for evaluation of various pollutants as the adsorption of Pn and Cr(VI) onto NanoBch-EDA. In this work, isotherm models as Langmuir, Freundlich, also Temkin and Dubinin-Radushkevitch (D-R) were investigated and discussed to explore more about the nature of the link between the active sites of NanoBch-EDA and the elimination of Pn and Cr(VI).
All parameters and equations of the above-mentioned models are listed out in Table 1. The Langmuir (Eq. 2) is utilized to give evidence about the occurrence of adsorption via monolayer, where the activation energy on the adsorbent surface is uniform. As well, it illustrates that when all sites of adsorption have been reacted with the adsorbate, the adsorption process will be obstructed after this instance. Therefore, the binding energy of all adsorption sites are equal and each site can only catch one adsorbate [64]. Freundlich model (Eq. 3) is the isotherm model which was excessively utilized for exploring the non-ideal behaviors of adsorption. Besides, Freundlich model is applied for describing the adsorption on heterogeneous surface. This model supposed that the distribution of sites during the adsorption is significantly having relevance to the heat of adsorption operation and the energy of these sites exhibits an influence on the adsorption process [65]. On the other hand, Dubinin-Radushkevich model (Eq. 4) is an experiential adsorption model which is ordinarily utilized in heterogeneous surfaces for discussing the adsorption technique with the distribution of Gaussian energy. This model is generally applied to distinguish between the binding reactions either physical adsorption of metal ions and chemical adsorption [66]. Lastly, Temkin model (Eq. 5) is viable for systems with moderate range of ion concentrations [67] and the influences of indirect interactions between adsorbate and adsorbent on the adsorption operation were taken into consideration through this model. Also for all molecules, the model supposed that the heat of adsorption (ΔHads) is linearly declined due to the increase in the coverage of surface [68].
Figure 8a–b presents Langmuir and Freundlich model for adsorption of both Pn and Cr(VI) onto NanoBch-EDA, respectively. From the listed values of R2 in Table 2, it was identified that the adsorption process of Pn onto NanoBch-EDA was best following the Langmuir model because the R2 value was superior in Langmuir model than the others models (R2 = 0.99), where Pn molecules were enveloped onto NanoBch-EDA surface in a monolayer distribution according to the higher value of correlation coefficient. Furthermore, as indicated in Table 2, the values of RL were determined to be < 1, ranging from RL = 0.045 to 0.488. Otherwise, according to the detected value of R2 = 0.98, Freundlich is the best fitting model for Cr(VI) adsorption onto NanoBch-EDA which postulate that the adsorption through heterogeneous multilayer [69]. Furthermore, the remarkable adsorption ability of NanoBch-EDA was demonstrated by the characterized value of the Freundlich constant (KF = 9.970 L mg−1) for Cr(VI). When the n-value is high (n > 1), the adsorption process is more powerful, which supports the NanoBch-EDA favorable adsorption process [70]. Table 2 compiles and demonstrates the calculated parameters from all models as R2, qmax, RL, KL, KT, KF, n, β, bT, and A. The values of maximum capacity qmax (mg g−1) for adsorption of Pn and Cr(VI) onto NanoBch-EDA were 21.93 and 79.37 mg g−1, respectively. At high concentration of Pn and Cr(VI), the percentage of Pn and Cr(VI) exclusion declined and these results explains that the active sites on NanoBch-EDA surface has become fully occupied and unable to catch more Pn and Cr(VI) molecules [69]. Based on the obtained data and isotherm parameters (Table 2), the calculated n = 1.974 and 1.757 to suggest that physical adsorption is the fundamental mechanism for adsorption of Pn and Cr(VI) onto NanoBch-EDA, respectively as illustrated in Scheme 1.
a Langmuir isotherm model for adsorption of prednisolone by NanoBch-EDA (Pn = 5–100 mg L−1, contact time = 30 min, dosage = 60 mg, volume = 20 mL and pH = 2). b Freundlich isotherm model for adsorption of Cr(VI) by NanoBch-EDA (Cr(VI) = 5–100 mg L.−1, contact time = 30 min, dosage = 20 mg, volume = 20 mL and pH = 2)
3.2.3 Impact of contact time
The influence of increasing in contact time between the NanoBch-EDA and Pn (25 mg L−1) in addition to Cr(IV) (10 mg L−1) was performed and discussed by various kinetic models including pseudo-first- and second-order, Elovich, and intraparticle diffusion. At first, pseudo-first order considered as the major model which characterize the interaction of liquid–solid and the solid substance capability for involving in the operation of adsorption. The linear equation (Eq. 6) in Table 1 epitomizes this model where qe and qt (mg g−1) are the amount of Pn (25 mg L−1) and Cr(IV) (10 mg L−1) adsorbed onto Artich-Bc-EDA at equilibrium and time t, respectively. The rate constant (K1, min−1) of pseudo-first-order was estimated from the straight line of the plot between ln (qe—qt) and t. On the other hand, the pseudo-second-order is the ultimate simplest and prevalent model which is utilized in the explanation of kinetic experiments as displayed by Eq. 7. The relation of this model was briefed through a straight line among (t/qt) against (t) time as validated in Fig. 9. The qe (mg g−1) is the equilibrium amounts of Pn and Cr(IV) adsorbed onto NanoBch-EDA as calculated from the slope of the gained straight line. The model rate constant K2 (g mg−1 min−1) is calculated from the intercept of the straight line. Besides, the intraparticle diffusion is another utilized model for describing the kinetic adsorption and was explained by Weber and Morris (Eq. 8). As in Table 1, the intraparticle constant kid (mg g−1 min−1/2) was determined from the slope of the plot of the adsorption amount (qt) of Pn and Cr(IV) versus (t1/2). Also, the surface width of sorption C (mg g−1) was identified from the intercept in this plot. Eventually, Elovich model was applied in the description of the chemisorption technique of heterogeneous methods. Equation 9 is employed to represent the linear formulation for Elovich model.
The pseudo-second-order model is displayed in Fig. 9 for elimination of Pn (25 mg L−1) and Cr(IV) (10 mg L−1) by NanoBch-EDA. Table 3 demonstrates all calculated parameters such as qe, K1, K2, Kid, C, and β, in addition to the correlation coefficients (R2) achieved from the plot by all previous models. The values of qtheor of Pn (25 mg L−1) and Cr(IV) (10 mg L−1) were found 7.86 and 9.38 mg g−1 to verify fully matching with the values of qe experimental (7.70 and 9.57 mg g−1), respectively. R2 values of the pseudo-second order are clearly greater than the R2 values which gained from other models. As a result, the pseudo-second order is the most appropriate model for explaining the adsorption processes of Pn (25 mg L−1) and Cr(IV) (10 mg L−1) onto NanoBch-EDA. Scheme 1a offers the conceivable ways for interaction among Pn molecules and NanoBch-EDA, where ion-pair mechanism would be formed between the highly positively charges on NanoBch-EDA surface as a result of the protonation of nitrogen-rich EDA molecules at pH 2 and the negatively charges on Pn surface. Additionally, Cr(VI) adsorption process onto NanoBch-EDA could be also proposed to occur over surface through ion-pair mechanism between Cr(VI) ions and the positively charged nitrogen functional as shown in Scheme 1b [71]. This means that EDA performs an essential function in the availability of positively charged in the adsorbent surface and therefore, facilitate the occurring of the proposed mechanism.
The collected results are obviously demonstrating that as the contact time of adsorbate (Pn and Cr(VI)) onto NanoBch-EDA increases. The adsorption of Pn onto NanoBch-EDA increases to 92.4% and full saturated for the active binding sites on NanoBch-EDA was occurred. Additionally, at the start the adsorption of Cr(VI) onto NanoBch-EDA is comparatively great at 60 min and reached to 95.7%, owing to the high abundance of active binding places on NanoBch-EDA [61]. At time > 60 min, there was a decrease from 95.7 to 92.7% as a result of the decline in the availability of binding sites on NanoBch-EDA based on the overcrowding, which rises the repulsive interactions between the functional groups on NanoBch-EDA to clarify the slight decrease in adsorption at longer time intervals [72, 73].
3.2.4 Thermodynamics and impact of temperature
The adsorption performance of NanoBch-EDA towards Pn and Cr(VI) were examined for discussing the impact of temperature on the adsorption operation. All achieved parameters from the thermodynamic experiments were determined and calculated using Van’t Hoff equations (Eq. 10) as given in Table 4. Figure 10 presents the equation of Van’t Hoff which was simplified by plotting ln KD against 1000/T, and from which the values of enthalpy (ΔH°) and entropy (ΔS°) could be identified from the gotten straight line and calculated from the slope (− ΔH°/R) and the intercept (ΔS°/R), respectively. The temperature factor was elevated from 288 to 333 K and revealed the enhancement in adsorption efficiency (%) for removal of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) onto NanoBch-EDA. The adsorption operations of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) onto NanoBch-EDA were detected to be spontaneous due to the negative value of ΔG° from Eq. 11. The ΔH° values were identified from the slope of Fig. 10 as 22.73 and 32.08 kJ mol−1 for adsorption of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) by NanoBch-EDA, respectively. Moreover, it was accomplished from the positive value of ΔH° that the adsorption operations of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) onto NanoBch-EDA were endothermic. The values of entropy (ΔS°) for adsorption of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) onto NanoBch-EDA were found 84.05 and 126.2 J mol−1 K−1, respectively. The computed positive values of ΔS° are attributed for enhancing entropy variation for elimination of Pn and Cr(VI) by NanoBch-EDA. Furthermore, the energy distribution among both adsorption and self-adsorption varied [74].
3.2.5 Interfering study
As displayed in Fig. 11, the influence of certain interfering salts including NaCl, KCl, NH4Cl, CaSO4, and MgSO4 was implemented to identifying the capability of NanoBch-EDA for Pn and Cr(VI) removal in presence of these salts. In this factor, 60 and 20 mg NanoBch-EDA were used as these doses are the optimum doses for Pn (25 mg L−1) and Cr(VI) (10 mg L−1), respectively. Removal of 92.2% of Pn (25 mg L−1) NanoBch-EDA was found in the absence of interfering salts, while their presence influenced the removal efficiency of (25 mg L−1) Pn by NanoBch-EDA. The existence of ions (Na+, K+, and NH+4) and (Ca+2 and Mg+2) led to a decrease in the effectiveness of NanoBch-EDA for extraction of Pn (25 mg L−1) by around 15% and 30%, respectively. This trend is mainly due to the ability of carboxylic group on NanoBch-EDA surface to interact and bind with (Na+, K+, and NH+4) for salts formation and so the ability of NanoBch-EDA was declined towards Pn (25 mg L−1). Additionally, Ca2+ and Mg+2 ions may bind and engage with the unoccupied positions on NanoBch-EDA and thus leading to a high drop in the adsorption percentage of Pn (25 mg L−1) onto NanoBch-EDA. These ions were also affected on the ability of NanoBch-EDA to remove Cr(VI) (10 mg L−1), but this impact was relatively small compared to their effect on Pn (25 mg L−1) elimination by NanoBch-EDA. The percentage extraction of Cr(VI) (10 mg L−1) by NanoBch-EDA decreased to (83.6%, 85.7%, and 85.5%), respectively In the presence of (Na+, K+, and NH+4), while the presence of Ca+2 and Mg+2 were characterized to decline the recovery of Cr(VI) (10 mg L−1) to 71% and 76.5%, respectively.
3.2.6 Regeneration and reusability of NanoBch-EDA
Regeneration of NanoBch-EDA-loaded Pn and NanoBch-EDA-loaded-Cr(VI) is very beneficial and economical method to recognize the possible contaminant desorption from the surface of NanoBch-EDA [75, 76]. Figure 12 shows the percentage removal of Pn were in the range of 75–90.0% by the recycled NanoBch-EDA after five cycles. The regeneration of NanoBch-EDA-loaded-Cr(VI) was deliberated through using HCl (0.1 mol L−1) in every cycle, as shown in Fig. 12, the recycled NanoBch-EDA removed Cr(VI) over five cycles in the range of 85–94.0%. This means that NanoBch-EDA exhibited high ability to remove Pn and Cr(VI) even after several number of reuses. Hence, the outlined results are referring that NanoBch-EDA as an appropriate and effective sorbent for elimination of Pn and Cr(VI).
3.2.7 Elimination of Pn and Cr(VI) by NanoBch-EDA from different waters
Several water species including tap, waste, and sea water were proceed to evaluate the effectiveness of NanoBch-EDA for application in water treatment applications of organic and inorganic pollutants as Pn and Cr(VI), respectively. The outcomes from this work are demonstrated in Table 5. Successful removal of Pn (25 mg L−1) from tap, waste, and sea water was established, respectively as 95%, 90%, and 92% by using 60 mg NanoBch-EDA. Recovery values 96%, 94.6%, and 97.3% of Cr(VI) (10 mg L−1) from tap, waste, and sea water were also confirmed when 20 mg NanoBch-EDA used, respectively.
4 Conclusion
In the current work, pyrolysis of artichoke leaves were done in a low oxygen environment at 350 °C to generate nanobiochar content (NanoBch), which was then enriched with ethylenediamine (EDA) as nitrogen-rich derivative to yield (NanoBch-EDA) as an appropriate and low-cost adsorbent for removal of prednisolone (Pn) and hexavalent chromium (Cr(VI)). The most notable function groups as COOH, OH, and C = C were confirmed from the FT-IR of NanoBch-EDA. At 25–800 °C, the thermal gravimetric analysis revealed that NanoBch-EDA exhibited lower thermal stability (total loss = 96.4%) than NanoBch (total loss = 81.8%). Results referred to the improvement in the elimination percentages of Pn and Cr(VI) due to the increase in the dose of NanoBch-EDA. Additionally, pH = 2 was listed as the superlative condition for Pn elimination and Cr(VI) by NanoBch-EDA as the highest adsorption of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) onto NanoBch-EDA. At this pH, ion-pair mechanism between the highly positively charged NanoBch-EDA surface resulting from the protonation of nitrogen-rich EDA molecules and the negatively charged Pn surface or Cr(VI) ions. Based on the obtained data from kinetic studies, it was concluded that pseudo-second-order was the finest model for this contact medium. It was also deduced from isotherms results that the elimination of Pn and Cr(VI) by NanoBch-EDA followed Langmuir and Freundlich models, respectively. Based on the values and signs of parameters obtained from thermodynamic experiments, the reaction was categorized as spontaneous, endothermic, and physisorption. Additionally, when (Na+, K+, and NH4+ Ca2+, and Mg2+) were present, the percentage extraction of Cr(VI) (10 mg L−1) by NanoBch-EDA decreased. The obtained results from this study demonstrated that NanoBch-EDA was effective and successful adsorbent for removal of Pn (25 mg L−1) and Cr(VI) (10 mg L−1) from different samples of water. Finally, Table 6 provides a good comparative assessment of the investigated NanoBch-EDA versus other previously reported studies [8, 23, 24, 77,78,79,80].
References
Cheng C, Jia M, Cui L, Li Y, Xu L, Jin X. Adsorption of Cr(VI) ion on tannic acid/graphene oxide composite aerogel: kinetics, equilibrium, and thermodynamics studies. Biomass Conversion Biorefinery. https://doi.org/10.1007/s13399-020-00899-4
Oraon A, Prajapati AK, Ram M, Saxena VK, Dutta S, Gupta AK (2022) Synthesis, characterization, and application of microporous biochar prepared from Pterospermum acerifolium plant fruit shell waste for methylene blue dye adsorption: the role of surface modification by SDS surfactant. Biomass Conversion Biorefinery. https://doi.org/10.1007/s13399-022-02320-8
Liang J, Chen Y, Cai M, Gan M, Zhu J (2021) One-pot pyrolysis of metal-embedded biochar derived from invasive plant for efficient Cr(VI) removal. J Environ Chem Eng 9:105714
Ngigi AN, Ok YS, Thiele-Bruhn S (2019) Biochar-mediated sorption of antibiotics in pig manure. J Hazard Mater 364:663–670
Jing C, Zhang Y, Zheng J, Ge S, Lin J, Pan D, Naik N, Guo Z (2022) In-situ constructing visible light CdS/Cd-MOF photocatalyst with enhanced photodegradation of methylene blue. Particuology 69:111–122
Jadhav P, Shinde S, Suryawanshi SS, Teli SB, Patil PS, Ramteke AA, Hiremath NG, Prasad NR (2020) Green AgNPs decorated ZnO nanocomposites for dye degradation and antimicrobial applications. Eng Sci 12:79–94
Aggrwal G, Salunke-Gawali S, Gejji SP, Nikalje M, Chakravarty D, Verma PL, Gosavi-Mirkute P, Harihar S, Jadhav M, Puranik VG (2021) Reactions of 2, 3-dibromonaphthalene-1,4-dione and pyridyl amines: X-ray structures, DFT investigations, and selective detection of the Hg2+ and Ni2+ ions. Eng Sci 14:78–93
Guo L, Zhang Y, Zheng J, Shang L, Shi Y, Wu Q, Liu X, Wang Y, Shi L, Shao Q (2021) Synthesis and characterization of ZnNiCr-layered double hydroxides with high adsorption activities for Cr(VI). Adv Compos Hybrid Mater 4:819–829
Allam EA, El-Sharkawy RM, Gizawy MA, Mahmoud ME (2021) Assembly of CeO2–MoO3–SiO2(CH2)3-(Alginate)2 As a novel nanocomposite for removal of MnII/CrVI and 56Mn/51Cr radionuclides from water. Mater Chem Phys 262:124278
Mahmoud ME, Nabil GM, Elweshahy SMT (2021) Novel NTiO2-chitosan@NZrO2-chitosan nanocomposite for effective adsorptive uptake of trivalent gadolinium and samarium ions from water. Powder Technol 378:246–254
Mahmoud ME, Abdelwahab MS (2021) One-step synthesis of zero-valent Sn nanoparticles and potential microwave remediation of lead from water. Mater Res Bull 134:11090
Allam EA, Ali ASM, Elsharkawy RM, Mahmoud ME (2021) Framework of nano metal oxides N-NiO@ N-Fe3O4@ N-ZnO for adsorptive removal of atrazine and bisphenol-A from wastewater: kinetic and adsorption studies. Environ Nanotechnol Monit Manag 16:100481
Mahmoud ME, El-Ghanam AM, Saad SR, Mohamed RHA (2020) Enhanced adsorption of levofloxacin and ceftriaxone antibiotics from water by assembled composite of nanotitanium oxide/chitosan/nano-bentonite. Mater Sci Eng C 108:110199
Mahmoud ME, Amira MF, Seleim SM, Nabil GN, Abouelanwar ME (2021) Multifunctionalized graphene oxide@ nanopolyaniline@ zirconium silicate nanocomposite for rapid microwable removal of dyes. J Nanostruct Chem 11:645–662
Caldwell DJ, D’Aco V, Davidson T, Kappler K, Murray-Smith RJ, Owen SF, Robinson PF, Simon-Hettich B, Straub JO, Tell T (2019) Environmental risk assessment of metformin and its transformation product guanylurea: II. occurrence in surface waters of Europe and the United States and derivation of predicted no-effect concentrations. Chemosphere 216:855–865
Xu D, Li Z, Wang P, Bai W, Wang H (2020) Aquatic plant-derived biochars produced in different pyrolytic conditions: spectroscopic studies and adsorption behavior of diclofenac sodium in water media. Sustain Chem Pharm 17:100275
Mahmoud ME, Saad SR, El-Ghanam AM, Mohamed RHA (2021) Developed magnetic Fe3O4–MoO3-AC nanocomposite for effective removal of ciprofloxacin from water. Mater Chem Phys 257:123454
Mahmoud ME, El-Ghanam AM, Saad SR, Mohamed RHA (2020) Promoted removal of metformin hydrochloride anti-diabetic drug from water by fabricated and modified nanobiochar from artichoke leaves. Sustain Chem Pharm 18:100336
Patel M, Kumar R, Kishor K, Mlsna T, Charles U, Pittman J, Mohan D (2019) Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem Rev 6:3510–3673
Weizel A, Schlüsener MP, Dierkes G, Wick A, Ternes TA (2020) Analysis of the aerobic biodegradation of glucocorticoids: Elucidation of the kinetics and transformation reactions. Water Res 174:115561
Albayati TM, Jassam AAA (2019) Synthesis and characterization of mesoporous materials as a carrier and release of prednisolone in drug delivery system. J Drug Deliv Sci Technol 53:101176
Jia A, Wu S, Daniels KD, Snyder SA (2016) Balancing the budget: accounting for glucocorticoid bioactivity and fate during water treatment. Environ Sci Technol 6:2870–2880
Flores-Céspedes F, Villafranca-Sánchez M, Fernández-Pérez M (2020) Alginate-based hydrogels modified with olive pomace and lignin to removal organic pollutants from aqueous solutions. Int J Biol Macromol 153:883–891
Bhattacharyya S, Banerjee P, Rathour RKS, Majumder SK, Das P, Datta S (2018) Comparative assessment on the removal of ranitidine and prednisolone present in solution using graphene oxide (GO) nanoplatelets. Desalin Water Treat 132:287–296
Fan ZL, Wu M, Chang H, Hu JY (2011) Behaviors of glucocorticoids, androgens and progestogens in a municipal sewage treatment plant: comparison to estrogens. Environ Sci Technol 45:2725–2733
Ammann AA, Macikova P, Groh KJ, Schirmer K, Suter MJF (2014) LC-MS/MS determination of potential endocrine disruptors of cortico signalling in rivers and wastewaters. Anal Bioanal Chem 406:7653–7665
Goh SXL, Goh EXY, Lee HK (2021) Sodium dodecyl sulfate-multi-walled carbon nanotubes-coated-membrane solid phase extraction of glucocorticoids in aqueous matrices. Talanta 221:121624
Rajeswari TR, Sailaja N (2014) Impact of heavy metals on environmental pollution. J Chem Pharm Sci 3:175–181
Hu H, Gao Y, Wang T, Sun L, Zhang YF, Li H (2019) Removal of hexavalent chromium, an analogue of pertechnetate, from aqueous solution using bamboo (Acidosasa edulis) shoot shell. J Radioanal Nucl Chem 321:427–437
Gholipour M, Hashemipour H, Mollashahi M (2011) Hexavalent chromium removal from aqueous solution via adsorption on granular activated carbon: adsorption, desorption, modelling and simulation studies. J Eng Appl Sci 6:10–18
Berihun D (2017) Removal of chromium from industrial wastewater by adsorption using coffee husk. J Mater Sci Eng 6:1000331
Nasrabadi T, Bidhendi GN, Karbassi A, Mehrdadi N (2010) Evaluating the efficiency of sediment metal pollution indices in interpreting the pollution of Haraz River sediments, southern Caspian Sea basin. Environ Monit Assess 171:395–410
Haruna A, Uzairu A, Harrison GFS (2011) Chemical fractionation of trace metals in sewage water–irrigated soils. Int J Environ Res 5:733–744
Serbaji MM, Azri C, Medhioub K (2012) Anthropogenic contributions to heavy metal distributions in the surface and sub-surface sediments of the Northern Coast of Sfax, Tunisia. Int J Environ Res 6:613–626
Manfe MM, Attar SJ, Parande M, Topare NS (2012) Treatment of Cr(VI) contaminated waste water using biosorbent Prunus amygdalus (Almond) nut shell carbon. Int J Chem Sci 10:609–618
Dave PN, Pandey N, Thomas H (2012) Adsorption of Cr(VI) from aqueous solutions on tea waste and coconut husk. Ind J Chem Technol 19:111–117
Aman T, Kazi AA, Sabri MU, Bano Q (2008) Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf B 63:116–121
Huang Y, Lee X, Macazo F, Grattieri M, Cai R, Minteer S (2018) Fast and efficient removal of chromium (VI) anionic species by a reusable chitosan-modified multiwalled carbon nanotube composite. Chem Eng J 339:259–267
Elmolla ES, Hamdy W, Kassem A, Abdel Hady A (2016) Comparison of different rice straw based adsorbents for chromium removal from aqueous solutions. Desalin Water Treat 57:6991–6999
Chakraborty R, Asthana A, Singh AK, Jain B, Susan ABH (2022) Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int J Environ Anal Chem 102:1–38
Afroze S, Sen TK (2018) A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut 229:225–229
Mahmoud ME, Osman MM, Abdel-Aal H, Nabil GM (2020) Microwave-assisted adsorption of Cr(VI), Cd(II) and Pb(II) in presence of magnetic graphene oxide-covalently functionalized-tryptophan nanocomposite. J Alloys Compd 823:153855
Zhao Z, Bai P, Du W, Liu B, Pan D, Das R, Liu C, Guo Z (2020) An overview of graphene and its derivatives reinforced metal matrix composites: preparation, properties and applications. Carbon 170:302–326
Li SJ, Cui JN, Wu X, Zhang X, Hu Q, Hou XH (2019) Rapid in situ microwave synthesis of Fe3O4/MIL-100(Fe) for aqueous diclofenac sodium removal through integrated adsorption and photodegradation. J Hazard Mater 373:408–416
Wang NN, Sun XY, Zhao Q, Yang Y, Wang P (2020) Leachability and adverse effects of coal fly ash: a review. J Hazard Mater 396:122725
Reis GS, Thue PS, Cazacliu BG, Lima EC, Sampaio CH, Quattrone M, Ovsyannikova E, Kruse A, Dotto GL (2020) Effect of concrete carbonation on phosphate removal through adsorption process and its potential application as fertilizer. J Clean Prod 256:120416
Caicedo DF, Reis GS, Lima EC, Brum IASD, Thue PS, Cazacliu BG, Lima DR, Santos AH, Dotto GL (2020) Efficient adsorbent based on construction and demolition wastes functionalized with 3-aminopropyltriethoxysilane (APTES) for the removal ciprofloxacin from hospital synthetic effluents. J Environ Chem Eng 8:103875
Low YW, Yee KF (2021) A review on lignocellulosic biomass waste into biochar-derived catalyst: current conversion techniques, sustainable applications and challenges. Biomass Bioenergy 154:106245
Wahi R, Zuhaidi NFQ, Yusof Y, Jamel J, Kanakaraju D, Ngaini Z (2017) Chemically treated microwave-derived biochar: An overview. Biomass Bioenergy 107:411–421
Wu H, Sun H, Han F, Xie P, Zhong Y, Quan B, Zhao Y, Liu C, Fan R, Guo Z (2022) Negative permittivity behavior in flexible carbon nano fibers poly dimethylsiloxane films. Eng Sci 17:113–120
Shakoor MB, Niazi NK, Bibi I, Murtaza G, Kunhikrishnan A, Seshadri B, Shahid M, Ali S, Bolan NS, Ok YS (2016) Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Crit Rev Environ Sci Technol 46:467–499
Ahmed I, Attar SJ, Parande MG (2012) Removal of hexavalent Chromium Cr(VI)) from industrial wastewater by using biomass adsorbent (rice husk carbone). Int J Adv Eng Stud 1:92–94
Park D, Yun YS, Ahn CK, Park JM (2007) Kinetics of the reduction of hexavalent chromium with the brown seaweed Ecklonia biomass. Chemosphere 66:939–946
Panwar NL, Pawar A (2022) Influence of activation conditions on the physicochemical properties of activated biochar: a review. Biomass Conversion Biorefinery 12:925–947
Sewu DD, Jung H, Kim SS, Lee DS, Woo SH (2019) Decolorization of cationic and anionic dye-laden wastewater by steam-activated biochar produced at an industrial-scale from spent mushroom substrate. Bioresour Technol 277:77–86
Jiang Z, Li JJ, Jiang D, Gao Y, Chen YK, Wang W, Cao B, Tao Y, Wang L, Zhang Y (2020) Removal of atrazine by biochar-supported zero-valent iron catalyzed persulfate oxidation: Reactivity, radical production and transformation pathway. Environ Res 184:109260
Sathvika T, Soni A, Sharma K, Praneeth M, Manasi V, Rajesh V, Rajesh N (2018) Potential application of Saccharomyces cerevisiae and Rhizobium immobilized in multiwalled carbon nanotubes to adsorb hexavalent chromium. Sci Rep 8:9862
Li B, Liu D, Lin XX, Wang S, Xu H, Wang J, Huang Y, Zhang S, Hu X (2020) Changes in biochar functional groups and its reactivity after volatile−char interactions during biomass pyrolysis. Energy Fuels 11:14291–14299
Fiol N, Villaescusa I (2009) Determination of sorbent point zero charge: usefulness in sorption studies. Environ Chem Lett 7:79–84
Tuan A, Nguyen VP, Ouakouak A, Nieva A, Bonifacio T, Doma J, Tran HN, Chao HP (2019) Effcient removal of Cr(VI) from water by biochar and activated carbon prepared through hydrothermal carbonization and pyrolysis:adsorption-coupled reduction mechanism. Water 11:2–14
Jobby R, Jha P, Yadav AK, Desai N (2018) Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: a comprehensive review. Chemosphere 207:255–266
Guo D, An Q, Xiao Z, Zhai S, Yang D (2018) Efficient removal of Pb(II), Cr(VI) and organic dyes by polydopamine modified chitosan aerogels. Carbohyd Polym 202:306–314
Koushkbaghi S, Zakialamdari A, Pishnamazi M, Ramandi HF, Aliabadi M, Irani M (2018) Aminated-Fe3O4 nanoparticles filled chitosan/PVA/PES dual layers nanofibrous membrane for the removal of Cr(VI) and Pb(II) ions from aqueous solutions in adsorption and membrane processes. Chem Eng J 337:169–182
Bajpai A, Frey K, Schneider WF (2020) Comparison of coverage-dependent binding energy models for mean-field microkinetic rate predictions. Langmuir 36:465–474
Singh S, Bajwa BS, Kaur I (2021) (Zn/Co)-zeolitic imidazolate frameworks: Room temperature synthesis and application as promising U(VI) scavengers — a comparative study. J Ind Eng Chem 93:351–360
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel (II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater 133:304–308
Nasrollahpour A, Moradi SE (2017) Hexavalent chromium removal from water by ionic liquid modified metal-organic frameworks adsorbent. Microporous Mesoporous Mater 243:47–55
Ringot D, Lerzy B, Chaplain K, Bonhoure JP, Auclair E, Larondelle Y (2007) In vitro biosorption of ochratoxin A on the yeast industry by-products: comparison of isotherm models. Bioresour Technol 98:1812–1821
Kahu S, Shekhawat A, Saravanan D, Jugade R (2016) Ionic solid-impregnated sulphate-crosslinked chitosan for effective adsorption of hexavalent chromium from effluents. Int J Environ Sci Technol 13:2269–2282
Mahmoud ME, Nabil GM, Zaki MM, Saleh MM (2019) Starch functionalization of iron oxide by-product from steel industry as a sustainable low cost nanocomposite for removal of divalent toxic metal ions from water. Int J Biol Macromol 137:455–468
Mahmoud ME, El-Sharkawy RM, Ibrahim GAA (2021) Promoted adsorptive removal of chromium(VI) ions from water by a green-synthesized hybrid magnetic nanocomposite (NFe3O4Starch-Glu-NFe3O4ED). RSC Adv 11:14829–14843
Achak M, Hafidi A, Ouazzani N, Sayadi S, Mandi L (2009) Low cost biosorbent “banana peel” for the removal of phenolic compounds from olive mill wastewater: kinetic and equilibrium studies. J Hazard Mater 166:117–125
Srivastava M, Ma LQ, Sabapathi BR, Srivastava P (2009) Effects of selenium on arsenic uptake in arsenic hyper accumulator Pterisvittata L. Bioresource Technol 100:1115–1121
Zhu S, Liu Y, Liu S, Zeng G, Jiang L, Zhou T, Zeng W, Li T, Yang C (2017) Adsorption of emerging contaminant metformin using graphene oxide. Chemosphere 179:20–28
Mahmoud ME, Abdelfattah AM, Fekry NA (2021) High performance of carbon quantum dots-decorated-polymeric nanocomposite for enhanced microwave adsorption of vanadium (V) from water. Ground Sustain Dev 14:100582
Lu D, Xu S, Qiu W, Sun Y, Liu X, Yang J, Ma J (2020) Adsorption and desorption behaviors of antibiotic ciprofloxacin on functionalized spherical MCM-41 for water treatment. J Clean Prod 264:121644
Zou H, Zhao J, He F, Zhong Z, Huang J, Zheng Y, Zhang Y, Yang Y, Yu F, Bashir MA, Gao B (2021) Ball milling biochar iron oxide composites for the removal of chromium (Cr(VI)) from water: performance and mechanisms. J Hazard Mater 413:125252
Zhou Y, Liu G, Liu J, Xiao Y, Wang T, Xue Y (2021) Magnetic biochar prepared by electromagnetic induction pyrolysis of cellulose: biochar characterization, mechanism of magnetization and adsorption removal of chromium (VI) from aqueous solution. Bioresour Technol 337:125429
Qu J, Wang Y, Tian X, Jiang Z, Deng F, Tao Y, Jiang Q, Wang L, Zhang Y (2021) KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: affecting factors, mechanisms and reusability exploration. J Hazard Mater 401:123292
Loulidi I, Boukhlifi F, Ouchabi M, Amar A, Jabri M, Kali A, Hadey C (2021) Assessment of untreated coffee wastes for the removal of chromium (VI) from aqueous medium. Int J Chem Eng 9977817
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohamed E. Mahmoud: conceptualization, methodology design, writing—editing—review the manuscript. Abdel Moneim El-Ghanam: writing—editing—review the manuscript. Shaimaa R. Saad: methodology, data analysis, writing, and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, M.E., El-Ghanam, A.M. & Saad, S.R. Sequential removal of chromium (VI) and prednisolone by nanobiochar- enriched-diamine derivative. Biomass Conv. Bioref. 14, 7011–7030 (2024). https://doi.org/10.1007/s13399-022-02888-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02888-1