Abstract
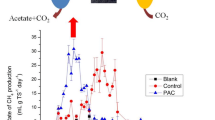
Agave plants are receiving increasing attention due to a wide range of products obtained from them. Besides, Agave processing generates lignocellulosic biomass (leaves and bagasse) and vinasses, all of them are wastes with a high organic matter content, which are suitable for methane production. However, Agave bagasse has been identified among the most recalcitrant lignocelluloses for biomethanization, while vinasses typically present low biodegradability indices. This study is aimed to improve methane production testing the inocula activated sludge (AS), pig manure (PM), and a mixture of them (M) at substrate-to-inoculum (S/I) ratios of 0.25, 0.50, 1.0, and 2.0, in terms of g volatile solids (VS)/gVS. The substrate consisted of a mixture of leaves, bagasse, and vinasses from the mezcal production. The study also analyzed microbial composition (bacteria and archaea) before and after anaerobic digestion and correlated performance with species abundance. AS reactors reached the highest methane production of 498 ± 67 mL (166 mL/gVS), followed by PM and M reactors that produced 188 ± 39 mL each (63 mL/gVS), all at a 0.25 S/I ratio. At a 0.50 S/I ratio or higher, the methane production stopped due to an insufficient quantity of microorganisms which were active during the process. AS reactors maintained the pH between 6.8 and 7.5 at all S/I ratios with a negligible volatile fatty acid accumulation. On the contrary, PM and M reactors led to volatile fatty acid accumulation as high as 12.2 g/L, so pH became acidic, ranging from 4.9 to 5.8. AS reactors contained the highest alpha diversity. The archaeal community in AS reactors consisted of Methanosarcina, Methanobrevibacter, and Methanospirillum. Unlike, Methanobrevibacter was the predominant genus in PM/M reactors. Pseudomonas and Clostridium were the predominant genera in the bacterial communities of AS reactors and PM/M reactors, respectively. The methane production positively correlated with Methanosarcina (r = 0.79) in AS reactors. On the contrary, the volatile fatty acid accumulation positively correlated with Methanobrevibacter (r = 0.57), Clostridium (r = 0.99), and Turicibacter (r = 0.96) in PM/M reactors. In sum, the AS inoculum at a 0.25 S/I ratio provided the proper quantity and type of microorganisms (such as Methanosarcina) and also the buffer capacity for improving notably the start-up of an anaerobic digester which treated the mixed agave wastes.




Similar content being viewed by others
Data availability
Data used for the research work will be shared upon request.
References
Davis SC, Dohleman FG, Long SP (2011) The global potential for Agave as a biofuel feedstock. GCB Bioenergy 3:68–78. https://doi.org/10.1111/j.1757-1707.2010.01077.x
Pérez-Pimienta JA, López-Ortega MG, Sanchez A (2017) Recent developments in Agave performance as a drought-tolerant biofuel feedstock: agronomics, characterization, and biorefining. Biofuel Bioprod Biorefin 11:732–748. https://doi.org/10.1002/bbb.1776
Robles-González V, Galíndez-Mayer J, Rinderknecht-Seijas N, Poggi-Varaldo HM (2012) Treatment of mezcal vinasses: A review. J Biotechnol 157:524–546. https://doi.org/10.1016/j.jbiotec.2011.09.006
Hoarau J, Caro Y, Grondin I, Petit T (2018) Sugarcane vinasse processing: Toward a status shift from waste to valuable resource A review. J Water Process Eng 24:11–25. https://doi.org/10.1016/j.jwpe.2018.05.003
Rodrigues Reis CE, Hu B (2017) Vinasse from Sugarcane Ethanol Production: Better Treatment or Better Utilization? Front Energy Res 5:7. https://doi.org/10.3389/fenrg.2017.00007
España-Gamboa E, Mijangos-Cortes J, Barahona-Perez L, Dominguez-Maldonado J, Hernández-Zarate G, Alzate-Gaviria L (2011) Vinasses: characterization and treatments. Waste Manag Res 29(12):1235–1250. https://doi.org/10.1177/0734242X10387313
Abraham A, Mathew AK, Park H, Choi O, Sindhu R, Parameswaran B, Sang BI (2020) Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour Technol 301:122725. https://doi.org/10.1016/j.biortech.2019.122725
Buitrón G, Hernández-Juárez A, Hernández-Ramírez MD, Sánchez A (2019) Biochemical methane potential from lignocellulosic wastes hydrothermally pretreated. Ind Crops Prod 139:111555. https://doi.org/10.1016/j.indcrop.2019.111555
Valdez-Vazquez I, Alatriste-Mondragón F, Arreola-Vargas J, Buitrón G, Carrillo-Reyes J, León-Becerril E, Mendez-Acosta HO, Ortíz I, Weber B (2020) A comparison of biological, enzymatic, chemical and hydrothermal pretreatments for producing biomethane from Agave bagasse. Ind Crops Prod 145:112160. https://doi.org/10.1016/j.indcrop.2020.112160
Pellera FM, Gidarakos E (2016) Effect of substrate to inoculum ratio and inoculum type on the biochemical methane potential of solid agroindustrial waste. J Environ Chem Eng 4(3):3217–3229. https://doi.org/10.1016/j.jece.2016.05.026
Ma X, Jiang T, Chang J, Tang Q, Luo T, Cui Z (2019) Effect of substrate to inoculum ratio on biogas production and microbial community during hemi-solid-state batch anaerobic co-digestion of rape straw and dairy manure. Appl Biochem Biotechnol 189(3):884–902. https://doi.org/10.1007/s12010-019-03035-9
Rouches E, Escudié R, Latrille E, Carrère H (2019) Solid-state anaerobic digestion of wheat straw: Impact of S/I ratio and pilot-scale fungal pretreatment. Waste Manage 85:464–476
Liu T, Sun L, Müller B, Schnürer A (2017) Importance of inoculum source and initial community structure for biogas production from agricultural substrates. Bioresour Technol 245:Part A, 768–777. https://doi.org/10.1016/j.biortech.2017.08.213
Hallaji SM, Kuroshkarim M, Moussavi SP (2019) Enhancing methane production using anaerobic co-digestion of waste activated sludge with combined fruit waste and cheese whey. BMC Biotechnol 19:19. https://doi.org/10.1186/s12896-019-0513-y
Elsayed M, Diab A, Soliman M (2021) Methane production from anaerobic co-digestion of sludge with fruit and vegetable wastes: effect of mixing ratio and inoculum type. Biomass Conv Bioref 11:989–998. https://doi.org/10.1007/s13399-020-00785-z
Ma G, Chen Y, Ndegwa P (2021) Association between methane yield and microbiota abundance in the anaerobic digestion process: A meta-regression. Renew Sustain Energy Rev 135:110212. https://doi.org/10.1016/j.rser.2020.110212
Zhang L, Yuan Y, Zhang Y, Liu Y (2021) Calcium hypochlorite pretreatment improves thermophilic digestion of waste activated sludge in an upflow anaerobic sludge blanket reactor. Sci Total Environ 151130. https://doi.org/10.1016/j.scitotenv.2021.151130
Xing BS, Wang XC (2021) High-rate mesophilic co-digestion with food waste and waste activated sludge through a low-magnitude increasing loading regime: Performance and microorganism characteristics. Sci Total Environ 777:146210. https://doi.org/10.1016/j.scitotenv.2021.146210
Chen Y, Yang H, Zou H, Sun T, Li M, Zhai J, He Q, Gu L, Tang WZ (2020) Effects of acid/alkali pretreatments on lignocellulosic biomass mono-digestion and its co-digestion with waste activated sludge. J Clean Prod 277:123998. https://doi.org/10.1016/j.jclepro.2020.123998
APHA (2012) Standard methods for the examination of water and wastewater, Washington, DC, USA
Cardeña R, Moreno G, Valdez-Vazquez I, Buitrón G (2015) Optimization of volatile fatty acids concentration for photofermentative hydrogen production by a consortium. Int J Hydrog Energy 40:17212–17223. https://doi.org/10.1016/j.ijhydene.2015.10.020
Martínez-Gutiérrez G, Ortiz-Hernández Y, Aquino-Bolaños T, Bautista-Cruz A, López-Cruz J (2015) Properties of Agave angustifolia Haw bagasse before and after its composting. Comun Sci 6(4):418–429. https://doi.org/10.14295/cs.v6i4.800
Pérez Del Río R, Caballero Caballero M, Hernández Gómez LH, Montes Bernabé JL (2013) Design and construction of Agave angustifolia Haw leaf shredder. Rev Cie Téc Agr 22(4):5–14
van Soest PV, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Int J Dairy Sci 74:3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
AOAC (2012) Official Methods of Analysis. AOAC International, Gaithersburg, Maryland, USA
Angelidaki I, Alves M, Bolzonella D, Borzacconi L, Campos JL, Guwy AJ, Kalyuzhnyi S, Jenicek P, Van Lier JB (2009) Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci Technol 59:927–934. https://doi.org/10.2166/wst.2009.040
Nopharatana A, Pullammanappallil PC, Clarke WP (2007) Kinetics and dynamic modelling of batch anaerobic digestion of municipal solid waste in a stirred reactor. Waste Manag 27(5):595–603. https://doi.org/10.1016/j.wasman.2006.04.010
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Shu D, He Y, Yue H, Wang Q (2016) Metagenomic and quantitative insights into microbial communities and functional genes of nitrogen and iron cycling in twelve wastewater treatment systems. Chem Eng J 290:21–30. https://doi.org/10.1016/j.cej.2016.01.024
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinform 26(2010):2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinform 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Edgar RC (2013) UPARSE: highly accurate OUT sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9. http://folk.uio.no/ohammer/past/
Figueroa-Escamilla L, Gonzalez-Martinez S, Campuzano R, Valdez-Vazquez I (2021) Methane production and bromatological characteristics of the different fractions of organic municipal solid waste. Detritus 15:13–23. https://doi.org/10.31025/2611-4135/2021.15095
Zhang J, Li W, Lee J, Loh KC, Dai Y, Tong YW (2017) Enhancement of biogas production in anaerobic co-digestion of food waste and waste activated sludge by biological co-pretreatment. Energy 137(15):479–486. https://doi.org/10.1016/j.energy.2017.02.163
Tian W, Chen Y, Shen Y, Zhong C, Gao M, Shi D, He Q, Gu L (2020) Effects of hydrothermal pretreatment on the mono- and co-digestion of waste activated sludge and wheat straw. Sci Tot Environ 732:139312. https://doi.org/10.1016/j.scitotenv.2020.139312
Potdukhe RM, Sahu N, Kapley A, Kumar R (2021) Co-digestion of waste activated sludge and agricultural straw waste for enhanced biogas production. Bioresour Technol Rep 15:100769. https://doi.org/10.1016/j.biteb.2021.100769
Abdel daiem MM, Hatata A, Galal OH, Said N, Ahmed D (2021) Prediction of biogas production from anaerobic co-digestion of waste activated sludge and wheat straw using two-dimensional mathematical models and an artificial neural network. Renew Energy 178:226–240. https://doi.org/10.1016/j.renene.2021.06.050
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58. https://doi.org/10.1016/j.biortech.2015.08.061
Werner JJ, Knights D, Garcia ML, Scalfone NB, Smith S, Yarasheski K, Cummings TA, Beers AR, Knight R, Angenent LT (2011) Bacterial community structures are unique and resilient in full-scale bioenergy systems. Proc Natl Acad Sci 108:4158–4163. https://doi.org/10.1073/pnas.1015676108
Li J, Liu W, Cai W, Wang B, Ajibade FO, Zhang Z, Tian X, Wang A (2019) Applying rhamnolipid to enhance hydrolysis and acidogenesis of waste activated sludge: retarded methanogenic community evolution and methane production. RSC Adv 9:2034–2041. https://doi.org/10.1039/C8RA08993K
Zhang L, Loh KC, Zhang J, Mao L, Tong YW, Wang CH, Dai Y (2019) Three-stage anaerobic co-digestion of food waste and waste activated sludge: identifying bacterial and methanogenic archaeal communities and their correlations with performance parameters. Bioresour Technol 285:121333. https://doi.org/10.1016/j.biortech.2019.121333
Pokój T, Klimiuk E, Bułkowska K, Kowal P, Ciesielski S (2020) Effect of individual components of lignocellulosic biomass on methane production and methanogen community structure. Waste Biomass Valor 11:1421–1433. https://doi.org/10.1007/s12649-018-0434-3
Danielsson R, Dicksved J, Sun L, Gonda H, Müller B, Schnürer A, Bertilsson J (2017) Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front Microbiol 226:1–15. https://doi.org/10.3389/fmicb.2017.00226
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Rychlik J, May T (2000) The effect of a methanogen, Methanobrevibacter smithii, on the growth rate, organic acid production, and specific ATP activity of three predominant ruminal cellulolytic bacteria. Curr Microbiol 40(3):176–180. https://doi.org/10.1007/s002849910035
Muñoz-Páez KM, Alvarado-Michi EL, Buitrón G, Valdez-Vazquez I (2019) Distinct effects of furfural, hydroxymethylfurfural and its mixtures on dark fermentation hydrogen production and microbial structure of a mixed culture. Int J Hydrog Energy 44:2289–2297. https://doi.org/10.1016/j.ijhydene.2018.04.139
De Paepe K, Verspreet J, Courtin CM, Van de Wiele T (2020) Microbial succession during wheat bran fermentation and colonisation by human fecal microbiota as a result of niche diversification. ISME J 14:584–596. https://doi.org/10.1038/s41396-019-0550-5
Rettedal E, Vilain S, Lindblom S, Lehnert K, Scofield C, George S, Clay S, Kaushik RS, Rosa AJ, Francis D, Brözel VS (2009) Alteration of the ileal microbiota of weanling piglets by the growth-promoting antibiotic chlortetracycline. Appl Environ Microbiol 75:5489–5495. https://doi.org/10.1128/AEM.02220-08
Rui J, Li J, Zhang S, Yan X, Wang Y, Li X (2015) The core populations and co-occurrence patterns of prokaryotic communities in household biogas digesters. Biotechnol Biofuels 8:158. https://doi.org/10.1186/s13068-015-0339-3
Habtewold J, Gordon R, Sokolov V, VanderZaag A, Wagner-Riddle C, Dunfield K (2018) Reduction in methane emissions from acidified dairy slurry is related to inhibition of Methanosarcina species. Front Microbiol 9:2806. https://doi.org/10.3389/fmicb.2018.02806
Puig-Castellví F, Cardona L, JouanRimbaudBouveresse D, Cordella CBY, Mazéas L, Rutledge DN, Chapleur O (2020) Assessment of the microbial interplay during anaerobic co-digestion of wastewater sludge using common components analysis. PLoS One 15:e0232324. https://doi.org/10.1371/journal.pone.0232324
Akyol Ç (2020) In search of the optimal inoculum to substrate ratio during anaerobic co-digestion of spent coffee grounds and cow manure. Waste Manag Res 38(11):1278–1283. https://doi.org/10.1177/0734242X20914731
Leser TD, Amenuvor JZ, Jensen TK, Lindecrona RH, Boye M, Møller K (2002) Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl Environ Microbiol 68:673–690. https://doi.org/10.1128/AEM.68.2.673-690.2002
Acknowledgements
A.V. Gómez-Guerrero thanks CONACYT for the scholarship. Jaime Pérez Trevilla is acknowledged for his technical assistance. This work was supported by the Instituto Politécnico Nacional (grant no. 20151315) and the DGAPAUNAM (PAPIIT project, grant no. IN102721).
Author information
Authors and Affiliations
Contributions
M Caballero-Caballero involved in conceptualization; supervision; funding acquisition; writing—review and editing. F Chiñas-Castillo took part in conceptualization; funding acquisition; writing — review and editing. AV Gómez-Guerrero: investigation; methodology; formal analysis; writing—first draft. I Valdez-Vazquez involved in conceptualization; methodology; supervision; funding acquisition; formal analysis; writing—final draft. R Alavez-Ramirez involved in supervision; resources; writing—review and editing. JL Montes Bernabe involved in supervision; resources; writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caballero-Caballero, M., Chiñas-Castillo, F., Gómez-Guerrero, A.V. et al. Activated sludge as inoculum improves methane production and community functionality during the anaerobic digestion of mixed agave wastes. Biomass Conv. Bioref. 14, 4635–4644 (2024). https://doi.org/10.1007/s13399-022-02718-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02718-4