Abstract
Polyphenolic stilbene glucosides are abundant in the fresh bark of Norway spruce (Picea abies [L.] Karst.) trees. Stilbene-rich bark extracts could be industrially utilized as preservatives due to their antioxidative, antifungal, and antibacterial properties. The postharvesting conditions, especially industrial debarking, influence the chemical properties of bark. Inherent variation in high-value compounds of bark is assumed to be offset by modifications within the bark supply chain; however, essential quantitative information is still rare. This study elucidated the magnitude of variation in the stilbenoid content and composition of Norway spruce bark due to (1) the geographical origin of Norway spruce seeds, (2) the geographical location of the growing site, (3) within-tree variability, and (4) industrial handling and pilot-scale extraction and fractioning processes. The inherent variation in stilbenoid content was large: the total average stilbenoid content of the inner bark varied from 70 to 110 mg/g of dry weight (DW). Sampling position in the stem and growing site explained over 50% of the total variance in stilbenoid content. Trees with a northern origin of seeds had a higher isorhapontin/astringin ratio than the trees with a southern origin of seeds, regardless of their growing site. Industrial bark from sawmills showed a significantly higher total stilbenoid content in winter than in summer, 22 mg/g and 1–3 mg/g DW, respectively. The inherent variation in the stilbenoid content was offset by the variation caused by the debarking process and experimental pilot-scale processing. To optimize the yield of stilbenoids from spruce bark, sampling of northern forests and short handling times in the supply chain are recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the forestry industry, bark is one of the major organic side-products from the debarking process at sawmills and pulp mills. The forestry industry in Finland produces ca. 7 million m3 of bark per year [1], which is mainly combusted to generate heat and energy. Transformation from fossil-based to bioeconomy strives to advance the utilization of bark side streams as a sustainable and vast biomass source for commercially valuable biochemicals and materials. The stilbenoids of spruce bark could be valorized for various commercial applications, such as antioxidants, antimicrobials, and preservatives, in cosmetics, techno-chemical products, or pharmaceuticals by applying environmentally benign biorefinery processes, including pressurized hot water extraction or supercritical fluid extraction, followed by further purification [2,3,4,5,6].
Norway spruce (Picea abies (L.) Karst.) is one of the dominant coniferous tree species in the boreal region. The bark of Norway spruce trees is a potential source of bioactive and protective compounds, such as glycosylated monomeric stilbene glucosides (trans-astringin, trans-isorhapontin, and trans-piceid), which are structurally similar to by far the most extensively studied stilbenoid, trans-resveratrol (Fig. 1). Resveratrol, piceatannol, and isorhapontigenin are the respective aglycone forms of the glucosides that appear as minor compounds in spruce bark [7]. In plants, stilbenoids serve as defensive compounds against a wide variety of environmental stressors, including protection from ultraviolet (UV) light and attacks by fungal pathogens and other microorganisms [8,9,10]. The inner bark of spruce contains 5–15% (dry weight, DW) stilbene glucosides [11,12,13].
Stilbenoids are known to vary according to growing site, within-stem location, and season of harvesting [11,12,13,14,15]. The high content of bark secondary metabolites may be a sign of ‘northern vigour’, i.e. trees have increased production of defence compounds in harsh environmental conditions, such as heavy snow load in winter or extreme temperatures due to the high latitude or altitude of the growing site, cf., [14]. It has been shown that the knots of Norway spruce have significantly higher lignan content close to the Arctic tree line in northern Finland than in southern locations, and thus, knot wood from northern mills is feasible to apply for biorefining [16]. However, to the best of our knowledge, no quantitative studies exist that simultaneously compare the magnitude of effects of seed origins and the effect of growth environment on bark stilbene chemistry in Norway spruce trees at the age of final felling. Furthermore, only a few systematic research reports have analysed the variability of bark secondary metabolites and changes in their content and composition within the industrial supply chain [15, 17,18,19,20]. Such information is, nevertheless, crucial for the commercial utilization of bark [6]. Several new wood-based biorefinery approaches are being planned, including the production of biochemicals from bark; thus, it is important to understand how the raw material should be selected, stored, and processed.
Stilbene glucosides can be extracted from bark with hot water under relatively mild conditions, even at 100 °C. Inner bark and outer bark contain different amounts of stilbene compounds [13,14,15], so extraction yield depends on relative portions of these parts, as well as the proportion of residual stem wood (with no stilbenes) after debarking. The yield of stilbene glucosides also depends on the preprocessing [17] and purification steps of bark [21]. Ultrafiltration has been shown to be a better concentration method than evaporation when spruce bark hot water extract is concentrated [22]. Extraction should also preferably be a part of a cascade process [23], in which extracted bark is further used in pyrolysis and anaerobic digestion to fully utilize this biomass.
Usually, studies related to plant physiology aspects and industrial utilization are carried out separately. Studies related to plant physiology usually include a representative sample size and provide detailed information on the variation in chemical composition.
On the other hand, for large-scale extraction studies, bark is usually collected from industrial sources, where compounds could have been degraded when stored in piles after debarking. In this study, we bridge the gap between biological studies and industrial applications to show the pathway of stilbenoids from biological sources to actual pilot-scale extraction and fractionation.
The aim of this study was to investigate how the stilbene glucoside content and composition of Norway spruce bark vary due to (1) the geographical origin of Norway spruce seeds (i.e. provenance), (2) the geographical location of the growing site of spruce provenances, and (3) within-tree variability (i.e. at different stem heights). Additionally, we examined (4) how bark stilbene content and composition vary during industrial storage and pilot-scale extraction and fractioning processes.
2 Materials and methods
A common garden experiment of ca. 83-year-old Norway spruce provenances was utilized. Trees representing four different provenances (two with northern Finnish origin, two with central European origin) grown in two distinct geographical locations in northern Finland and southern Finland were harvested. Inner bark from four different vertical sample discs on each stem was extracted and analysed for stilbene glucoside content and composition by gas chromatography–mass spectrometry (GC–MS). Industrial bark from sawmills was used for pilot-scale extractions. Whole bark was extracted with hot water by using different process parameters and purification steps with XAD7HP adsorbent. The effects of different processing steps on stilbene glucoside content and composition in bark and resulting extracts were analysed by gas chromatography with flame-ionization detectors (GC-FID) and high-performance liquid chromatography (HPLC).
2.1 Experimental stands
Figure 2 shows the locations for seed origins and the geographical locations of the provenance trial experiment stands in Finland selected for this study. During the late 1920s, seed material was collected from superior individual trees of Norway spruce stands in central Europe and Finland and grown into seedlings in Finland, and provenance trial experiments were then established in the early 1930s in Finland (Fig. 2, Table 1). For this study, four provenances were selected, all replicated at two geographical locations of the provenance trial experiment: Schmiedefeld/Thüringer Wald (named ‘Preußen’), Schilbach (named ‘Sachsen’), ‘Simo’, and ‘Sodankylä’ (Table 1). Additionally, trees representing one local provenance from both the south and the north growing sites (i.e. Kivalo and Punkaharju, respectively) were sampled.
Location of the P. abies seed origins in Germany (‘Sachsen’ and ‘Preußen’) and Finland (‘Simo’ and ‘Sodankylä’) (left) and research forests in northern and southern Finland (Kivalo and Punkaharju, respectively) where the provenances were grown (right). Additional sample trees representing local provenances were also harvested from both growth sites in Finland (pink and purple characters)
2.2 Bark material from provenance trials
Norway spruce (Picea abies [L.] Karst.) trees (83 years old) were harvested in northern Finland (Kivalo research forest, 66°2′N, 26°4′E, 140 m above sea level) and southern Finland (Punkaharju research forest, 61°45′N, 29°23′E, 113 m above sea level), representing four different provenances of experimental design and two additional local provenances. Three sample trees without defects per provenance were harvested, and four sample discs per tree were collected along the trunk (stem base 0.3 m, height class (H) 1; breast height (1.3 m, H2); base of living crown (H3); and 75% on the stem (H4)) (Fig. 3). The discs were stored at − 20 °C until further processing. From the discs, the inner bark was separated from the outer bark with a knife based on visually observed differences in tissue colour and softness [14, 15]. The inner bark was freeze-dried and ground using a ball mill (Retsch MM400, Retsch GmbH, Haan, Germany) and stored at − 20 °C prior to use.
2.3 Bark from sawmills
Industrial Norway spruce bark originated from sawmills located in Pietarsaari (63°7′N, 22°7′E) and Haapajärvi (63°7′N, 25°3′E) in Finland. Typically, the wood supply for a sawmill originates from an area around the sawmill within a radius of a few hundred kilometres. Pilot extractions were conducted in two separate series (Fig. 4). The first series, consisting of four extraction batches, was completed between February 25 and 28, 2019, with bark originating from a Pietarsaari mill. Bark was retrieved on February 15, 2019, by a debarker immediately after it had been peeled off from the sawlogs. The material was stored frozen in ambient winter conditions. Fines from the material were removed by screening with a 4 m2 screen with a 20 × 20 mm mesh size.
The second series consisted of five extraction batches. Bark for these extractions was harvested in summer and originated from the Haapajärvi sawmill. Bark was retrieved on September 4, 2019, by a debarker immediately after the debarking of trees and industrial shredding of bark. For two of the extraction batches, bark was further comminuted with a shredder having a screen of 25 × 25 mesh. Bark was stored at + 5 °C prior to extractions, which were carried out between September 9 and 13, 2019.
2.4 Analysis of stilbene glucosides in the bark from Norway spruce provenance trials
Approximately 20 mg of bark powder was extracted with an acetone–water solution (95:5, v/v, including 0.2 mg mL−1 heptadecanoic acid as an internal standard) in an ultrasonic water bath (USC300TH, VWR, Radnor, PA, USA) for 30 min. After sonication, the tubes were centrifuged at 3000 rpm for 5 min, and 1 mL of supernatant from each sample was pipetted into an autosampler vial. The samples were evaporated with N2, silylated with 0.5 mL of N-trimethylsilyl imidazole (TMSi in pyridine, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), and kept in a heater at 60 °C for 30 min or at room temperature overnight before analysis. The silylated extracts were analysed by GC–MS (Agilent Hewlett-Packard 6890 and Hewlett-Packard 5973 MSD, EIMS 70 eV; Agilent, Santa Clara, CA, USA) equipped with a Zebron ZB SemiVolatiles capillary column. Quantitative analysis of the stilbene glucosides (trans-astringin, trans-isorhapontin, trans-piceid) was performed using an internal standard (heptadecanoic acid, C:17) and authentic compounds as external standards (for detailed methodological description, see [14, 15]).
2.5 Comparison of laboratory- and pilot-scale extractions of Norway spruce bark from sawmills
Stilbene glucosides were extracted first in the laboratory, and a response surface model was created to find optimal conditions for the pilot-scale extractions. An accelerated solvent extractor (ASE-100, Dionex, USA) was used for the lab-scale extractions. Scaled-up extractions were performed in February 2019 for the winter bark and September 2019 for the summer bark. Pilot-scale extractions were carried out with a 300-l reactor [24] for 40–80 min at 60–85 °C in batch mode for the winter bark and 40–47 min at 90–120 °C for summer bark. Altogether, 80–100 kg of fresh bark was added into the reactor, which equals approximately 40 kg of dry bark. A total extract of 230–250 l and 210–250 l of winter and summer bark was collected into a 1000-l container. A subsample of each extraction was collected and stored in a freezer at − 20 °C prior to further chemical analyses. Approximately 36–38 l of winter bark extract was eluted in an XAD7HP adsorbent column to remove carbohydrates [25]. For summer bark, extracts (22–59 l) were eluted through a column, the column was washed with water, and polyphenols were eluted out of adsorbent using 95% ethanol.
2.6 Analysis of stilbenoids from the bark and extracts from the piloting studies
Bark samples (both before and after the pilot extractions) were first extracted with an accelerated solvent extractor (ASE 100) with hexane and subsequently with acetone–water (70/30) to remove lipophilic and hydrophilic compounds, respectively. The ASE extractions were performed at 120 °C and 1500 psi, with a static extraction time of 10 min and one cycle. Approximately 3 mg of the acetone–water extract was dried together with internal standards (100 µg of heneicosanoic acid and betulinol in acetone). A total of 500 µg of pyridine and 300 µg of the silylation reagent N-trimethylsilyl imidazole (TMSI in pyridine, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) were added, and the mixture was kept in an oven at 70 °C for 1 h to silylate it. The derivatized samples were analysed quantitatively and qualitatively with an Agilent 6850 GC-FID and Hewlett Packard 5973 GC–MS equipped with an HP-5 column (30 m × 0.32 mm with 0.25 μm film), respectively. The samples were injected at 290 °C and detected at 300 °C. The temperature programme used was 100 °C (1.5 min), 6 °C/min to 180 °C, 4 °C/min to 290 °C (13 min), and 4 °C/min to 300 °C (20 min).
Crude and resin-treated extracts were analysed for their stilbene glucoside and aglycone content by GC-FID/MS. Approximately 0.5 mL of the extracts was first dried under nitrogen flow with 100 µg of heneicosanoic acid (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and betulinol (Sigma-Aldrich, St. Louis, MO, USA) in acetone added as internal standards. In the case of the predried resin-treated extracts from Jokioinen, approximately 6 mg of the dried extract was weighed, to which the internal standards were added and dried. Silylation and analyses of samples were carried out as in the case of the bark samples.
Approximately 4 mg of the predried and resin-treated extractives was weighed for HPLC analysis of stilbenoids. The samples were dissolved in UHQ water and filtered through a 0.2-µm PTFE filter. An Agilent 1290 LC instrument equipped with a ZORBAX StableBond column (80 Å C18, 2.1 mm × 100 mm, 1.8 µm, 1,200 bar), a ZORBAX SB-C18 UHPLC guard column (2.1 mm, 1.8 µm), 1290 Infinity II Diode Array Detector, and a 6460 triple quadrupole mass spectrometer (LC/DAD/QQQQ) was used for analyses. The LC columns were kept at 30 °C. Two solvents were used for the mobile phase: (A) 0.1% formic acid in UHQ water and (B) 0.1% formic acid in acetonitrile. Trans-piceid (0.05–0.8 mg/mL) was used as an external standard for the quantitative analysis of stilbenoids. LC analyses were carried out as described in [18].
2.7 Statistical analysis
In the provenance experiment, various stilbenes were modelled separately using the same linear mixed model with the following effects (hierarchy levels of the data: i = tree, j = height class of a tree):
A log-transformed stilbene Y (for a height class of a tree) was explained by the fixed effects of seed provenance (Provenance: Preußen, Sachsen, Simo, or Sodankylä), growing site (Site: Kivalo or Punkaharju), height class (Height: 1, 2, 3, or 4), their two-way interaction effects, and the intercept (int). The random effect of tree (Tree) with autoregressive covariance structure between height classes for random errors (e) was added to the model to account for correlated Y measurements from the same tree. Random terms (Tree and e) were assumed to be normally distributed.
Additionally, the variance component model (using all effects as random except the intercept) was used to roughly estimate the proportion of the total variation (variance) explained by each effect [26]. The fixed and random effects of the original model (above) were separated with different colours in the variance component plot.
In the final analysis, 87–95 height class observations were included in the model development.
The local provenances (Kivalo and Punkaharju) were excluded from the analysis because they existed in only one growing site. The local provenances (Kivalo and Punkaharju) were excluded from the analysis because they existed in only one growing site.
In the industrial bark data, various stilbenes were modelled separately using almost the same linear mixed model with the following effects (hierarchy levels of the data: i = extract, j = sample of an extract, k = treatment stage of a sample of an extract):
A log-transformed stilbene Y (for a treatment stage of a sample of an extract) was explained by the fixed effects of treatment stage (TreatmentStage: 1 = pretreatment, 1 + 2 = pretreatment + extraction, 1 + 2 + 3 = pretreatment + extraction + adsorbent, or 1 + 2 + 3 + 4 = pretreatment + extraction + adsorbent + drying), temperature during extraction (Temperature: °C, regression coefficient β), their interaction effect, and the intercept (int). Although temperature was not significant (p > 0.05) in some stilbene models, it was included to show the effect in the prediction plots. The interaction effect was included only if it was significant. Random effects of extract (Extract) and sample (Sample) with autoregressive covariance structure between treatment stages for random errors (e) were added in the model to account for the correlated Y measurements from the same extract and sample. Random terms (Extract, Sample, and e) were assumed to be normally distributed. Winter-collected bark (32 observations) and summer-collected bark (80 observations) were analysed separately because of the different treatment stages. Because an extraction temperature effect was not possible in the first treatment stage (pretreatment), the mean temperature of the other treatment stages was used as the constant value for the first treatment stage (separately defined for the winter and summer bark), which kept the stilbene mean of the first treatment stage unchanged in multiple comparisons.
The Tukey–Kramer method was used in multiple comparisons of class means, which were log-scale predictions by the fixed part of the model. For interpretation, the means with their 95% confidence intervals were backtransformed to the original scale. The GLIMMIX procedure of SAS software (version 9.4) was used in the analyses.
3 Results and discussion
3.1 Content and composition of bark stilbene glucosides in Norway spruce provenance trials
We hypothesized that (1) the origin of provenance does not affect the content and composition of stilbenoids of Norway spruce bark; (2) trees on northern growing sites have a higher total amount of stilbenes; (3) within-stem variation is larger than the between-provenances and between-site variation; and (4) inherent variation in the stilbene content and composition of bark is largely offset by the variation due to material processing steps from harvesting and logistics to debarking and experimental pilot-scale extraction and fractionation. That is, (5) the stilbene content in industrial bark was hypothesized to be lower than that in fresh, intact bark because storage has inevitably occurred before industrial debarking of trees. We also hypothesized that (6) increasing the extraction temperature increases the extraction yield. An increase in extraction temperature favours solubilization and diffusion and thus facilitates extraction [27, 28]. However, the presence of thermolabile compounds set the upper limits for the extraction temperature. Here, we present the results and discuss them.
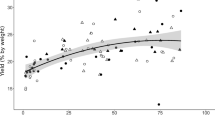
In the provenance trial, the extraction of Norway spruce inner bark by acetone/water (95:5) yielded 1–3 mg/g, 30–50 mg/g, and 20–70 mg/g of stilbene glucosides trans-piceid, trans-astringin, and trans-isorhapontin, respectively (dry inner bark weight, DW) (Fig. 5). The total average stilbene content of the inner bark varied from 70 to 110 mg/g (DW). Our results agree with earlier studies showing that the total stilbene content of Norway spruce inner bark varies between 20 and 120 mg/g (DW) in trees grown under southern and northern Finnish climatic conditions [6, 12,13,14,15].
The average model predictions of stilbene glucoside amount analysed by GC–MS of the hydrophilic acetone/water (95:5) extracts of Norway spruce inner bark obtained from provenance trial experiment in two growing sites: total amount of stilbene glucosides (A), trans-astringin (B), trans-isorhapontin (C), and trans-piceid (D) (mg g−1 DW ± 95% CI); and the ratio of isorhapontin to astringin content (E). For descriptions of the height classes, provenances, and growing sites, see Figs. 2 and 3
On average, the trees growing on the northern site had a 25% higher total content of stilbene glucosides than trees on the southern site, and this difference was statistically significant (Supplementary material, Table S1). Moreover, the northern origin of seeds (provenances Sodankylä and Simo) resulted in ca. 2–32% higher total amount of stilbene glucosides than the southern origin of seeds (provenances Sachsen and Preußen). The effect of growing site was significant for isorhapontin and piceid compounds but was negligible for astringin.
The bark collected from the local provenances in the sites showed the opposite: the southern site had a 26% higher total content of stilbene glucosides in bark than the northern site (Supplementary material, Table S2). The total content was at the same level, varying from 8 to 10% (DW). However, tree-to-tree variation was relatively large. Astringin was the dominant compound in the northern site, whereas isorhapontin was the dominant stilbene glucoside in bark from the southern site (Supplementary Table S2).
The effect of provenance on total stilbene content was significant at the level of α = 0.1. For all separately analysed trans-stilbene glucosides of inner bark (isorhapontin, astringin, and piceid), the effect of provenance was also significant (Figs. 5 and 6; Table S1). Interestingly, the content of astringin in inner bark decreased with increasing latitude of seed origin. In contrast, the content of isorhapontin increased with increasing latitude of seed origin; thus, the isorhapontin:astringin ratio was significantly higher for northern provenances than for southern provenances at both growing sites (Fig. 6). The isorhapontin:astringin ratio seems to be genetically determined rather than an environmentally driven property of the inner bark.
The average model predictions of stilbene glucoside amount analysed by GC–MS of the hydrophilic acetone/water (95:5) extracts of Norway spruce inner bark obtained from provenance trial experiment in two growing sites as a function of height class on the stem: total amount of stilbene glucosides (A), trans-isorhapontin (B), trans-astringin (C), trans-piceid (D) (mg g−1 DW ± 95% CI); and the ratio of isorhapontin to astringin content (E). For descriptions of the height classes, provenances, and growing sites, see Figs. 2 and 3
This phenomenon was also evident in the results of variance component analysis: provenance factor explained the majority of the total variance in isorhapontin and piceid content, as well as in the isorhapontin:astringin ratio (Fig. 7). In contrast, most of the total variation in astringin content was explained by tree-to-tree differences and sampling height on the stem, followed by provenance effects and residual variation (Fig. 7).
Proportion of the total variance in stilbene glucoside content as explained by the effects of the random-components model in the provenance experiment (original fixed effects in blue and random effects in red): total amount: total amount of stilbene glucosides (A), trans-isorhapontin (B), trans-astringin (C), trans-piceid (D) (mg g−1 DW ± 95% CI); and the ratio of isorhapontin to astringin content (E). For descriptions of the height classes, provenances, and growing sites, see Figs. 2 and 3
The total stilbene content in inner bark increased with increasing sampling height on the stem, and the effect of height on stilbene content was significant (Table S1). On the top of the stem (i.e. 75% of stem), the total stilbene amount was on average 56% higher than that on the stem base (Figs. 5 and 6). The sampling height impact was also significant for all individually analysed stilbene compounds except for isorhapontin.
Growth site conditions at different latitudes differ significantly during the growing season in terms of photoperiod, temperature, and light quality, i.e. spectral distribution of light and the level of UV radiation [29]. All these factors have marked effects on both tree growth and the biosynthesis of secondary metabolites. However, long-term studies on the effects of gene–environment interactions on the bark secondary metabolites of Norway spruce trees in the Northern Hemisphere are missing. In general, long-term studies analysing the climate impact and the effect of plant origin on secondary metabolites of mature coniferous tree species are very scarce. To the best of our knowledge, this is the first common garden study that examines the effects and interactions of the growing environment and the origin of seeds on the content and composition of bark secondary metabolites in mature Norway spruce trees.
Similar to our study, Oleszek et al. [30] showed that flavonoid content and composition in the needles of Pinus sylvestris were determined by the genetic origin of the seed of the trees. They found that the 80-year-old trees of northern origin (63°50′N) had the lowest levels of flavonoids, while the trees with southern origin (40°30′N) had higher flavonoid contents in the common garden design, where all the origins were grown at the study sites at latitudes 51°37′N and 52°15′N. In contrast, a positive relationship was detected for phenolic content in Juniperus communis needles with increasing latitude (59 N°–69 N°) [31].
The genetic component in total stilbene content was not clear in the analyses, whereas in isorhapontin, it was substantial, as shown by the small and nonsignificant effect of tree in the analysis of variance (Fig. 7). However, due to limited data and complex analysis with many factors that may partially include genetic components (e.g. provenance), one cannot draw conclusions on the proportion of genetic variation in the traits. Stilbene variation in conifers has been shown to have a strong genetic basis; e.g. in Scots pine, the genetic component of the variance has been over 70% in the heartwood [32], and a strong genetic correlation has been found between stilbene content in different parts of organisms, e.g. heartwood vs. induced stilbenes in needles after wounding [33].
In addition to trans-stilbene glucosides, cis-stilbenes, dimers and larger structures have been detected in, e.g., pathogen-attacked bark [8, 15, 34,35,36,37,38]. Biological factors may thus play a significant role in modifying bark properties during the long lifespan of trees, reaching up to 60- to 90-year rotation lengths in northern commercial forestry. Due to their long life, conifers have evolved a variety of defence mechanisms to shield themselves against pests and pathogens. Norway spruce integrates preformed (constitutive) and inducible defence compounds and mechanical (structural) barriers against biotic invaders and abiotic stressors.
Consequently, UV light appears to affect trans-stilbene glucosides by forming the corresponding cis-transformations and phenanthrenes, cf., [6, 21, 39,40,41] but also smaller oligomers and degrading the stilbene glucosides [21]. In nature, UV-light-driven changes do not presumably take place inside the intact living cells of healthy inner bark because the outer bark (periderm) efficiently protects them, while also shielding vascular cambium and photoassimilating conducting phloem from detrimental light impacts.
3.2 Stilbenoid content and composition in bark extracts from a sawmill
The extracts of summer-collected bark contained 0.3–0.8 mg/g, 1.2–5.9 mg/g, and 0.8–3.3 mg/g of stilbene glucosides trans-piceid, trans-astringin, and trans-isorhapontin, respectively (dry bark weight, DW) (Fig. 8). Extraction of winter-collected bark from a sawmill by acetone/water (70:30) yielded 1–1.5 mg/g, 2.3–6.4 mg/g, and 2.4–4.2 mg/g stilbene glucosides trans-piceid, trans-astringin, and trans-isorhapontin, respectively (dry bark weight, DW) (Fig. 9).
The total stilbene (glucosides and aglycones) content was ca. 22 mg/g DW and 1–3 mg/g DW in winter- and summer-collected industrial bark, respectively. The higher total stilbenoid content in winter-collected bark may partially be related to the seasonal variation in stilbenoid content. Jyske et al. [14] detected slightly higher contents of stilbene glucosides in Norway spruce inner bark samples from midsummer to autumn and winter than in those from spring and early summer. The winter- and summer-collected batches of the current study also differed with respect to their origin. The content of stilbenoids in summer-collected bark from sawmills was notably lower than the contents detected in studies of fresh Norway spruce bark samples that were analysed immediately after tree felling. For example, according to Halmemies et al. [18], the content of stilbenoids in summer-collected bark from sawlogs was approximately 1% immediately after tree felling. However, the timing in their study differed to some extent, as sampling occurred in May. According to their results, the stilbenoid content was 2.4-fold higher in winter- than in summer-collected bark, and it was at the same level as in this study.
Before debarking at a mill, sawlogs are usually stored for 4–5 weeks [42]. Crushing of bark in the sawmill after debarking releases soluble contents as the cells are damaged. This leads to an increase in the surface area of the biomass, which further promotes microbial growth and chemical reactions. In the present study, a delay occurred between debarking and extraction of bark even though the supply chain was kept as fast as possible. Furthermore, summer-collected bark was stored at + 5 °C for the 5–13-day period from industrial debarking to hot water extraction. At the time of pilot-scale extractions, it was evident that microbiological processes leading to the losses of extractives were ongoing, and this was observed by the increased temperature of the bark batches. Winter bark is stored in cold, ambient winter conditions, which slows down, or even inhibits, microbial degradation [43]. The results of Jyske et al. [15] demonstrated that the contents of stilbene glucosides and aglycones in Norway spruce bark rapidly declined during the storage of timber logs. The rate of change in stilbene content decrease was higher in summer than in winter.
After extraction, the total stilbene (glucoside and aglycones) content in the solid extraction residue of winter- and summer-collected bark was 8.7–30 mg/g DW and 1.1–6.2 mg/g DW, respectively. In extractions, 7–11% of bark solid content was dissolved, although extraction at 120 °C was an exception, as the amount of total dissolved solids was 17%. The high stilbenoid content in extracted bark may partially be explained by the findings of Rencoret et al. [44]. They proved that the hydroxystilbene glucosides isorhapontin and, at lower levels, astringin and piceid are incorporated into the lignin polymer in Norway spruce (Picea abies) bark through β-ether bonds. According to those investigators, the corresponding aglycones isorhapontigenin, piceatannol, and resveratrol were released from the MWL (milled wood lignin) sample of bark by reductive cleavage that cleaves β-ether bonds in lignin. It could be speculated that further laboratory-scale extraction after pilot processing could have released stilbenoids bound to lignin.
Pilot-scale hot water extractions of winter bark yielded between 6 and 13 mg/g DW stilbenes, whereas yield was between 3 and 12 mg/g DW for bark collected in summer. Extraction temperature had a significant effect on the yield of stilbenoids other than trans-rhapontigenin and trans-piceatannol in the case of bark collected in winter (Figs. 8 and 9; Supplementary material, Table S3a). A higher extraction temperature was favourable for the extraction of stilbenoids. The only exception was piceid, as temperatures above 90 °C seemed to have a negative effect on its extraction yield (Fig. 8). More experiments to find the optimal extraction conditions on a pilot scale are required in future research. Additionally, the liquid–solid ratio should be considered in further studies.
Treatment stages had a significant effect on the stilbenoid yield (Figs. 8 and 9; Supplementary material, Tables S3a, S3b). Extract treatment with adsorbent caused significant losses of stilbenoids. Thus, this extraction treatment method is not optimal/suitable for the enrichment of stilbenoids and is probably more suited for the enrichment and purification of condensed tannins in extracts [45]. In particular, the addition of the water-rinsing phase to resin treatment (Fig. 8) had a negative effect on stilbenoid yield, while it enhanced the removal of carbohydrates from the extract (results not shown).
Based on our study, the obtained yield of stilbenoids from industrial Norway spruce bark from sawmills was limited to approximately 1% (DW), which was only one tenth of the typical stilbenoid content of fresh bark. Stilbenoids are more sensitive to degradation during storage than condensed tannins of Norway spruce bark [15]. From the utilization point of view, stilbenoids and tannins would be best used as extracts containing both types of phenolic compounds, as the economic feasibility of extraction and purification at the industrial scale requires sufficient yields of biologically active components. Our recent research proposes that the phenol-rich extracts of Norway spruce bark are active in several ways, showing, e.g., antioxidant activity and capacity to prevent lipid oxidation in the liposome model [46], and antidiabetic, antiviral, and anti-inflammatory potential while being nontoxic in the in vitro profile [47, 48], thus allowing further use in food or cosmetics models, among others.
4 Conclusions
Based on this study, the natural variation in stilbene content was substantial: the total average stilbene content of the inner bark varied from 70 to 110 mg/g of dry weight, with significant effects of geographic origin, growing site, and sampling location along the stem (from stem base to upper part of the crown). Sampling location and growing site explained over 50% of the total variance in total stilbene content. Trees with a northern origin of seeds had a higher isorhapontin/astringin ratio than the trees with a southern origin of seeds, regardless of their growing site. Industrial bark from sawmills contained substantial amounts of stilbenes with large variation due to storage: the total stilbene (glucosides and aglycones) content was 22 mg/g DW and 1–3 mg/g DW stilbenoids in industrial bark obtained in winter and summer, respectively. Thus, biological variation in the stilbene content was offset by the variation caused by the debarking process and pilot-scale extraction and fractionation. Northern forests and short handling times are recommended to optimize stilbene yields. However, extraction and fractionation processes for stilbenoids require further research.
References
Statistics Finland (OSF) (2021) Natural Resources Institute Finland, Forest industries’ wood consumption. Helsinki: Natural Resources Institute Finland. https://stat.luke.fi/en/wood-consumption (accessed 16 March 2022)
Shibutani S, Samejima M, Doi S (2004) Effects of stilbenes from bark of Picea glehnii (Sieb. et Zucc) and their related compounds against feeding behaviour of Reticulitermes speratus (Kolbe). J Wood Sci 50:439–544. https://doi.org/10.1007/s10086-003-0583-1
Metsämuuronen S, Siren H (2014) Antibacterial compounds in predominant trees in Finland: review. J Bioprocess Biotech 4:167. https://doi.org/10.4172/2155-9821.1000167
Reinisalo M, Kårlund A, Koskela A, Kaarniranta K, Karjalainen RO (2015) Polyphenol stilbenes: molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxid Med Cell Longev 2015:340520. https://doi.org/10.1155/2015/340520
Jansone Z, Muizniece I, Blumberga D (2017) Analysis of wood bark use opportunities. Energy Procedia 128:268–274. https://doi.org/10.1016/j.egypro.2017.09.070
Latva-Mäenpää H (2017) Bioactive and protective polyphenolics from roots and stumps of conifer trees (Norway spruce and Scots pine). https://helda.helsinki.fi/handle/10138/186254
Mulat DG, Latva-Mäenpää H, Koskela H, Saranpää P, Wähälä K (2014) Rapid chemical characterisation of stilbenes in the root bark of Norway spruce by off-line HPLC/DAD-NMR. Phytochem Anal 25:529–536. https://doi.org/10.1002/pca.2523
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–375. https://doi.org/10.1111/j.1469-8137.2005.01436.x
Krokene P (2015) Conifer defense and resistance to bark beetles. In: Vega FE, Hofstetter RW (eds) Biology and ecology of native and invasive species. Elsevier Academic Press, San Diego, pp 177–207
Gabaston J, Richard T, Biais B, Waffo-Teguo P, Pedrot E, Jourdes M, Corio-Costet M-F, Mérillon J-M (2017) Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind Crops Prod 103:267–273. https://doi.org/10.1016/j.indcrop.2017.04.009
Krogell J, Holmbom B, Pranovich A, Hemming J, Willför S (2012) Extraction and chemical characterization of Norway spruce inner and outer bark. Nord Pulp Pap Res J 27:6–17. https://doi.org/10.3183/npprj-2012-27-01-p006-017
Latva-Mäenpää H, Laakso T, Sarjala T, Wähälä K, Saranpää P (2013) Variation of stilbene glucosides in bark extracts obtained from roots and stumps of Norway spruce (Picea abies [L.] Karst.). Trees 27:131–139. https://doi.org/10.1007/s00468-012-0780-x
Jyske T, Laakso T, Latva-Mäenpää H, Tapanila T, Saranpää P (2014) Yield of stilbene glucosides from the bark of young and old Norway spruce stems. Biomass Bioenergy 71:216–227. https://doi.org/10.1016/j.biombioe.2014.10.005
Jyske TM, Suuronen JP, Pranovich AV et al (2015) Seasonal variation in formation, structure, and chemical properties of phloem in Picea abies as studied by novel microtechniques. Planta 242:613–629. https://doi.org/10.1007/s00425-015-2347-8
Jyske T, Brännström H, Sarjala T, Hellström J, Halmemies E, Raitanen J-E, Kaseva J, Lagerquist L, Eklund P, Nurmi J (2020) Fate of antioxidative compounds within bark during storage: a case of Norway spruce logs. Molecules 25:4228. https://doi.org/10.3390/molecules25184228
Piispanen R, Willför S, Saranpää P, Holmbom B (2008) Variation of lignans in Norway spruce (Picea abies [L.] Karst.) knotwood: within-stem variation and the effect of fertilisation at two experimental sites in Finland. Trees 22:317–328. https://doi.org/10.1007/s00468-007-0186-3
Tamminen T, Ruuskanen M, Grönqvist S (2017) The influence of softwood bark origin on tannin recovery by hot‐water extraction. In: Proceedings of the 19th International Symposium on Wood, Fibre and Puling Chemistry, Porto Seguro, Brazil, 28 August–1 September 2017
Halmemies ES, Brännström HE, Nurmi J, Läspä O, Alén R (2021) Effect of seasonal storage on single-stem bark extractives of Norway spruce (Picea abies). Forests 12(6):736
Routa J, Brännström H, Laitila J (2020) Effects of storage on dry matter, energy content and amount of extractives in Norway spruce bark. Biomass and bioenergy 143.
Jylhä P, Halmemies E, Hellström J, Hujala M, Kilpeläinen P, Brännström H (2021) The effect of thermal drying on the contents of condensed tannins and stilbenes in Norway spruce (Picea abies [L.] Karst.) sawmill bark. Industrial crops and products 173
Välimaa A-L, Honkalampi-Hämäläinen U, Pietarinen S, Willför S, Holmbom B, von Wright A (2007) Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int J Food Microbiol 115:235–243. https://doi.org/10.1016/j.ijfoodmicro.2006.10.031
Ding T, Bianchi S, Ganne-Chédeville C, Kilpeläinen P, Haapala A, Räty T (2017) Lifecycle assessment of tannin extraction from spruce bark. IForest 10:807–814. https://doi.org/10.3832/ifor2342-010
Rasi S, Kilpeläinen P, Rasa K, Korpinen R, Raitanen J-E, Vainio M, Kitunen V, Pulkkinen H, Jyske T (2019) Cascade processing of softwood bark with hot water extraction, pyrolysis and anaerobic digestion. Bioresour Technol 292:7. https://doi.org/10.1016/j.biortech.2019.121893
Kilpeläinen P, Hautala S, Byman O, Tanner J, Korpinen R, Lillandt M, Pranovich A, Kitunen V, Willför S, Ilvesniemi H (2014) Pressurized hot water flow-through extraction system scale up from the laboratory to the pilot scale. Green Chem 12(6):3186–3194
Varila T, Brännström H, Kilpeläinen P, Hellström J, Romar H, Nurmi J, Lassi U (2020) From Norway spruce bark to carbon foams: characterization, and applications. BioResources 15(2):3651–3666
Stroup WW, Milliken GA, Claassen EA, Wolfinger RD (2018) SAS for mixed models: introduction and basic applications. SAS Institute Inc., Cary, NC, USA
Seidel V (2012) Initial and bulk extraction of natural products isolation. Methods Mole Biol 864:27–41. https://doi.org/10.1007/978-1-61779-624-1_2
Zhang Q-W, Li L-G, Ye W-C (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med 13:20. https://doi.org/10.1186/s13020-018-0177-x
Jaakola L, Hohtola A (2010) Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ 33:1239–1247. https://doi.org/10.1111/j.1365-3040.2010.02154.x
Oleszek W, Stochmal A, Karolewski P, Simonet AM, Macias FA, Tava A (2002) Flavonoids from Pinus sylvestris needles and their variation in trees of different origin grown for nearly a century at the same area. Biochem Syst Ecol 30:1011–1022
Martz F, Peltola R, Fontanay S, Duval RE, Julkunen-Tiitto R, Stark S (2009) Effect of latitude and altitude on the terpenoid and soluble phenolic composition of juniper (Juniperus communis) needles and evaluation of their antibacterial activity in the boreal zone. J Agric Food Chem 57(20):9575–9584
Partanen J, Harju AM, Venäläinen M, Kärkkäinen K (2011) Highly heritable heartwood properties of Scots pine: possibilities for selective seed harvest in seed orchards. Can J For Res 41(10):1993–2000
Harju AM, Venäläinen M, Laakso T, Saranpää P (2009) Wounding response in xylem of Scots pine seedlings shows wide genetic variation and connection with the constitutive defence of heartwood. Tree Physiol 29(1):19–25. https://doi.org/10.1093/treephys/tpn006
Viiri H, Annila A, Kitunen V (2001) Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus Ceratocystis polonica. Trees 15(2):112–122
Lieutier F, Brignolas F, Sauvard D, Yart A, Galet C, Brunet M et al (2003) Intra- and inter-provenance variability in phloem phenols of Picea abies and relationship to a bark beetle associated fungus. Tree Physiol 23(4):247–256
Li SH, Nagy NE, Hammerbacher A, Krokene P, Niu XM, Gershenzon J, Schneider B (2011) Localization of phenolics in phloem parenchyma cells of Norway spruce (Picea abies). ChemBioChem 13:2707–2713
Hammerbacher A, Ralph SG, Bohlmann J, Fenning TM, Gershenzon J, Schmidt A (2011) Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol 157(2):876–890
Hammerbacher A, Schmidt A, Wadke N, Wright LP, Schneider B et al (2013) A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol 162(3):1324–1336
Buckles RE (1955) Illumination of cis- and trans-stilbenes in dilute solutions. J Am Chem Soc 77:1040–1041. https://doi.org/10.1021/ja01609a073
Mallory FB, Wood CS, Gordon JT (1964) Photochemistry of stilbenes. III. Some aspects of the mechanism of photocyclization to phenanthrenes. J Am Chem Soc 86:3094–3102. https://doi.org/10.1021/ja01069a025
Latva-Mäenpää H, Wufu R, Mulat D, Sarjala T, Saranpää P, Wähälä K (2021) Stability and photoisomerization of stilbenes isolated from the bark of Norway spruce roots. Molecules 26(4):15
Lukkari J, Hyppölä A, Kärkkäinen M, Lipponen P, Mäkelä M, Paananen S, et al (2004) Puun laadun säilyttäminen. [Storing the quality of the wood]. Metsäteho Oy [In Finnish]
Krigstin S, Wetzel S (2016) A review of mechanisms responsible for changes to stored woody biomass fuels. Fuel 175:75–86
Rencoret J, Duarte N, Marques G, Gutiérrez A, Kim H, Gominho J, Pereira H, Ralph J, Del Río JC (2019) Hydroxystilbene glucosides are incorporated into Norway spruce bark lignin. Plant Phys 180:1310–1321
Zeller WE (2019) Activity, purification, and analysis of condensed tannins: current state of affairs and future endeavors. Crop Sci 59(3):886–904
Raitanen J-E, Järvenpää E, Korpinen R, Mäkinen S, Hellström J, Kilpeläinen P, Liimatainen J, Ora A, Tupasela T, Jyske T (2020) Tannins of conifer bark as Nordic piquancy—sustainable preservative and aroma? Molecules 25:567. https://doi.org/10.3390/molecules25030567
Pap N, Reshamwala D, Korpinen R, Kilpeläinen P, Fidelis M, Furtado MM, Granato D et al (2021) Toxicological and bioactivity evaluation of blackcurrant press cake, sea buckthorn leaves and bark from Scots pine and Norway spruce extracts under a green integrated approach. Food Chem Toxicol 153:112284. https://doi.org/10.1016/j.fct.2021.112284
Granato D, Reshamwala D, Korpinen R, Azevedo L, do Araújo Vieira Carmo M, Mendanha Cruz T, Boscacci Marques M, Wen M, Zhang L, Marjomäki V, Kilpeläinen P (2022) From the forest to the plate – hemicelluloses, galactoglucomannan, glucuronoxylan, and phenolic-rich extracts from unconventional sources as functional food ingredients. Food Chemistry 81:132284. https://doi.org/10.1016/j.foodchem.2022.132284
Acknowledgements
Ms. Kaija Puputti, Ms. Irmeli Luovula, Mr. Kalle Kaipanen, Mr. Tapio Nevalainen, Mr. Tapio Järvinen, and MSc. Fernando Urbano Tenorio are thanked for their skilful technical assistance. Dr. Sonja Kujala is thanked for the comments and suggestions that substantially enhanced the quality of the work.
Funding
Open access funding provided by Natural Resources Institute Finland (LUKE). This work is a part of the Luke strategic research project Green Chemistry. Financial support was also received from the EU/Interreg/Botnia-Atlantica, the Regional Council of Ostrobothnia, and Region Västerbotten (n:o 20201484), and the Academy of Finland (n:o 305763, n:o 342250).
Author information
Authors and Affiliations
Contributions
Conceptualization: TJ, PS, HB; funding acquisition and resources: TJ, HB, PS; investigation: TJ, PS, TL, EH, HB, PK, JH; project administration and supervision: TJ, HB, PS; writing—original draft: TJ, HB, PK, JH; writing—review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• In common garden experiments, stilbenoid content was higher at the northern site than at the southern site, suggesting a strong influence of the environment on stilbenoid production.
• Genetic differences between provenances were found: northern origins had a higher isorhapontin/astringin ratio in bark than southern origins.
• Industrial bark contained markedly fewer stilbenoids than bark from fresh cut trees.
• To obtain a high yield of stilbenoids, the supply chain needs to be optimized.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jyske, T., Brännström, H., Halmemies, E. et al. Stilbenoids of Norway spruce bark: does the variability caused by raw-material processing offset the biological variability?. Biomass Conv. Bioref. 14, 5085–5099 (2024). https://doi.org/10.1007/s13399-022-02624-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02624-9