Abstract
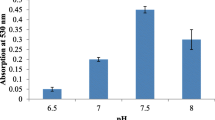
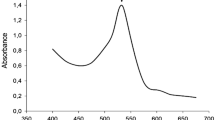
The bacterial secondary metabolite prodigiosin is known to possess some exceptional features that include its role as antimicrobial, antifungal, antiviral, anticancer, antimalarial, insecticidal, immunosuppressive, antidiabetic, and antiprotozoal. Besides, this bright red-hued pigment is also a potent biodye and a worthy replacement to the synthetic dyes which otherwise create havoc due to its non-biodegradable nature. However, the biosynthesis of this multifaceted compound is limited by its high-production cost and poor yield, which is largely due to the requirement of expensive commercial growth media. Therefore, devising formulations using cheaper raw materials that yet deliver the same or even better nutritive values is the need of the hour to promote bioproduction. In this study, soybean meal was primarily utilized as the sole source of nutrition for Serratia marcescens and 8.567 g/L prodigiosin was obtained when 2% w/v soybean meal medium was used which was 5.19-fold higher than the maximum titer achieved in the commercial growth media used in the study. To further enhance prodigiosin biosynthesis, the soybean meal medium was supplemented with sucrose and glycine, the concentration of which was optimized using the Box-Behnken design (BBD) model. Lastly, 9.632 g/L prodigiosin was obtained upon using soybean meal 2.477 % w/v; sucrose 0.864 % w/v; glycine 187.377 mg/L, and incubation time 96 h 2 min, all of which were optimized through the BBD model. The approach provides environment-friendly management, which is cost-effective and supports circular economy.






Similar content being viewed by others
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
References
Singh BP, Rateb ME, Rodriguez-Couto S, Polizeli M, Li WJ (2019) Editorial: microbial secondary metabolites: recent developments and technological challenges. Front Microbiol 10:914. https://doi.org/10.3389/fmicb.2019.00914
Jimtha CJ, Jishma P, Sreelekha S, Chithra S, Radhakrishnan E (2017) Antifungal properties of prodigiosin producing rhizospheric Serratia sp. Rhizosphere 3:105–108. https://doi.org/10.1016/j.rhisph.2017.02.003
Gohil N, Bhattacharjee G, Singh V (2020) Synergistic bactericidal profiling of prodigiosin extracted from Serratia marcescens in combination with antibiotics against pathogenic bacteria. Microb Pathog 149:104508. https://doi.org/10.1016/j.micpath.2020.104508
Bhatia SK, Lee BR, Sathiyanarayanan G, Song HS, Kim J, Jeon JM, Kim JH, Park SH, Yu JH, Park K, Yang YH (2016) Medium engineering for enhanced production of undecylprodigiosin antibiotic in Streptomyces coelicolor using oil palm biomass hydrolysate as a carbon source. Bioresour Technol 217:141–149. https://doi.org/10.1016/j.biortech.2016.02.055
Zhou W, Zeng C, Liu R, Chen J, Li R, Wang X, Bai W, Liu X, Xiang T, Zhang L, Wan Y (2016) Antiviral activity and specific modes of action of bacterial prodigiosin against Bombyx mori nucleopolyhedrovirus in vitro. Appl Microbiol Biotechnol 100:3979–3988. https://doi.org/10.1007/s00253-015-7242-5
Lin C, Jia X, Fang Y, Chen L, Zhang H, Lin R, Chen J (2019) Enhanced production of prodigiosin by Serratia marcescens FZSF02 in the form of pigment pellets. Electron J Biotechnol 40:58–64. https://doi.org/10.1016/j.ejbt.2019.04.007
Arivizhivendhan KV, Mahesh M, Boopathy R, Patchaimurugan K, Maharaja P, Swarnalatha S, Regina Mary R, Sekaran G (2016) Synthesis of surface-modified iron oxides for the solvent-free recovery of bacterial bioactive compound prodigiosin and its algicidal activity. J Phys Chem B 120:9685–9696. https://doi.org/10.1021/acs.jpcb.6b03926
Liang TW, Chen SY, Chen YC, Chen CH, Yen YH, Wang SL (2013) Enhancement of prodigiosin production by Serratia marcescens TKU011 and its insecticidal activity relative to food colorants. J Food Sci 78:M1743-1751. https://doi.org/10.1111/1750-3841.12272
Han SB, Kim HM, Kim YH, Lee CW, Jang ES, Son KH, Kim SU, Kim YK (1998) T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. Int J Immunopharmacol 20:1–13. https://doi.org/10.1016/s0192-0561(97)00062-3
Sajjad W, Ahmad S, Aziz I, Azam SS, Hasan F, Shah AA (2018) Antiproliferative, antioxidant and binding mechanism analysis of prodigiosin from newly isolated radio-resistant Streptomyces sp. strain WMA-LM31. Mol Biol Rep 45:1787–1798. https://doi.org/10.1007/s11033-018-4324-3
Pradeep S, Sarath Josh MK, Balachandran S, Sudha Devi R, Sadasivam R, Thirugnanam PE, Doble M, Anderson RC, Benjamin S (2014) Achromobacter denitrificans SP1 produces pharmaceutically active 25C prodigiosin upon utilizing hazardous di(2-ethylhexyl)phthalate. Bioresour Technol 171:482–486. https://doi.org/10.1016/j.biortech.2014.08.077
Castro AJ (1967) Antimalarial activity of prodigiosin. Nature 213:903–904. https://doi.org/10.1038/213903a0
Darshan N, Manonmani HK (2015) Prodigiosin and its potential applications. J Food Sci Technol 52:5393–5407. https://doi.org/10.1007/s13197-015-1740-4
Giri AV, Anandkumar N, Muthukumaran G, Pennathur G (2004) A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol 4:11. https://doi.org/10.1186/1471-2180-4-11
Choi SY, Lim S, Yoon K-h, Lee JI, Mitchell RJ (2021) Biotechnological activities and applications of bacterial pigments violacein and prodigiosin. J Biol Eng 15:1–16. https://doi.org/10.1186/s13036-021-00262-9
Sreedharan HE, Harilal CC, Pradeep S (2020) Response surface optimization of prodigiosin production by phthalate degrading Achromobacter denitrificans SP1 and exploring its antibacterial activity. Prep Biochem Biotechnol 50:564–571. https://doi.org/10.1080/10826068.2020.1712659
Liu W, Yang J, Tian Y, Zhou X, Wang S, Zhu J, Sun D, Liu C (2021) An in situ extractive fermentation strategy for enhancing prodigiosin production from Serratia marcescens BWL1001 and its application to inhibiting the growth of Microcystis aeruginosa. Biochem Eng J 166:107836. https://doi.org/10.1016/j.bej.2020.107836
Pandey A, Soccol CR, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresour Technol 74:69–80. https://doi.org/10.1016/S0960-8524(99)00142-X
Voora V, Larrea C, Bermudez S (2020) Global Market Report: Soybeans. https://www.iisd.org/system/files/2020-10/ssi-global-market-report-soybean.pdf. Accessed 17 October 2021
Khambhati K, Gohil N, Bhattacharjee G, Panchasara H, Singh V (2019) An equation for biomimicking macromolecular crowding using Escherichia coli MG1655 strain. Biophys Chem 254:106244. https://doi.org/10.1016/j.bpc.2019.106244
Wei YH, Chen WC (2005) Enhanced production of prodigiosin-like pigment from Serratia marcescens SMdeltaR by medium improvement and oil-supplementation strategies. J Biosci Bioeng 99:616–622. https://doi.org/10.1263/jbb.99.616
Kalivoda EJ, Stella NA, Aston MA, Fender JE, Thompson PP, Kowalski RP, Shanks RM (2010) Cyclic AMP negatively regulates prodigiosin production by Serratia marcescens. Res Microbiol 161:158–167. https://doi.org/10.1016/j.resmic.2009.12.004
Chen WC, Yu WJ, Chang CC, Chang JS, Huang SH, Chang CH, Chen SY, Chien CC, Yao CL, Chen WM (2013) Enhancing production of prodigiosin from Serratia marcescens C3 by statistical experimental design and porous carrier addition strategy. Biochem Eng J 78:93–100. https://doi.org/10.1016/j.bej.2013.02.001
Gulani C, Bhattacharya S, Das A (2012) Assessment of process parameters influencing the enhanced production of prodigiosin from Serratia marcescens and evaluation of its antimicrobial, antioxidant and dyeing potentials. Malays J Microbiol 8:116–122. https://doi.org/10.21161/mjm.03612
Van Eys J, Offner A, Bach A (2004) Manual of quality analyses for soybean products in the feed industry. https://ussec.org/wp-content/uploads/2012/09/Manual-of-Quality-Analyses-2nd-edition.pdf. Accessed 17 October 2021
Banaszkiewicz T (2011) Nutritional value of soybean meal. In: El-Shemy H (ed) Soybean and nutrition. IntechOpen, pp 1–20. https://doi.org/10.5772/23306
Mukherjee R, Chakraborty R, Dutta A (2016) Role of fermentation in improving nutritional quality of soybean meal - a review. Asian-Australas J Anim Sci 29:1523–1529. https://doi.org/10.5713/ajas.15.0627
Su WT, Tsou TY, Liu HL (2011) Response surface optimization of microbial prodigiosin production from Serratia marcescens. J Taiwan Inst Chem Eng 42:217–222. https://doi.org/10.1016/j.jtice.2010.05.009
de Araujo HW, Fukushima K, Takaki GM (2010) Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules 15:6931–6940. https://doi.org/10.3390/molecules15106931
Scott RH, Qadri SM, Williams RP (1976) Role of L-proline in the biosynthesis of prodigiosin. Appl Environ Microbiol 32:561–566. https://doi.org/10.1128/aem.32.4.561-566.1976
Tao JL, Wang XD, Shen YL, Wei DZ (2005) Strategy for the improvement of prodigiosin production by a Serratia marcescens mutant through fed-batch fermentation. World J Microbiol Biotechnol 21:969–972. https://doi.org/10.1007/s11274-004-7257-z
Fender JE, Bender CM, Stella NA, Lahr RM, Kalivoda EJ, Shanks RM (2012) Serratia marcescens quinoprotein glucose dehydrogenase activity mediates medium acidification and inhibition of prodigiosin production by glucose. Appl Environ Microbiol 78:6225–6235. https://doi.org/10.1128/AEM.01778-12
Stella NA, Fender JE, Lahr RM, Kalivoda EJ, Shanks RM (2012) The LysR transcription factor, HexS, is required for glucose inhibition of prodigiosin production by Serratia marcescens. Adv Microbiol 2:511–517. https://doi.org/10.4236/aim.2012.24065
Suryawanshi RK, Patil CD, Borase HP, Salunke BK, Patil SV (2014) Studies on production and biological potential of prodigiosin by Serratia marcescens. Appl Biochem Biotechnol 173:1209–1221. https://doi.org/10.1007/s12010-014-0921-3
Abdul Manas NH, Chong LY, Tesfamariam YM, Zulkharnain A, Mahmud H, AbangMahmod DS, Mohamad Fuzi SFZ, Wan Azelee NI (2020) Effects of oil substrate supplementation on production of prodigiosin by Serratia nematodiphila for dye-sensitized solar cell. J Biotechnol 317:16–26. https://doi.org/10.1016/j.jbiotec.2020.04.011
Elkenawy NM, Yassin AS, Elhifnawy HN, Amin MA (2017) Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol Rep (Amst) 14:47–53. https://doi.org/10.1016/j.btre.2017.04.001
Panesar R, Kaur S, Panesar PS (2015) Production of microbial pigments utilizing agro-industrial waste: a review. Curr Opin Food Sci 1:70–76. https://doi.org/10.1016/j.cofs.2014.12.002
Montaner B, Navarro S, Pique M, Vilaseca M, Martinell M, Giralt E, Gil J, Perez-Tomas R (2000) Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Pharmacol 131:585–593. https://doi.org/10.1038/sj.bjp.0703614
Sun Y, Wang L, Pan X, Osire T, Fang H, Zhang H, Yang ST, Yang T, Rao Z (2020) Improved prodigiosin production by relieving CpxR temperature-sensitive inhibition. Front Bioeng Biotechnol 8:344. https://doi.org/10.3389/fbioe.2020.00344
Kurbanoglu EB, Ozdal M, Ozdal OG, Algur OF (2015) Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz J Microbiol 46:631–637. https://doi.org/10.1590/S1517-838246246220131143
Wang SL, Wang CY, Yen YH, Liang TW, Chen SY, Chen CH (2012) Enhanced production of insecticidal prodigiosin from Serratia marcescens TKU011 in media containing squid pen. Process Biochem 47:1684–1690. https://doi.org/10.1016/j.procbio.2011.07.010
Soybean meal (2021) Market Insider. https://markets.businessinsider.com/commodities/soybean-meal-price. Accessed 25 October 2021
Sucrose (2021) Merck, Catalogue no. S9378-1KG, CAS no. 57-50-1. https://www.sigmaaldrich.com/US/en/product/sigma/s9378?context=product. Accessed 25 October 2021
Glycine (2021) Merck, Catalogue no. W328707-1KG, CAS no. 54-40-6. https://www.sigmaaldrich.com/US/en/product/aldrich/w328707?context=product. Accessed 25 October 2021
Gohil N, Bhattacharjee G, Gayke M, Narode H, Alzahrani KJ, Singh V (2021) Enhanced production of violacein by Chromobacterium violaceum using agro-industrial waste soybean meal. J Appl Microbiol. https://doi.org/10.1111/jam.15277
Acknowledgements
N.G., G.B., R.K., V.P., K.K., J.G., R.M., and V.S. thank Indrashil University, Rajpur, Mehsana, India for providing the infrastructure facility to carry out this study.
Funding
N.G. acknowledges the Indian Council of Medical Research, Government of India for financial assistance as Senior Research Fellowship (File No. 5/3/8/63/ITR-F/2020). The financial support from Gujarat State Biotechnology Mission (GSBTM) (Project ID: 5LY45F), Gujarat, India to G.B. and V.S. is duly acknowledged. R.K. and V.P. gratefully acknowledge the Education Department, Government of Gujarat for the SHODH fellowship. K.J.A. acknowledges a grant from Taif University Researchers Supporting Program (Project number: TURSP-2020/128), Taif University, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The present work has been applied for Indian patent vide number IN202121025727 dated 09.06.2021.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gohil, N., Bhattacharjee, G., Kalariya, R. et al. Biovalorization of agro-industrial waste soybean meal for the production of prodigiosin by Serratia marcescens. Biomass Conv. Bioref. (2021). https://doi.org/10.1007/s13399-021-02102-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-021-02102-8