Abstract
Current research indicates that n-3 polyunsaturated fatty acids (PUFAs) bind to polar lipids (phospholipids and glycolipids) seem to exert a greater bioavailability compared to their neutral forms. The aim of this work was to obtain eicosapentaenoic acid (EPA) rich polar lipids from the saponifiable lipids (SLs) extracted from the microalga Nannochloropsis sp. (33.4 ± 0.1% of EPA; 60 ± 0.6% polar lipids) by fractionation using silica-gel columns and importantly, non-polar and polar (ethanol) non-toxic solvents. Nowadays, few studies have been conducted towards the extraction and purification of polar lipids. Firstly, the solvent type for obtaining the neutral saponifiable lipid (NSL) fraction (ethyl acetate, EA, butyl acetate, BA) and the SL/silica-gel, SL/BA, and SL/ethanol ratios were optimized in a small silica-gel cartridge (0.69 g silica gel). The optimized conditions were an SL/silica-gel ratio of 22.6 mg/g, an SL/BA ratio of 1.56 mg/mL and an SL/ethanol ratio of 0.312 mg/mL. Next, the fractionation scale was increased to a column containing 10 g of silica-gel. At this scale, a BA SL fraction was obtained with 96.2 ± 0.5% of NSLs, and an ethanol SL fraction containing 97.7 ± 0.3% of polar lipids and 44.9 ± 0.2% of EPA. In the ethanol fraction, 86.6 ± 0.2% of the polar lipids and 71.5 ± 0.4% of the EPA from the SL microalgal extract were recovered. Consequently, EPA-rich polar lipids were obtained at high yields and purities, which could be used as a source of n-3 PUFAs with greater bioavailability than those based on neutral lipids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
There is ample scientific evidence on the health benefits of omega-3 polyunsaturated fatty acids (n-3 PUFAs), especially docosahexaenoic acid (DHA, 22:6n3) and eicosapentaenoic acid (EPA, 20:5n3) [1]. These benefits include their ability to reduce cardiovascular problems [2], decrease triglyceride levels, and increase HDL (high-density lipoprotein, “good cholesterol”) levels and anti-inflammatory agents [3]. All these benefits, together with the lack of n-3 PUFAs in the diets of Western countries [4], explain why the market for products rich in these acids is expanding [5]. Whether the n-3 PUFAs are used as an ingredient in food, drugs, or cosmetics, or are a product in themselves (a nutritional supplement), it is convenient to start from raw materials containing a high concentration of n-3 PUFAs such as fish, krill, and microalgal oils.
It is also important to take into account the chemical form in which these PUFAs are presented since this influences their bioavailability; their technological properties as components in food, drugs, and cosmetics; and their oxidative stability. Current research indicates that n-3 PUFAs that bind to polar lipids, such as phospholipids (PLs) and glycolipids (GLs), seem to exert differential bioavailability and biological effects when consumed compared to neutral forms of n-3 PUFAs [6,7,8]. Polar lipids are excellent emulsifying agents due to their amphiphilic nature, which makes them useful both in food products and as excipients for drugs and cosmetics [9]. In addition, several studies suggest that n-3 PUFAs show greater resistance to oxidation when they are part of polar lipids [10]. Polar lipids have been described as potent bioactive compounds, and therefore, the interest in understanding their bioactive mechanisms and the extent of their bioactive potential have increased. Several studies have pointed to PLs and GLs, especially those esterified with n-3 PUFAs, as possessing antioxidant, anti-inflammatory, anti-obesity, anti-tumor, anti-viral and anti-bacterial activity [11].
Microalgae are the unicellular microorganisms ultimately responsible for the n-3 PUFA content in krill and fish. In microalgal biomasses, PUFAs are frequently found in a higher proportion in polar lipids, which are those that mainly form cell membranes (structural lipids) [12]. Multiple bioactivities have been reported for microalgal polar lipids. Thus, for example, the anti-inflammatory properties of bioactive lipids were evaluated using crude extracts from microalgae such as Chlorella vulgaris, Ch. ovalis, Nannochloropsis oculata, N. granulata, N. oceanica, Phaedactylum tricornutum and Amphidinium carterae [11]. Nannochloropsis sp. has also received a great attention from researchers in recent years due to the complexity and abundance of its lipid structures. Among other aspects, this species is characterized by high EPA contents. Recently, the company Qualitas Health (USA) has put Almega®PL on the market, a plant-based product derived from Nannochloropsis. It is the first photosynthetic source of n-3 PUFAs available for human consumption in the USA. Besides offering a natural source of EPA alone (25%), it contains polar lipids (15%) rich in GLs and PLs, thus providing nutritional characteristics that are different from other forms of n-3 PUFAs [13].
According to Conde et al. [11], further investigations are needed to obtain complete knowledge of the mechanism of action of microalgal polar lipids as bioactive compounds and these investigations need isolated compounds. This requires specific food grade and environmentally friendly methods for extracting and separating microalgal polar lipids need to be investigated and employed according to guidelines for oral consumption in order to further support such health-related nutraceutical applications of microalgae-derived lipid bioactives. At this respect, several studies obtained EPA-rich polar lipids from microalgae by simultaneously extracting and fractionating the saponifiable lipids (SLs), and by extraction of the SLs followed by a subsequent fractionation. Jiménez Callejón et al. [14] applied a simultaneous extraction and fractionation of SLs from the microalga Nannochloropsis sp. using low-toxicity solvents. Firstly, hexane was used to obtain an NSL lipid-rich fraction (87% of the total SLs in this fraction). Secondly, an EPA and polar-lipid-enriched fraction was obtained using ethanol (of the total fatty acids, 88% of SLs were polar lipids and up to 35% EPA). Other authors separated the lipids using silica-gel cartridges at large scale after an extraction step. Devos et al. [15] separated SLs from the microalga Isochrysis galbana using chloroform to elute the NSLs, chloroform/methanol (5:1 v/v) for the GLs and methanol for the PLs. Antonopoulos et al. [16] obtained purified polar lipids (mainly PLs) from the archaeon Thermoplasma acidophilum DMS 1728/10217 using diethylaminoethyl (DEAE) cellulose column chromatography with chloroform/methanol 7:3 (v/v) as the eluent. However, low and high-toxicity solvents were used in these works; such solvents would not be suitable for use in the food industry, where processes using non-toxic or GRAS (Generally Recognized as Safe) solvents need to be developed. Jiménez Callejón et al. [17] used these types of solvents along with newer techniques such as supercritical fluid extraction (SFE) and pressurized liquid extraction (PLE) to obtain EPA and polar-lipid-enriched fractions from Nannochloropsis sp. biomass. The best results were obtained carrying out an initial NSL extraction by SFE using CO2 with 10 wt% ethanol as the co-solvent, at 35 MPa, 50 °C, a flow of 8 g CO2/min, and an extraction time of 8 h; this procedure obtained a lipid fraction containing 70% of NSLs. Subsequently, in the second extraction step with pressurized ethanol, at 10 MPa, 125 °C, and three extraction cycles of 5 min, an SL extract containing 85% of polar lipids and up to 39% of EPA was obtained. This SL extract contained 77% of the GLs, 71% of the PLs, and 62% of the EPA from the microalgal biomass. By a similar procedure, Monte et al. [18] recovered the 94% of polar lipids from the microalga Dunaliella salina using heptane, an ethanol–water mixture and acetone.
A further aim of this work was to obtain EPA-rich polar lipids from the microalga Nannochloropsis sp. by fractionating the SLs previously extracted by PLE using silica-gel column chromatography and non-toxic solvents. Firstly, the fractionation was developed in small silica-gel cartridges; once the optimal conditions were determined, the fractionation scale was increased 14.5-fold. This method was compared with the results obtained in the previous works that also used low-toxicity or non-toxic solvents. To the best of our knowledge, few studies have analyzed lipid fractionation by column chromatography using non-toxic solvents.
2 Materials and methods
2.1 Microalgae and chemicals
Lyophilized biomass from the microalga Nannochloropsis sp. was purchased from Monzón Biotech S.L. (Huesca, Spain). This microalga was chosen for its EPA and polar-lipid-rich lipid fraction. The complete lipid composition is described in Section 3.1. “Extraction of SLs from the microalgal biomass: composition of the extracted SLs.”
The solvents used were ethanol (96% v/v), hexane (95%), ethyl acetate (99.8%), n-butyl acetate (99.5%), acetone, methanol, and chloroform, all of analytical grade, from Panreac AppliChem S.A. (Barcelona, Spain). Nonadecanoic acid (19: 0) was used as the internal standard in the fatty acids analysis by gas chromatography (GC) (Fluka Analytical, Sigma-Aldrich, St. Louis, MO, USA) and acetyl chloride as the catalyst in the prior methylation (Fluka Analytical). Hydrochloric acid (37%, analytical grade) and magnesium chloride 6-hydrate (both from Panreac AppliChem S.A) were used for the determination of the total lipids. Aluminum oxide (Alumina A-50, Sigma, St. Louis, MO, USA) was used for the cell disruption of the microalgal biomass. Finally, silica-gel cartridges were used for the SL fractionation. The Sep-pack Silica Plus Long cartridge (690 mg sorbent, 55–105 µm, WAT020520, Waters Corporation, Milford, MA) was used for the SL fractionations at small scale and for the analytical determinations (Section 2.4.2.) while the Sep-pack Silica 35 cc Vac cartridge (10 g sorbent, 55–105 µm, WAT043355 Waters Corporation, Milford, MA) was used for the large-scale SL fractionations.
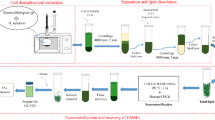
2.2 Biomass pretreatment and lipid extraction from the microalgal biomass
Prior to extraction, 300 g of dry Nannochloropsis sp. biomass was mixed with aluminum oxide (1:1 w/w) and pretreated in a bead mill for 4 h (from now on, this mixture will be referred to as the sample). The lipids were extracted from the pretreated biomass with ethanol using pressurized liquid extraction (PLE) in an accelerated solvent extractor (ASE 100, Thermo-Fisher Scientific, USA). The extraction cells used have a volume of 10 mL. Cellulose filters were placed in the cells to prevent any clogging of the outlets. In each extraction, 5 g of sample (2.5 g of lyophilized microalgal biomass along with 2.5 g of aluminum oxide) were used. The extractions were conducted at 10.3 MPa and 125 °C. The static extraction time (the period in which static extraction occurs) was set to 5 min and the number of extraction cycles to 3. The flush volume was set to 100%, expressed as a percentage of the cell volume, divided among the number of extraction cycles. The nitrogen purge time was 60 s. Under these conditions, the ethanol consumption was approximately 9.6 mL/g of dry biomass [17].
2.3 Fractionation of extracted SLs by silica-gel chromatography
The lipids previously extracted from the microalgal biomass were separated into NSLs and polar lipids (GLs and PLs), first at the small scale by eluting the microalgal lipids in cartridges containing 0.69 g of silica gel. In a typical experiment, ethanol from a sample containing 15.6 mg of SLs (amount determined by gas chromatography; GC, 22.6 mg SL/g of silica-gel) was evaporated under a nitrogen stream. Then, SLs were suspended in 0.5 mL of ethyl acetate or butyl acetate. SL/silica-gel ratios between 11.3 mg/g (7.8 mg/0.69 g) and 33.9 mg/g (23.4 mg/0.69 g) were tested. The samples were loaded into a silica-gel cartridge and eluted with 10 mL of ethyl or butyl acetate (1.56 mg SL/mL) to try to elute the NSLs. SL/ethyl or butyl acetate ratios between 0.26 mg/mL (7.8 mg/30 mL) and 2.23 mg/mL (15.6 mg/7 mL) were tested. Then, 50 mL of ethanol were used to try to collect the polar lipids (GLs and PLs) (0.312 mg SLs/mL). SL/ethanol ratios between 0.312 mg/mL (15.6 mg/50 mL) and 0.52 mg/mL (15.6 mg/30 mL) were tested. The GC analysis of each fraction gave the SLs recovered and the fatty acid profile of each fraction. The analytical fractionation into NSLs, GLs, and PLs, following the method described in Section 2.4.2. “Determination of neutral saponifiable lipids (NSLs), glycolipids (GLs) and phospholipids (PLs),” gave the percentage of each lipidic class with respect to the total SLs and the fatty acid profile of that fraction (NSLs, GLs, and PLs).
Once the optimal conditions were determined at the small scale, the fractionation scale was increased approximately 14.5-fold, carrying out the fractionation in a 10 g silica-gel column. Prior to each fractionation, the column was conditioned using 30 mL of butyl acetate. In a typical experiment, ethanol from a sample containing 225.4 mg of SLs (determined by GC; 22.5 mg SL/g of silica-gel) was evaporated under a nitrogen stream. Next, SLs were suspended in 15 mL of butyl acetate. The samples were loaded into the silica-gel column and eluted with 145 mL of butyl acetate (1.55 mg SL/mL) to try to elute the NSLs, and next with 725 mL of ethanol to try to collect the polar lipids (GLs and PLs) (0.311 mg SL/mL).
2.4 Analytical methods
2.4.1 Determination of the total lipid (TL), saponifiable lipid (SL), and fatty acid composition
TLs comprise both SLs (lipids containing fatty acids) and non-saponifiable lipids. SLs comprise neutral saponifiable lipids (NSLs, such as acylglycerols) and polar lipids (such as glycolipids, GLs, and phospholipids, PLs). The TL content of the microalgal biomass was determined using the Kochert method [19], which is based on the extraction of lipids from lyophilized biomass with chloroform/methanol (2:1 v/v), the final step of which involves weighing.
The SLs were determined by direct transesterification of the lipids in the microalgal biomass to transform all the SLs into fatty acid methyl esters (FAMEs), which were then analyzed by gas chromatography (GC). To determine the SL content of the biomass samples, lyophilized algal biomass (three 10 mg samples) was directly transesterified in the presence of 1 mL of hexane and 0.125 mg of internal standard (nonadecanoic acid, 19:0) using 1 mL of a 1:20 v/v solution of acetyl chloride in methanol. The reactions were conducted in tubes heated at 105 °C for 20 min for trans methylation. Next, the mixture was cooled to room temperature and 1 mL of water was added. The tubes were then agitated and centrifuged. Two phases were formed, with the upper one (hexane) containing the FAMEs obtained from the SLs present in the microalgal biomass. These FAMEs were analyzed by GC following the method described by Rodríguez et al. [18]. This analysis was carried out in an Agilent Technologies 6890 N chromatograph (Santa Clara, USA) equipped with a capillary column of fused silica OmegaWax™ (0.25 mm × 30 m, 0.25 μm standard film, Supelco, Bellefonte, PA) and a flame ionization detector (FID). Nitrogen was the carrier gas at a flow rate of 58.1 mL/min and a split ratio of 1:40. The injector and detector temperatures were set at 250 and 260 °C, respectively. The oven temperature was initially set at 150 °C for 3 min, then programmed to increase to 240 °C at a rate of 7.5 °C/min and then set at 240 °C for 12 min. This analysis gave the SL content per unit mass of dry biomass and the fatty acid composition of microalgal SLs. The fatty acid composition of the SLs, including the EPA content, was determined by comparing the retention times to those of the PUFA-3 Menhaden oil standard (Supelco, Bellefonte, PA, USA), analyzed under the same conditions [20].
The amounts of SLs obtained by pressurized ethanol extraction and their fatty acid profile (the weight percentage of each fatty acid with respect to the total fatty acids in the SL fraction) were determined by methylation and GC analysis of the samples. In this case, 0.5 mL of ethanolic extract, previously set to a known volume, was firstly dried under a nitrogen stream and then mixed with 1 mL of hexane before sample methylation (following the procedure previously described).
The amounts of SLs in each fraction eluted from the silica-gel columns and their fatty acid profiles were also determined by methylation and GC analysis of the samples. Similarly, 0.5 mL of ethyl acetate, butyl acetate, or ethanolic fractions were firstly dried under a nitrogen stream and then mixed with 1 mL of hexane before sample methylation. The SL yield (wt%) in each fraction is defined as the percentage of SLs in the fraction with respect to the total SLs contained in the original lipidic extract (obtained by PLE). In the same way, the EPA yield (wt%) in the fractions is defined as the percentage of EPA in the fraction with respect to the total EPA amount contained in the original lipidic extract.
2.4.2 Determination of neutral saponifiable lipids (NSLs), glycolipids (GLs), and phospholipids (PLs)
The TLs extracted from the microalgal biomass and some of the lipidic fractions eluted from the silica-gel columns were separated into NSLs, GLs, and PLs following the Kates’ procedure [21]. This fractionation was carried out by eluting the microalgal lipids in silica-gel cartridges (Sep-pack silica plus long cartridges, 690 mg sorbent, 55–105 μm, WAT020520). Samples containing around 10 mg of SLs were evaporated under a nitrogen stream and suspended in 0.5 mL of chloroform. The samples were loaded into the silica-gel cartridge and eluted with 30 mL of chloroform to collect the NSL fraction. Then, 30 mL of acetone was used along with 20 mL of chloroform:methanol (85:15 v/v) to collect the GLs; finally, 30 mL of methanol was used to elute the PL fraction. GC analysis of all the fractions gave the percentage of each lipidic class with respect to the total SLs and their fatty acid profile [20].
2.5 Statistical analysis
All the experiments were carried out in triplicate and the analyses were carried out in duplicate, so the results were expressed as the arithmetic mean ± the standard deviation of six analyses. The results were evaluated in terms of ANOVA using the Statgraphics 18 software. P-values below 0.05 were considered statistically significant.
3 Results and discussion
3.1 Extraction of SLs from the microalgal biomass: composition of the extracted SLs
The Nannochloropsis sp. microalgal biomass used contained 12.8 ± 0.9 wt% of SLs and 24.1 ± 0.1 wt% of TLs (both with respect to the biomass dry weight). The SLs were extracted by pressurized ethanol following the extraction method detailed in Section 2.2. (Biomass pretreatment and lipid extraction from the microalgal biomass). Using this procedure, the SLs were extracted with an SL yield of 100 wt% with respect to the SL contained in the Nannochloropsis sp. biomass. The SL purity of this extract was 30 wt%, which indicate that, along with the SLs, pressurized ethanol extracts important amounts of other lipidic and non-lipidic compounds. This high SL yield and low SL purity are due to the high solvent power and low selectivity of ethanol as an extraction solvent [22], characteristics that can increase when the extraction is carried out at high temperature and pressure.
Table 1 shows the fatty acid composition of the SLs extracted using PLE. There were no significant differences between the fatty acid profile of the extracted SLs and those of the biomass (data not shown), which indicates that the extraction method did not alter the fatty acid profile. For this extraction, ethanol was chosen because previous studies have demonstrated that the solvent extracts both neutral and polar lipids [23]. EPA (20:5n3) accounted for 33.4 wt% of the total fatty acids in the extract.
Table 1 shows that the fractionation of SLs extracted from the biomass resulted in 40 wt% of the SLs being NSLs, 39.2 wt% being GLs, and 20.8 wt% being PLs. This table also shows that polar lipids were much richer in EPA (54.9 wt% GLs and 24.2% wt% PLs of the total fatty acids in each lipidic class; the average EPA content in the polar lipids being 44.3%) than the NSLs (9.8 wt%). This means that 87.1% of the total EPA is contained in the polar lipids (70.6% in the GLs and 16.5% in the PLs). Polar lipids are richer in PUFAs (18:2n6, 20:4n6, and EPA) than NSLs, and the latter are richer in saturated and monounsaturated fatty acids (14:0, 16:0, 16:1n7, and 18:1n9) than polar lipids. This is common in photoautotrophic algae because the PUFAs contained within them are mainly accumulated in complex polar lipids constituting the cell membranes [24, 25]. The EPA distribution between the polar lipids and the NSLs found in this biomass means that the polar-lipid concentration, or the fractionation of SLs into NSLs and polar lipids, occurs alongside a certain EPA concentration in the polar-lipid fraction.
3.2 Fractionation of the microalgal SL extract in a small silica-gel cartridge
The aim of this study was to choose a non-toxic solvent for the first fractionation step and to maximize the SL/silica-gel and SL/solvent ratios for extracting the NSLs (in the first fractionation step) and the polar lipids (in the second fractionation step), maintaining NSL and polar-lipid recoveries around 100%. This study was carried out at the small scale using a cartridge containing 0.69 g of silica gel.
3.2.1 Influence of the solvent type and SL/solvent ratio on the first fractionation step
For analytical purposes, chloroform is the solvent used to elute NSLs in the silica-gel cartridge (dielectric constant at 25 °C, ε = 4.8). Likewise, hexane (ε = 2.0) is a non-polar solvent that is commonly used to extract neutral lipids. However, these solvents are classified as “highly hazardous” and “hazardous” in the CHEM21 solvent guide of classical solvents [26]. For this reason, ethyl acetate and butyl acetate were chosen for testing in this fractionation step. These solvents are non-polar (ε = 6.0 and 5.1, respectively) and are classified as “recommended” in the CHEM21 solvent guide.
Figure 1 shows the SL yields and the EPA contents of the SL fractions obtained in the silica-gel cartridge using the non-polar solvents ethyl and butyl acetate, with the objective of separating only the NSL fraction of microalgal SLs. In the first experiment (an SL/ethyl acetate ratio of 0.26 mg/mL), 56.4% of the SLs were extracted; however, this percentage is higher than the NSL content of the fractionated extract (40%, Fig. 1, NSL extract); moreover, its EPA content (25.2%) is much higher than the EPA content of the NSLs in the microalgal extract (9.8%, Fig. 1, NSL extract). This result seems to indicate that, along with the NSLs, an important amount of polar lipids were also extracted. For this reason, a higher SL/ethyl acetate ratio was tested (0.39 mL/mg). However, similar results (with no significant differences) were obtained, which could indicate that the ethyl acetate solvent is too polar (ε = 6.0 versus ε = 2.0 and 4.8 for hexane and chloroform, respectively) to extract only the NSLs. Consequently, we also tested butyl acetate, a solvent with a lower polarity (ε = 5.1). Using this solvent, we tested SL/solvent ratios of 0.26, 0.39, and 0.78 mg/mL. Figure 1 shows that, using the latter, a SL yield of 45.7% was obtained, which is only a little higher than the NSL content of the microalgal SL extract (40%); furthermore, its EPA content (10.9%) is close to the EPA content of the extract’s NSLs (9.8%, with no significant differences). These results suggest that, under these conditions, the NSLs were separated from the extract in their entirety. To verify this, an analytical fractionation of the SL fraction was performed, separated with 0.78 mg SLs/mL of butyl acetate (Section 2.4.2. “Determination of neutral saponifiable lipids (NSLs), glycolipids (GLs), and phospholipids (PLs)”). Figure 2 shows that this fraction (the butyl acetate fraction) contains 96.8% NSLs, and only 2.7% GLs and 0.5% PLs. Therefore, butyl acetate and a SL/solvent ratio of 0.78 were chosen to optimize the SL fractionation. In this small-scale fractionation, the NSLs contain 9.4% of EPA, practically equal to that of the NSLs in the extract (9.8%, Table 1). The higher EPA content of the total SL fraction (10.9%) is due to the high EPA content of the small amounts of GLs extracted (45.3%).
Influence of the solvent type (ethyl acetate, EA or butyl acetate, BA) and SL/solvent ratio (mg/mL) on the SL yield (wt% of the SL in the microalgal extract) and on the EPA content of the extracted SLs (wt% of the total fatty acids) in the first fractionation step, with the goal of extracting the NSLs (these results are compared with the NSL content of the microalgal SL extract, 40%, and the EPA content of the NSLs, 9.8%, Table 1, NSL extract). The fractionations were carried out in a 0.69 g silica-gel cartridge, loading 7.8 mg of SLs (an SL/silica-gel ratio of 11.3 mg/g), which were eluted with 30, 20, and 10 mL of ethyl or butyl acetate. The different letters, a, b, c, d and a´, b´, c´, d´, show significant differences in the SL yield and the EPA content between the NSLs from the microalgal SLs and the extracts from the first extraction step, respectively (α = 0.05)
Analytical determination of the NSL, GL, PL, and EPA contents of the butyl acetate and ethanol fractions separated in a silica-gel cartridge (small scale). The results are compared with the NSL, GL, PL, and EPA contents of the initial SL microalgal extract. Fractions were eluted with an SL/butyl acetate ratio of 0.78 mg/mL and an initial SL/ethanol ratio of 0.156 mg/mL. The different letters, a,b,c, d,e,f, g,h,i, and j,k,l, show significant differences in the NSL, GL, PL, and EPA content between the initial SL extract and butyl acetate and ethanol fractions, respectively (α = 0.05)
A second elution step of the residual SL extract from the first fractionation step was carried out using 50 mL of ethanol (0.156 mg of the initial SL/mL of ethanol, ε = 24) in order to obtain a polar-lipid-rich fraction. Under these conditions, the SL yield obtained in this second elution was 56.4% of the initial SLs. Therefore, after the two consecutive extraction steps, the SLs were eluted quantitatively (an SL yield of 46.2% with butyl acetate and 56.4% with ethanol). This second ethanolic fraction was also analytically fractionated to verify that it was rich in polar lipids. Figure 2 shows that this fraction (the ethanol fraction) contained 94.3% polar lipids (64.2% GLs and 30.1% PLs) and only 5.6% NSLs. This fraction contained 42.3% of the EPA, which is similar to the EPA content of the polar lipids in the microalgal extract (44.3%, calculated from the EPA content of the GLs and PLs; Table 1) and higher than the EPA content of the SL extract (33.4%; Table 1 and Fig. 2).
These results confirm that SLs extracted from the microalga Nannochloropsis sp. can be fractionated into NSLs and polar lipids in a silica-gel column using the non-toxic solvents butyl acetate and ethanol. The concentration of the polar lipids occurs together with an appreciable enrichment in EPA, which is predictable considering the distribution of EPA between the neutral and polar lipids in the microalgal biomass (Table 1).
3.2.2 Influence of the SL/silica-gel ratio
Prior experiments were carried out loading 7.8 mg of the SLs into a cartridge containing 0.69 g of silica gel (an SL/silica-gel ratio of 11.3 mg/g). In order to increase this ratio, higher amounts of SLs were loaded into the same small silica-gel cartridge. In these experiments, the SL/butyl acetate and SL/ethanol ratios were kept constant at the previously tested values (0.78 and 0.156 mg/mL, respectively) increasing both the amounts of SLs and solvents. It is probable that the higher the SL load, the higher the content of polar lipids in the NSL fraction eluted with butyl acetate; this is because the excess load of SLs will not be retained by the stationary phase.
Figure 3 shows that doubling the SL/stationary-phase ratio (from 11.3 to 22.6 mg/g) leads to a slight increase in the SL yield in the first fractionation step (from 42.3 to 44.8 wt%, with no significant differences). However, its EPA content increased appreciably (up to 20.1%), which seems to indicate that greater amounts of polar lipids are being eluted. A further increase in the SL/stationary-phase ratio (28.2 to 33.9 mg/g) gave higher SL yields in this first fractionation step (48.7% to 53.4%, respectively; Fig. 3). A comparison of these SL yields with the NSL content of the microalgal extract (40%) suggests that increased amounts of polar lipids are eluted in this first step, together with NSLs. For this reason, the SL/stationary-phase ratio of 22.6 mg/g was chosen for the subsequent experiments. In the second fractionation step using this initial SL/stationary-phase ratio with ethanol, the SL yield was 55.1%, and its EPA content was 41.5% (Fig. 3). These values are not much lower than the polar-lipid content of the initial extract, and its corresponding EPA content (60% and 44.3%, respectively).
Influence of the SL/silica-gel ratio (mg/g) on the SL yield and on the EPA content obtained in the butyl acetate fraction (the first fractionation step) and the ethanol fraction (the second fractionation step). The results are compared with the NSLs and polar lipids, and their EPA contents, in the SL microalgal extract (SL extract; Table 1). Fractionations were carried out in the 0.69 g silica-gel cartridge, keeping the SL/solvent ratios constant: 0.78 mg/mL for butyl acetate and 0.156 mg/mL for ethanol. The different letters, a, b, c, d and a´, b´, c´, d´, show the significant differences in the SL yields and in the EPA contents between the microalgal SLs and the extracts from the first fractionation step, respectively (α = 0.05). The same applies to e, f, g and e´, f´, g´, h´, which refer to extracts from the second fractionation step
3.2.3 Influence of the SL/solvent ratio on both fractionation steps
Once the SL/stationary-phase ratio was optimized (22.6 mg/g), increasing the SL/solvents ratio was studied in both fractionation steps to save on solvent usage. Figure 4A shows that the SL yield decreased as the SL/butyl acetate ratio increased. At a 1.56 mg/mL ratio, the SL yield (39.1%) was similar to the NSL content of the microalgal SL extract (40%, with no significant differences). Likewise, the EPA content of this fraction (10.1%) was similar to the EPA content of the NSLs (9.8%, with no significant differences). At a higher SL/butyl acetate ratio, the EPA content was slightly higher than the EPA content of the NSLs. Therefore, a SL/butyl acetate ratio of 1.56 mg/mL was chosen to obtain a rich NSL fraction, as indicated by the SL yield, and its EPA contents.
Influence of the SL/solvent ratio on the SL yield and on the EPA content. A Comparing the SL yield and EPA contents of the fractions eluted using increasing SL/butyl acetate ratios with the NSL content (and its EPA content) of the SL extract. B Comparing the SL yield and EPA contents of the fractions eluted using increasing SL/ethanol ratios with the polar-lipid content and its EPA content of SL extract; fractionation carried out after a first fractionation step using 1.56 mg SL/mL of butyl acetate. The different letters, a, b, c and a´, b´, c´, d´, show significant differences in the SL yield and the EPA content between the NSLs of the microalgal SLs and the extracts from the first fractionation step, respectively (α = 0.05). The same applies to e, f and e´, f´, g´, h´, which refer to the polar lipids of the microalgal SLs and the extracts from the second fractionation step
An optimization of the SL/ethanol ratio was also carried out for the second fractionation step. In these experiments, the SL/butyl acetate ratio was kept constant at 1.56 mg/mL. In the previous experiments, the SL/ethanol ratio was 0.156 mg/mL (Fig. 3). Figure 4B shows that the SL yield decreased when the SL/ethanol ratio increased from 0.312 to 0.347 mg/mL and higher values. At a ratio of 0.312 mg/mL, a SL yield of 61.5% was obtained (similar to the polar-lipid content of the SL extract, 60%, with no significant differences); this ethanolic fraction contained 46.2% EPA, which is similar (with no significant differences) to the EPA content of the polar lipids contained in the microalgal lipidic extract (44.3%). Taking into account the SL yield obtained with butyl acetate (39.1% with a 1.56 mg/mL ratio; Fig. 4A), 100% of the SLs loaded into the silica-gel cartridge were recovered. At higher SL/ethanol ratios, less than 100% of the SLs were recovered. For this reason, a ratio of 0.312 mg/mL was chosen to scale the fractionation process.
Therefore, for scaling up, SL/butyl acetate and SL/ethanol ratios of 1.56 mg/mL and 0.312 mg/mL, respectively, were chosen to achieve the highest possible purity in both fractions and to try to recover 100% of the microalgal SLs. Figure 4 shows that higher SL/butyl acetate and SL/ethanol ratios could be used to save on solvents but at the cost of obtaining lower purities and yields of NSLs and polar lipids in each of the fractions.
3.3 Scaling up the SL fractionation
Once the optimal conditions were determined, the fractionation was scaled up to a column containing 10 g of silica gel. The relationship between the stationary phase amounts in both columns (10/0.69 = 14.5) was used as the scaling-up factor to increase the SL load and solvent amounts. The optimized ratios for the SL/silica gel (22.6 mg/g), SL/butyl acetate (1.56 mg/mL), and SL/ethanol (0.312 mg/mL) were likewise scaled up (Figs. 3 and 4). Figure 5 shows the results of this fractionation in the 10 g silica-gel column. In this case, the amount of SL fractionated was 225.4 mg with loaded butyl acetate and ethanol volumes of 145 mL and 725 mL, respectively. Regarding the butyl acetate fraction, an SL yield of 41.1% was achieved. This result is similar (with no significant differences) to that obtained at the small scale (39.1%) and to the NSL content of the microalgal extract (40%). However, the EPA content was slightly higher (16.2% vs 10.1%) at the large scale. With regard to the ethanol fraction, Fig. 5 shows an SL yield of 53.2%, lower than that obtained at the small scale (61.5%). On the other hand, the EPA content (44.9%) was similar to that obtained at the small scale and to that of the microalgal polar-lipid EPA content (with no significant differences). Again, this result seems to indicate that the ethanolic fraction is very rich in polar lipids and should have a fatty acid profile similar to the one for microalgal polar lipids (Table 1).
Comparison of the SL yields and the EPA content in the butyl acetate and ethanol fractions at the small and large scale (0.69 and 10 g of silica gel) with the NSL, polar-lipid, and EPA contents of the SL extract. Fractionations were carried out using the following ratios: SL/stationary phase 22.6 mg/g, SL/butyl acetate 1.56 mg/mL, and SL/ethanol 0.312 mg/mL. The different letters, a, b and a´, b´, show significant differences in the SL yield and the EPA content between the NSLs of the microalgal SLs and the extracts from the first fractionation step (butyl acetate), respectively (α = 0.05). The same applies to c, d and c´, d´, which refer to the polar lipids of the microalgal SLs and the extracts from the second fractionation step (ethanol)
To determine the contents of the lipidic species in the butyl acetate and ethanol fractions, an analytical determination of the NSLs, GLs and PLs was carried out (Section 2.4.2, “Determination of neutral saponifiable lipids (NSLs), glycolipids (GLs) and phospholipids (PLs).” Fig. 6 shows that the fraction eluted with butyl acetate was very rich in NSLs (96.2% NSLs and only 7.4% GLs and 0.0% PLs). This high NSL content means that 98.8% of the NSLs contained in the microalgal SL extract were recovered in this butyl acetate fraction.
Analytical determination of the NSL, GL, PL, and EPA contents in the butyl acetate and ethanol fractions separated in a silica-gel column at large scale (a 10 g silica-gel column) and a comparison of these results with the NSL, GL, PL, and EPA contents of the initial SL microalgal extract. Fractions were eluted with an SL/silica-gel ratio of 22.6 mg/g, an SL/butyl acetate ratio of 1.56 mg/mL, and an SL/ethanol ratio of 0.312 mg/mL. The different letters, a,b,c, d,e,f, g,h,i, and j,k,l, show significant differences in the NSL, GL, PL, and EPA content between the initial SL extract and butyl acetate and ethanol fractions, respectively (α = 0.05)
Figure 6 also shows that the ethanolic fraction obtained in the large-scale column contained 97.6% of polar lipids (64.8% GLs and 32.8% PLs) and only 2.4% of NSLs. This high polar-lipid content means that 86.6% of the polar lipids contained in the microalgal SL extract were recovered in the ethanolic fraction. This fraction contains 44.9% of EPA, which is equal to the EPA content of the polar lipids in the microalgal extract (44.3%; Table 1), and significantly higher than the EPA content of the biomass extract (33.4%; Table 1). This result again confirms that polar-lipid separation occurs along with appreciable EPA enrichment, which is predictable considering the lipidic composition of the Nannochorolsis sp. microalgal biomass. This high EPA content means that 71.5% of the EPA contained in the microalgal SL extract was recovered in this ethanolic fraction along with the polar lipids.
Thus, in this work, a simple microalgal lipid fractionation method was developed, using a methodology and solvents allowed in the food industry, which allows obtaining EPA-rich polar lipid (PLs and GLs) concentrates with high purity and recovery yield.
Finally, Table 2 shows that the polar lipids were also richer in PUFAs (18:2n6, 20:4n6, and EPA) (55.4%) than the NSLs (19.7%) whereas the latter were richer in saturated and monounsaturated fatty acids (14:0, 16:0, 16:1n7, and 18:1n9) (80.3%) than the polar lipids (44.5%). This PUFA composition mean that 74% of the PUFAs present in the microalgal biomass were recovered in the polar-lipid fraction. This is the result of the fractionation carried out, and the fatty acid distribution between the NSLs and the polar lipids in the microalgal biomass used. In this biomass, the NSLs contain 85.5% of the saturated and monounsaturated fatty acids, and 80% of the biomass’s total PUFAs are contained in the polar lipids.
It is difficult to compare these results with those obtained by other authors because, as far as we know, there are no works covering this area. Devos et al. [15] extracted the total lipids from the microalga Isochrysis galbana (13.5% of the total fatty acids are DHA) using the Bligh and Dyer method; these lipids were then fractionated into NLs, GLs, and PLs in a column with 20 g of silica gel, the amount allowed for the treatment of lipids extracted from about 6 g of biomass. The three lipidic classes were eluted with 30 mL of chloroform for the NLs, 120 mL of chloroform/methanol 5:1 (v/v) for the GLs, and 45 mL of methanol for the PLs. Therefore, these authors used toxic solvents and did not provide data on the lipid yields. The fatty acid profiles of both the NSLs and the GLs are not very different from those of the total lipids. However, as with the higher EPA content that we found in the polar lipids of Nannochloropsis sp., they found that the PLs from I. galbana were particularly rich in DHA (75% of the total DHA was present in the PLs), and these authors used this PL fraction as a source of DHA. Antonopoulos et al. [16] obtained purified polar lipids (mainly PLs) from the archaeon Thermoplasma acidophilum DSM 1728/10217. The GL fraction was cleanly separated from the PLs by diethylaminoethyl (DEAE) cellulose column chromatography in columns with inner diameters of 1.5 and 2.5 cm, and lengths of 20–27 cm. The NLs and GLs were eluted with chloroform/methanol 7:3 (v/v) (4–5 column volumes) whereas the PLs were eluted with five column volumes of chloroform/methanol 7:3 containing 4 g of ammonium acetate and 51 mL 25% of aqueous ammonia. The final purification of the main glycophospholipids in a silica-gel column with mixtures of chloroform/methanol/water 80:25:3 (v/v/v) or petroleum ether/2-propanol/water 80:70:8 (v/v/v) proved an efficient method for obtaining the main glycophospholipids in a pure state and in a relatively large quantity. Up to 150 mg of purified PLs were obtained in a single run in a column with a diameter of 2.5 cm. However, these researchers also used toxic solvents and two chromatographic steps were required.
Table 3 compares the results obtained in this work with those obtained in previous works [14, 17] using direct extraction from Nannochloropsis sp. lyophilized biomass (initially extracting the NSL using a non-polar solvent and then the EPA-rich polar lipids using ethanol). Table 3 also shows the solvent consumption in mL/g of polar lipids obtained for the different procedures. Firstly, Table 3 shows that, in all cases, the NSL contents of the microalgal biomass were similar to the SL yields obtained in the first fractionation step using the non-polar solvent, these fractions thus being very rich in NSLs. Undoubtedly, in this work, the fraction obtained using the non-polar solvent was richer in NSLs (96.2% of the SLs of this fraction were NSLs). However, this good NSL separation required a large volume of butyl acetate (1233 mL/g of polar lipids obtained, calculated from the SL/butyl acetate ratio of 1.56 mg/mL). In any case, considering the results obtained at the small scale (Fig. 4A), it is estimated that this butyl acetate consumption could be reduced by approximately 30% using an SL/butyl acetate ratio of 2.23 mg/mL, although the SL yield would decrease (37.8%) and the subsequent ethanolic fraction would be poorer in polar lipids.
Table 3 also shows that the polar-lipid contents of microalgal SLs were similar to the SL yields obtained in the second fractionation step using ethanol, and that these fractions were very rich in polar lipids. Furthermore, the ethanolic fraction obtained in this work is richer in polar lipids (97.7%), but again, this high purity is achieved at the expense of a greater ethanol consumption (6167 mL/g of polar lipids obtained, calculated from the SL/ethanol ratio of 0.312 mg/mL). In the same way, considering the results obtained at the small scale (Fig. 4B), it is estimated that this ethanol consumption could be reduced by approximately 40% using an SL/ethanol ratio of 0.52 mg/mL, although the SL yield would decrease (54.5%) and possibly this ethanolic fraction would be poorer in polar lipids.
4 Conclusions
In this work, the SLs extracted from the microalga Nannochloropsis sp. (33.4% EPA) were fractionated in silica-gel columns to obtain an initial fraction rich in NSLs (using a non-polar solvent) and then a second SL fraction high in EPA-enriched polar lipids (using a polar solvent). In both steps, non-toxic solvents (which are allowed in the food industry) were used. The method was first developed in small cartridges containing 0.69 g of silica gel. At this small scale, the SL/silica-gel, SL/butyl acetate, and SL/ethanol ratios were optimized to obtain high polar-lipid and EPA contents and recoveries in the polar-lipid fraction. This optimized fractionation (with an SL/silica-gel ratio of 22.6 mg/g, an SL/butyl acetate ratio of 1.56 mg/mL and an SL/ethanol ratio of 0.312 mg/mL) was scaled up to a column containing 10 g of silica gel. Under these conditions, an ethanolic SL fraction containing 97.7% of polar lipids (64.8% GLs and 32.9% PLs) was obtained. In this fraction, 86.6% of the polar lipids contained in the original microalgal extract were recovered. This ethanolic polar-lipid fraction contained 44.9% of EPA of the total fatty acids and 71.5% of the EPA contained in the microalgal SL extract was recovered. With this method, high NSL, polar-lipid, and EPA contents and recoveries can be obtained although it consumes considerable amounts of solvents. These consumption levels can be reduced by around 30–40% but at the cost of reducing both the purity and the recovery yields.
5 Data availabity
Not Applicable.
References
Swanson D, Block R, Mousa S (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3:1–7. https://doi.org/10.3945/an.111.000893
Lee JH, O’Keefe JH, Lavie CJ, Harris WS (2009) Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat Rev Cardiol 6:753–758. https://doi.org/10.1038/nrcardio.2009.188
Calder PC (2012) The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res 56:1073–1080. https://doi.org/10.1002/mnfr.201100710
FAO (Food and Agriculture Organization (2010) Paper 91.
Finco A, Mamani L, Carvalho J, de Melo Pereira G, Thomaz-Soccol V, Soccol C (2017) Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit Rev Biotechnol 37:656–671. https://doi.org/10.1080/07388551.2016.1213221
Rossmeisl M, Macek Z, Kuda O, Bryhn M, Kopecky J (2012) Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: possible role of endocannabinoids. Plos One 7:e38834. https://doi.org/10.1371/journal.pone.0038834
Tang X, Li ZJ, Xu J, Xue Y, Li JZ, Wang JF, Yanagita T, Xue CH, Wang YM (2012) Short term effects of different omega-3 fatty acid formulation on lipid metabolism in mice fed high or low fat diet. Lipids Health Dis 11:70–78. https://doi.org/10.1186/1476-511X-11-70
Lordan R, Redfern S, Tsoupras A, Zabetakis I (2020) Inflammation and cardiovascular disease: are marine phospholipids the answer? Food Funct 11:2861. https://doi.org/10.1039/c9fo01742a
van Hoogevest P, Wendel A (2014) The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur J Lipid Sci Technol 116:1088–1107. https://doi.org/10.1002/ejlt.201400219
Burri L, Hoem N, Banni S, Berge K (2012) Marine omega-3 phospholipids: metabolism and biological activities. Int J Mol Sci 13:15401–15419. https://doi.org/10.3390/ijms131115401
T.A. Conde, I. Zabetakis, A. Tsoupras, I. Medina, M. Costa, J. Silva, B. Neves, P. Domingues, M.R. Domingues (2021) Microalgal lipid extract have potential to modulate the inflammatory response: a critical review. Int. J. Mol. Sci. 22 . https://doi.org/10.3390/ijms22189825
E. Molina, F. García, F.G. Acién 1999 Production of EPA from Phaeodactylum tricornutum, in: chemicals from Microalgae. Edited by Zvi Cohen. Taylor & Francis, London, pp 57–92.
Rao A, Briskey D, Nalley JO, Ganuza E (2020) Omega-3 eicosapentaenoic acid (EPA) rich extract from the microalga Nannochloropsis decreases cholesterol in healthy individuals: a double-Blind, randomized, placebo-controlled, three-month supplementation study. Nutrients 12:1869. https://doi.org/10.3390/nu12061869
Jiménez Callejón MJ, Robles Medina A, González Moreno PA, Esteban Cerdán L, OrtaGuillén S, Molina Grima E (2020) Simultaneous extraction and fractionation of lipids from the microalga Nannochloropsis sp. for the production of EPA-rich polar lipid concentrates. J Appl Phycol 32:1117–1128. https://doi.org/10.1007/s10811-020-02037-z
Devos M, Poisson L, Ergan F, Pencreac’h G (2006) Enzymatic hydrolysis of phospholipids from Isochrysisgalbana for docosahexaenoic acid enrichment. Enz Microb Technol 39:548–554. https://doi.org/10.1016/j.enzmictec.2005.08.040
Antonopoulos E, Freisleben H, Krisnamurti D, Esuningtyas A, Mulyanto C, Ridwan R, Freisleben S (2013) Fractionation and purification of membrane lipids from the archaeon Thermoplama acidophilum DSM 1728/10217. Sep Purif Technol 110:119–126. https://doi.org/10.1016/j.seppur.2013.03.014
Jiménez Callejón MJ, Robles Medina A, Macías Sánchez MD, González Moreno PA, Navarro López E, Esteban Cerdán L, Molina Grima E (2022) Supercritical fluid extraction and pressurized liquid extraction processes applied to EPA-rich polar lipid recovery from the microalga Nannochloropsis sp. Algal Res 62:102586. https://doi.org/10.1016/j.algal.2021.102586
Monte J, Ribeiro C, Parreira C, Costa L, Brive L, Casal S, Brazinha C, Crespo JG (2020) Biorefinery of Dunaliella salina: sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour Technol 297:122509. https://doi.org/10.1016/j.biortech.2019.122509
G. Kochert, Quantitation of the macromolecular components of microalgae. In: Hellebust, J, Cragie, S (Eds.), Handbook of Phycological Methods. Physiological and Biochemical Methods. Cambridge University Press, London, 1978.
Jiménez MJ, Robles A, Macías MD, Hita E, Esteban L, González PA, Molina E (2014) Extraction of saponifiable lipids from wet microalgal biomass for biodiesel production. Bioresour Technol 169:198–205. https://doi.org/10.1016/j.biortech.2014.06.106
M. Kates 1986 Techniques of lipidology isolation, analysis, and identification of lipids. Edited by Burdon R.H. and van Knippenber PH Elsevier Science Publishers, Amsterdam, p. 190.
Navarro López E, Robles Medina A, González Moreno PA, Jiménez Callejón MJ, Esteban Cerdán L, Martín Valverde L, Molina Grima E (2015) Enzymatic production of biodiesel from Nannochloropsisgaditana lipids: influence of operational variables and polar lipid content. Bioresour Technol 187:346–353. https://doi.org/10.1016/j.biortech.2015.03.126
Jiménez MJ, Robles A, Macías MD, Esteban L, González PA, Navarro E, Hita E, Molina E (2020) Obtaining highly pure EPA-rich lipids from dry and wet Nannochloropsis gaditana microalgal biomass using ethanol, hexane and acetone. Algal Res 45:101729. https://doi.org/10.1016/j.algal.2019101729
López D, Belarbi EH, Rodríguez J, Segura CI, Giménez A (1998) Acyl lipids of three microalgae. Phytochemistry 47:1473–1481. https://doi.org/10.1016/S0031-9422(97)01080-7
F. Guihéneuf, M. Schmid, D.B. Stengel, Lipids and fatty acids in algae: extraction, fractionation into lipid classes, and analysis by gas chromatography coupled with flame ionization detector (GC-FID) in: Dagmar B. Stengel and Solène Connan (eds.), Natural products from marine algae: methods and Protocols, Methods in Molecular Biology, vol. 1308, Chapter 11, pp. 173–190. Springer Science+Business Media, New York, 2015.
Prat D, Wells A, Hayler J, Sneddon H, McElroy R, Abou-Shehada S, Dunn PJ (2016) CHEM21 selection guide of classical- and less classical-solvents. Green Chem 18:288–296. https://doi.org/10.1039/c5gc01008j
Rodríguez J, Belarbi E, García J, López D (1998) Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol Technol 12:689–691. https://doi.org/10.1023/A:1008812904017
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was supported by grants from the Ministerio de Economía, Industria y Competitividad (Spain), Project CTQ2017-85613-R, which, in turn, was co-funded by FEDER (The European Regional Development Fund).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by María D. Sánchez Macías and Elvira Navarro López. The first draft of the manuscript was written by María D. Sánchez Macías. The lastest version of the manuscript was written by María José Jiménez Callejón and Alfonso Robles Medina. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sánchez, M.D.M., Callejón, M.J.J., Medina, A.R. et al. Obtaining EPA-rich polar lipids from microalga Nannochloropsis sp. by silica-gel chromatography using non-toxic solvents. Biomass Conv. Bioref. 13, 15519–15530 (2023). https://doi.org/10.1007/s13399-022-02520-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02520-2