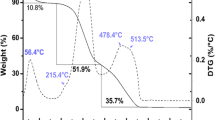
Abstract
Because of their detrimental impacts on water quality and human health, dyes’ ubiquitous presence in the aquatic environment has aroused concerns over the last few decades concerning the methods to be employed to eradicate these emerging pollutants. In the ongoing study, raw cactus fruit peel (RCFP) is used as a low-cost, environmentally friendly sorbent to remove the hazardous dye Basic Red 46 (BR46) from aqueous solutions in the current study. The raw biosorbent was firstly characterized using FTIR spectroscopy, SEM, BET, pHpzc and Boheme titration techniques. Respectively, the influencing operational parameters such as pH, biosorbent dosage, stirring speed, temperature, contact time and initial dye concentration were tweaked to obtain a maximum adsorption capacity of 82.58 mg g−1. The Freundlich model was found to better represent the biosorption isotherm, whereas the pseudo-second-order model describes well the kinetic biosorption data obtained. The biosorption process of BR46 onto RCFP was spontaneous and exothermic, and the reuse study revealed the possibility of regeneration of RCFP after 3 successive cycles using HCl as a chemical reagent. Furthermore, the results revealed that raw cactus fruit peel is an untapped potential supply in Algeria, with the potential to provide low-cost environmental removal of additional toxins in the wastewater treatment domain.














Similar content being viewed by others
References
Salleh MAM, Mahmoud DK, Karim WAWA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280(1–3):1–13
Eren E (2009) Investigation of a basic dye removal from aqueous solution onto chemically modified Unyebentonite. J Hazard Mater 166(1):88–93
Kaci MM, Nasrallah N, Atmani F, Kebir M, Guernanou R, Soukeur A, Trari M (2021) Enhanced photocatalytic performance of CuAl2O4 nanoparticles spinel for dye degradation under visible light. Res Chem Intermed 47:3785–3806
Senturk HB, Ozdes D, Duran C (2010) Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunusdulcis) as a low cost biosorbent. Desalination 252(1–3):81–87
Vilar VJ, Botelho CM, Boaventura RA (2007) Methylene blue adsorption by algal biomass based materials: biosorbents characterization and process behaviour. J Hazard Mater 147(1–2):120–132
Yu L, Luo YM (2014) The adsorption mechanism of anionic and cationic dyes by Jerusalem artichoke stalk-based mesoporous activated carbon. J Environ Chem Eng 2(1):220–229
Dawood S, Sen TK (2012) Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res 46(6):1933–1946
Tang B, Lin Y, Yu P, Luo Y (2012) Study of aniline/ɛ-caprolactam mixture adsorption from aqueous solution onto granular activated carbon: kinetics and equilibrium. Chem Eng J 187:69–78
Noli F, Kapashi E, Kapnisti M (2019) Biosorption of uranium and cadmium using sorbents based on Aloe vera wastes. J Environ Chem Eng 7(2):102985
Anastopoulos I, Kyzas GZ (2014) Agricultural peels for dye adsorption: a review of recent literature. J Mol Liq 200:381–389
Saranya N, Ajmani A, Sivasubramanian V, Selvaraju N (2018) Hexavalent chromium removal from simulated and real effluents using Artocarpusheterophyllus peel biosorbent-batch and continuous studies. J Mol Liq 265:779–790
Kais H, YeddouMezenner N, Trari M (2020) Biosorption of rifampicin from wastewater using cocoa shells product. Sep Sci Technol 55(11):1984–1993
Jain SN, Tamboli SR, Sutar DS, Jadhav SR, Marathe JV, Shaikh AA, Prajapati AA (2020) Batch and continuous studies for adsorption of anionic dye onto waste tea residue: kinetic, equilibrium, breakthrough and reusability studies. J Clean Prod 252:119778
Kumar S, Narayanasamy S, Venkatesh RP (2019) Removal of Cr (VI) from synthetic solutions using water caltrop shell as a low-cost biosorbent. Sep Sci Technol 54(17):2783–2799
Stavrinou A, Aggelopoulos CA, Tsakiroglou CD (2018) Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: adsorption kinetics and equilibrium isotherms as a tool. J Environ Chem Eng 6(6):6958–6970
Ammar C, El-Ghoul Y, Jabli M (2021) Characterization and valuable use of Calotropis gigantea seedpods as a biosorbent of methylene blue. International Journal of Phytoremediation 1–10
Al-Ghamdi YO, Jabli M, Soury R, Khan SA (2021) Synthesis of copper oxide nanoparticles using Pergularia tomentosa leaves and decolorization studies. International Journal of Phytoremediation 1–13
Sebeia N, Jabli M, Ghith A, Saleh TA (2020) Eco-friendly synthesis of Cynomorium coccineum extract for controlled production of copper nanoparticles for sorption of methylene blue dye. Arab J Chem 13(2):4263–4274
Sebeia N, Jabli M, Ghith A, Elghoul Y, Alminderej FM (2019) Production of cellulose from Aegagropila Linnaei macro-algae: chemical modification, characterization and application for the bio-sorption of cationic and anionic dyes from water. Int J Biol Macromol 135:152–162
Akkari, I., Graba, Z., Bezzi, N., Merzeg, F. A., Bait, N., & Ferhati, A. (2021). Raw pomegranate peel as promise efficient biosorbent for the removal of Basic Red 46 dye: equilibrium, kinetic, and thermodynamic studies. Biomass Conversion and Biorefinery 1–14
Saenz C (2000) Processing technologies: an alternative for cactus pear (Opuntia spp.) fruits and cladodes. J Arid Environ 46(3):209–225
Shetty AA, Rana MK, Preetham SP (2012) Cactus: a medicinal food. J Food Sci Technol 49(5):530–536
Berraaouan A, Abderrahim Z, Hassane M, Abdelkhaleq L, Mohammed A, Mohamed B (2015) Evaluation of protective effect of cactus pear seed oil (Opuntiaficus-indica L. MILL.) against alloxan-induced diabetes in mice. Asian Pac J Trop Med 8(7):532–537
Macharia M (2011) Effectiveness of cactus biomass and its combusted products in removal of colour, turbidity and selected metal ions from contaminated water. Kenyatta University, Nairobi, Kenya, MS thesis
Kumar R, Barakat MA (2013) Decolourization of hazardous brilliant green from aqueous solution using binary oxidized cactus fruit peel. Chem Eng J 226:377–383
Mohamed SK, Alazhary AM, Al-Zaqri N, Alsalme A, Alharthi FA, Hamdy MS (2020) Cost-effective adsorbent from arabinogalactan and pectin of cactus pear peels: kinetics and thermodynamics studies. Int J Biol Macromol 150:941–947
Gebrezgiher, M., & Kiflie, Z. (2020). Utilization of cactus peel as biosorbent for the removal of reactive dyes from textile dye effluents. J Environ Pub Health 2020
Shoushtarian F, Moghaddam MRA, Kowsari E (2020) Efficient regeneration/reuse of graphene oxide as a nanoadsorbent for removing basic Red 46 from aqueous solutions. J Mol Liq 312:113386
Konicki W, Hełminiak A, Arabczyk W, Mijowska E (2018) Adsorption of cationic dyes onto Fe@ graphite core–shell magnetic nanocomposite: equilibrium, kinetics and thermodynamics. Chem Eng Res Des 129:259–270
Senoussi H, Bouhidel KE (2018) Feasibility and optimisation of a batch mode capacitive deionization (BM CDI) process for textile cationic dyes (TCD) removal and recovery from industrial wastewaters. J Clean Prod 205:721–727
Boehm HP (2002) Surface oxides on carbon and their analysis: a critical assessment. Carbon 40(2):145–149
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57(385471):1100–1107
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Actaphysiochim URSS 12:327–356
Olgun A, Atar N (2009) Equilibrium and kinetic adsorption study of Basic Yellow 28 and Basic Red 46 by a boron industry waste. J Hazard Mater 161(1):148–156
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Nasrullah A, Bhat AH, Naeem A, Isa MH, Danish M (2018) High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int J Biol Macromol 107:1792–1799
Mokhtar N, Aziz EA, Aris A, Ishak WFW, Ali NSM (2017) Biosorption of azo-dye using marine macro-alga of Euchema Spinosum. J Environ Chem Eng 5(6):5721–5731
Daoud M, Benturki O, Girods P, Donnot A, Fontana S (2019) Adsorption ability of activated carbons from Phoenix dactylifera rachis and Ziziphus jujube stones for the removal of commercial dye and the treatment of dyestuff wastewater. Microchem J 148:493–502
Gundogdu A, Duran C, Senturk HB, Soylak M, Imamoglu M, Onal Y (2013) Physicochemical characteristics of a novel activated carbon produced from tea industry waste. J Anal Appl Pyrol 104:249–259
Mishra S, Yadav SS, Rawat S, Singh J, Koduru JR (2019) Corn husk derived magnetized activated carbon for the removal of phenol and para-nitrophenol from aqueous solution: interaction mechanism, insights on adsorbent characteristics, and isothermal, kinetic and thermodynamic properties. J Environ Manage 246:362–373
Xiao Z, Yuan M, Yang B, Liu Z, Huang J, Sun D (2016) Plant-mediated synthesis of highly active iron nanoparticles for Cr (VI) removal: investigation of the leading biomolecules. Chemosphere 150:357–364
Yuan W, Cheng J, Huang H, Xiong S, Gao J, Zhang J, Feng S (2019) Optimization of cadmium biosorption by Shewanella putrefaciens using a Box-Behnken design. Ecotoxicol Environ Saf 175:138–147
Wang T, Sun H (2013) Biosorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis. Environ Sci Pollut Res 20(10):7450–7463
Argun ME, Dursun S, Karatas M, Gürü M (2008) Activation of pine cone using Fenton oxidation for Cd (II) and Pb (II) removal. Bioresour Technol 99(18):8691–8698
Foletto EL, Weber CT, Bertuol DA, Mazutti MA (2013) Application of papaya seeds as a macro-/mesoporous biosorbent for the removal of large pollutant molecule from aqueous solution: equilibrium, kinetic, and mechanism studies. Sep Sci Technol 48(18):2817–2824
Omorogie MO, Babalola JO, Unuabonah EI, Song W, Gong JR (2016) Efficient chromium abstraction from aqueous solution using a low-cost biosorbent: Naucleadiderrichii seed biomass waste. J Saudi Chem Soc 20(1):49–57
Garcia-Reyes RB, Rangel-Mendez JR (2010) Adsorption kinetics of chromium (III) ions on agro-waste materials. Bioresour Technol 101(21):8099–8108
Ofomaja AE, Naidoo EB, Modise SJ (2009) Removal of copper (II) from aqueous solution by pine and base modified pine cone powder as biosorbent. J Hazard Mater 168(2–3):909–917
Cheruiyot GK, Wanyonyi WC, Kiplimo JJ, Maina EN (2019) Adsorption of toxic crystal violet dye using coffee husks: equilibrium, kinetics and thermodynamics study. Sci Afr 5:e00116
Banerjee S, Chattopadhyaya MC (2017) Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab J Chem 10:S1629–S1638
Nandi BK, Goswami A, Purkait MK (2009) Removal of cationic dyes from aqueous solutions by kaolin: kinetic and equilibrium studies. Appl Clay Sci 42(3–4):583–590
Shabaan OA, Jahin HS, Mohamed GG (2020) Removal of anionic and cationic dyes from wastewater by adsorption using multiwall carbon nanotubes. Arab J Chem 13(3):4797–4810
Saleh TA, Gupta VK (2012) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interface Sci 371(1):101–106
Yao Y, Xu F, Chen M, Xu Z, Zhu Z (2010) Adsorption behavior of methylene blue on carbon nanotubes. Bioresour Technol 101(9):3040–3046
Yao Y, Bing H, Feifei X, Xiaofeng C (2011) Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem Eng J 170(1):82–89
Ruthiraan M, Abdullah EC, Mubarak NM, Noraini MN (2017) A promising route of magnetic based materials for removal of cadmium and methylene blue from waste water. J Environ Chem Eng 5(2):1447–1455
Naushad M, Alqadami AA, AlOthman ZA, Alsohaimi IH, Algamdi MS, Aldawsari AM (2019) Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J Mol Liq 293:111442
Deniz F, Saygideger SD (2011) Removal of a hazardous azo dye (Basic Red 46) from aqueous solution by princess tree leaf. Desalination 268(1–3):6–11
Badmus MAO, Audu TOK, Anyata BU (2007) Removal of lead ion from industrial wastewaters by activated carbon prepared from periwinkle shells (Typanotonusfuscatus). Turk J Eng Environ Sci 31(4):251–263
Enniya I, Rghioui L, Jourani A (2018) Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain Chem Pharm 7:9–16
Malkoc E, Nuhoglu Y, Dundar M (2006) Adsorption of chromium (VI) on pomace—an olive oil industry waste: batch and column studies. J Hazard Mater 138(1):142–151
Rambabu K, Bharath G, Banat F, Show PL (2020) Biosorption performance of date palm empty fruit bunch wastes for toxic hexavalent chromium removal. Environ Res 187:109694
Rambabu K, Banat F, Nirmala GS, Velu S, Monash P, Arthanareeswaran G (2019) Activated carbon from date seeds for chromium removal in aqueous solution. Desalin Water Treat 156:267–277
Bagdonavicius V, Nikulin MS (2011) Chi-squared goodness-of-fit test for right censored data. Int J Appl Math Stat 24(1):1–11
Guedidi H, Reinert L, Soneda Y, Bellakhal N, Duclaux L (2017) Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab J Chem 10:S3584–S3594
Weng CH, Pan YF (2007) Adsorption of a cationic dye (methylene blue) onto spent activated clay. J Hazard Mater 144(1–2):355–362
Funding
The authors would like to thank the Algerian Ministry of High Education and the University of Bajaia for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akkari, I., Graba, Z., Bezzi, N. et al. Biosorption of Basic Red 46 using raw cactus fruit peels: equilibrium, kinetic and thermodynamic studies. Biomass Conv. Bioref. 14, 1825–1836 (2024). https://doi.org/10.1007/s13399-022-02354-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02354-y