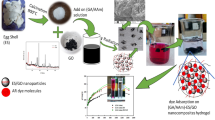
Abstract
The polymeric composite adsorbent was successfully synthesized by grafting polyamide on eggshell (CMP). The performance of the CMP composite as an adsorbent was studied for the methylene blue (MB) dye removal in a batch mode system. The effects of several factors such as adsorbent dosage, initial concentration of MB dye, and initial solution pH on the efficiency of MB removal were investigated. Kinetic studies were implemented to understand the adsorption mechanism. The maximum adsorption capacity was achieved under the conditions of an initial concentration of 200 ppm, the adsorbent dosage of 0.01 mg, and solution pH of 6 at room temperature. The pseudo-second-order kinetic model showed best fitted the experimental data. The adsorption capacity of the CMP was 345 ± 4 mg/g at 298 K for MB dye. The adsorption mechanisms included π-π interactions, electrostatic attraction, interaction with functional groups, and formation of complexes. The CMP showed simultaneous removal of MB dye and toxic metals such as Pb, Cr, Ni, Cd, Hg, and As. The CMP composite can be potentially used as an efficient and low-cost biosorbent for the removal of MB dye and heavy metals.









Similar content being viewed by others
References
Jack Clifton II, Leikin JB (2003) Methylene blue. Am J Ther 10:289–291
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov 3:275–290
Narwade VN, Khairnar RS, Kokol V (2018) In situ synthesized hydroxyapatite—cellulose nanofibrils as biosorbents for heavy metal ions removal. J Polym Environ 26:2130–2141
Moreno-Sader K, García-Padilla A, Realpe A, Acevedo-Morantes M, Soares JBP (2019) Removal of heavy metal water pollutants (Co2+ and Ni2+) using polyacrylamide/sodium montmorillonite (PAM/Na-MMT) nanocomposites. ACS Omega 4:10834–10844
Saleh TA (2020) Nanomaterials: classification, properties, and environmental toxicities. Environ Technol Innov 20:101067
Saleh TA (2021) Protocols for synthesis of nanomaterials, polymers, and green materials as adsorbents for water treatment technologies. Environ Technol Innov 24:101821
Saleh TA (2018) Simultaneous adsorptive desulfurization of diesel fuel over bimetallic nanoparticles loaded on activated carbon. J Clean Prod 172:2123–2132
Saleh TA (2020) Trends in the sample preparation and analysis of nanomaterials as environmental contaminants. Trends Environ Anal Chem 28:e00101
Mouni L, Belkhiri L, Bollinger J-C, Bouzaza A, Assadi A, Tirri A et al (2018) Removal of methylene blue from aqueous solutions by adsorption on Kaolin: kinetic and equilibrium studies. Appl Clay Sci 153:38–45
Saleh TA, Sarı A, Tuzen M (2002) Simultaneous removal of polyaromatic hydrocarbons from water using polymer modified carbon. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-02163-9
T.A. Saleh, M Mustaqeem, M Khaled, Water treatment technologies in removing heavy metal ions from wastewater: a review. Environ Nanotechnol Monit Manag :100617. https://doi.org/10.1016/j.enmm.2021.100617.
Saleh TA (2017) Insights into carbon nanotube-metal oxide composite: embedding in membranes, Int J Sci. Res Environ Sci Toxicol 2(1):1–4
Bin-Dahman OA, Saleh TA (2020) Synthesis of carbon nanotubes grafted with PEG and its efficiency for the removal of phenol from industrial wastewater. Environ Nanotechnol Monit Manag 13:100286
Saleh TA, Elsharif AM, Bin-Dahman OA (2021) Synthesis of amine functionalization carbon nanotube-low symmetry porphyrin derivatives conjugates toward dye and metal ions removal. J Mol Liq 340:117024
Sankaran R, Show PL, Ooi C-W, Ling TC, Shu-Jen C, Chen S-Y et al (2020) Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes, Clean Technol. Environ. Policy 22:773–786
Burakov AE, Galunin EV, Burakova IV, Kucherova AE, Agarwal S, Tkachev AG et al (2018) Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol Environ Saf 148:702–712
Van Thuan T, Quynh BTP, Nguyen TD, Bach LG (2017) Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surfaces and Interfaces 6:209–217
Javadian H, Ruiz M, Saleh TA, Sastre AM (2020) Ca-alginate/carboxymethyl chitosan/Ni0. 2Zn0. 2Fe2. 6O4 magnetic bionanocomposite: Synthesis, characterization and application for single adsorption of Nd+ 3, Tb+ 3, and Dy+ 3 rare earth elements from aqueous media. J Mol Liq 306:112760
de Oliveira AP, Módenes AN, Bragião ME, Hinterholz CL, Trigueros DEG, G. de O. Isabella, (2018) Use of grape pomace as a biosorbent for the removal of the Brown KROM KGT dye. Bioresour Technol Rep 2:92–99
Tsai W-T, Hsien K-J, Hsu H-C, Lin C-M, Lin K-Y, Chiu C-H (2008) Utilization of ground eggshell waste as an adsorbent for the removal of dyes from aqueous solution. Bioresour Technol 99:1623–1629
Kyzas GZ, Lazaridis NK, Mitropoulos AC (2012) Removal of dyes from aqueous solutions with untreated coffee residues as potential low-cost adsorbents: equilibrium, reuse and thermodynamic approach. Chem Eng J 189:148–159
El-Bindary AA, El-Sonbati AZ, Al-Sarawy AA, Mohamed KS, Farid MA (2014) Adsorption and thermodynamic studies of hazardous azocoumarin dye from an aqueous solution onto low cost rice straw based carbons. J Mol Liq 199:71–78
Hazzaa R, Hussein M (2015) Adsorption of cationic dye from aqueous solution onto activated carbon prepared from olive stones. Environ Technol Innov 4:36–51
Fernández-López JA, Angosto JM, Roca MJ, Miñarro MD (2019) Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci Total Environ 653:55–63
Noreen S, Bhatti HN (2014) Fitting of equilibrium and kinetic data for the removal of Novacron Orange P-2R by sugarcane bagasse. J Ind Eng Chem 20:1684–1692
Zhang Y-J, Ou J-L, Duan Z-K, Xing Z-J, Wang Y (2015) Adsorption of Cr (VI) on bamboo bark-based activated carbon in the absence and presence of humic acid. Colloids Surf A Physicochem Eng Asp 481:108–116
Alhussary BN, Taqa GA, Taqa AAA (2020) Preparation and characterization of natural nano hydroxyapatite from eggshell and seashell and its effect on bone healing. J Appl Vet Sci 5:25–32
Tshizanga N, Aransiola EF, Oyekola O (2017) Optimisation of biodiesel production from waste vegetable oil and eggshell ash, South African. J Chem Eng 23:145–156
Bandhavya GB, Sandeep K, Bindhushree GB (2017) An experimental study on partial replacement of cement with egg shell powder in concrete. Int Res J Eng Technol 4:2017
Wazir A, Gul Z, Hussain M (2018) Comparative study of various organic fertilizers effect on growth and yield of two economically important crops, potato and pea. Agric Sci 9:703
Zaman T, Mostari M, Al Mahmood MA, Rahman MS (2018) Evolution and characterization of eggshell as a potential candidate of raw material. Cerâmica. 64:236–241
Cree D, Rutter A (2015) Sustainable bio-inspired limestone eggshell powder for potential industrialized applications. ACS Sustain Chem Eng 3:941–949
Carvalho J, Araújo J, Castro F (2011) Alternative low-cost adsorbent for water and wastewater decontamination derived from eggshell waste: an overview. Waste Biomass Valoriz 2:157–167
Al-Ghouti MA, Salih NR (2018) Application of eggshell wastes for boron remediation from water. J Mol Liq 256:599–610
Dayanidhi K, Vadivel P, Jothi S, Eusuff NS (2020) Facile synthesis of silver@ eggshell nanocomposite: a heterogeneous catalyst for the removal of heavy metal ions, toxic dyes and microbial contaminants from water. J. Environ. Manage. 271:110962
Zhang Y, Hong X, Cao X, Huang X, Hu B, Ding S, Lin H (2021) Functional porous organic polymers with conjugated triaryl triazine as the core for superfast adsorption removal of organic dyes. ACS Appl Mater Interfaces 13(5):6359–6366
Rezania H, Vatanpour V, Salehi E, Gavari N, Shockravi A, Ehsani M (2019) Wholly heterocycles-based polyamide–sulfide containing pyridine and thiazole rings: a super-adsorbent polymer for lead removal. J Polym Environ 27:1790–1800
Dotto GL, Santos JMN, Tanabe EH, Bertuol DA, Foletto EL, Lima EC et al (2017) Chitosan/polyamide nanofibers prepared by Forcespinning® technology: a new adsorbent to remove anionic dyes from aqueous solutions. J Clean Prod 144:120–129
Moradi E, Ebrahimzadeh H, Mehrani Z, Asgharinezhad AA (2019) The efficient removal of methylene blue from water samples using three-dimensional poly (vinyl alcohol)/starch nanofiber membrane as a green nanosorbent. Environ Sci Pollut Res 26:35071–35081
Saleh TA, Sarı A, Tuzen M (2021) Development and characterization of bentonite-gum arabic composite as novel highly-efficient adsorbent to remove thorium ions from aqueous media. Cellulose 28(16):10321–10333
Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R (2013) Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—a review. Prog Polym Sci 38:1232–1261. https://doi.org/10.1016/J.PROGPOLYMSCI.2013.02.003
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238
Arami M, Limaee NY, Mahmoodi NM (2006) Investigation on the adsorption capability of egg shell membrane towards model textile dyes. Chemosphere 65:1999–2008
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Tempkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR 12:327
Ali I, S.A. AL-Hammadi, T.A. Saleh, (2018) Simultaneous sorption of dyes and toxic metals from waters using synthesized titania-incorporated polyamide. J Mo. Liq 269:564–571
Elzain AA, El-Aassar MR, Hashem FS, Mohamed FM, Ali ASM (2019) Removal of methylene dye using composites of poly (styrene-co-acrylonitrile) nanofibers impregnated with adsorbent materials. J Mol Liq 291:111335
Saleh TA (2015) Mercury sorption by silica/carbon nanotubes and silica/activated carbon: a comparison study. J Water Supply Res Technol 64:892–903
Abualnaja KM, Alprol AE, Abu-Saied MA, Mansour AT, Ashour M (2021) Studying the adsorptive behavior of poly (acrylonitrile-co-styrene) and carbon nanotubes (nanocomposites) impregnated with adsorbent materials towards methyl orange dye. Nanomaterials 11:1144
Sousa HR, Silva LS, Sousa PAA, Sousa RRM, Fonseca MG, Osajima JA et al (2019) Evaluation of methylene blue removal by plasma activated palygorskites. J Mater Res Technol 8:5432–5442
Qi C, Zhao L, Lin Y, Wu D (2018) Graphene oxide/chitosan sponge as a novel filtering material for the removal of dye from water. J Colloid Interface Sci 517:18–27
He J, Cui A, Deng S, Chen JP (2018) Treatment of methylene blue containing wastewater by a cost-effective micro-scale biochar/polysulfone mixed matrix hollow fiber membrane: performance and mechanism studies. J Colloid Interface Sci 512:190–197
Allen SJ, Koumanova B (2005) Decolourisation of water/wastewater using adsorption. J Univ Chem Technol Metall 40:175–192
Moreno-Castilla C (2004) Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon N Y 42:83–94
Wong S, AbdGhafar N, Ngadi N, Razmi FA, Inuwa IM, Mat R et al (2020) Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 10:1–13
Murcia-Salvador A, Pellicer JA, Rodríguez-López MI, Gómez-López VM, Núñez-Delicado E, Gabaldón JA (2020) Egg by-products as a tool to remove direct Blue 78 dye from wastewater: kinetic, equilibrium modeling, thermodynamics and desorption properties. Materials (Basel) 13:1262
El-Kemary MA, El-mehasseb IM, Shoueir KR, El-Shafey SE, El-Shafey OI, Aljohani HA et al (2018) Sol-gel TiO2 decorated on eggshell nanocrystal as engineered adsorbents for removal of acid dye. J Dispers Sci Technol 39:911–921
Saleh TA, Tuzen M, Sarı A (2018) Polyamide magnetic palygorskite for the simultaneous removal of Hg (II) and methyl mercury; with factorial design analysis. J Environ Manage 211:323–333
Mittal H, Maity A, Ray SS (2015) Synthesis of co-polymer-grafted gum karaya and silica hybrid organic–inorganic hydrogel nanocomposite for the highly effective removal of methylene blue. Chem Eng J 279:166–179. https://doi.org/10.1016/J.CEJ.2015.05.002
Agarwal S, Tyagi I, Gupta VK, Golbaz F, Golikand AN, Moradi O (2016) Synthesis and characteristics of polyaniline/zirconium oxide conductive nanocomposite for dye adsorption application. J Mol Liq 218:494–498
Guesmi Y, Agougui H, Lafi R, Jabli M, Hafiane A (2018) Synthesis of hydroxyapatite-sodium alginate via a co-precipitation technique for efficient adsorption of Methylene Blue dye. J Mol Liq 249:912–920
Li Y, Zhang Y, Wang G, Li S, Han R, Wei W (2018) Reed biochar supported hydroxyapatite nanocomposite: characterization and reactivity for methylene blue removal from aqueous media. J Mol Liq 263:53–63
Arora C, Soni S, Sahu S, Mittal J, Kumar P, Bajpai PK (2019) Iron based metal organic framework for efficient removal of methylene blue dye from industrial waste. J Mol Liq 284:343–352
Ma T, Wu Y, Liu N, Wu Y (2020) Hydrolyzed polyacrylamide modified diatomite waste as a novel adsorbent for organic dye removal: adsorption performance and mechanism studies. Polyhedron. 175:114227
Panda J, Sahoo JK, Panda PK, Sahu SN, Samal M, Pattanayak SK et al (2019) Adsorptive behavior of zeolitic imidazolate framework-8 towards anionic dye in aqueous media: combined experimental and molecular docking study. J Mol Liq 278:536–545
Maazinejad B, Mohammadnia O, Ali GAM, Makhlouf ASH, Nadagouda MN, Sillanpää M et al (2020) Taguchi L9 (34) orthogonal array study based on methylene blue removal by single-walled carbon nanotubes-amine: adsorption optimization using the experimental design method, kinetics, equilibrium and thermodynamics. J Mol Liq 298:112001
Zhang Z, Xu X (2014) Wrapping carbon nanotubes with poly (sodium 4-styrenesulfonate) for enhanced adsorption of methylene blue and its mechanism. Chem Eng J 256:85–92
Jawad AH, Ismail K, Ishak MAM, Wilson LD (2019) Conversion of Malaysian low-rank coal to mesoporous activated carbon: structure characterization and adsorption properties, Chinese. J Chem Eng 27:1716–1727
Acknowledgements
The author TA Saleh thanks KFUPM, Saudi Arabia, for the support (Project No. DF201019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bin-Dahman, O.A., Saleh, T.A. Synthesis of polyamide grafted on biosupport as polymeric adsorbents for the removal of dye and metal ions. Biomass Conv. Bioref. 14, 2439–2452 (2024). https://doi.org/10.1007/s13399-022-02382-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02382-8