Abstract
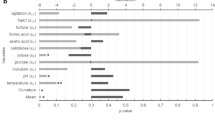
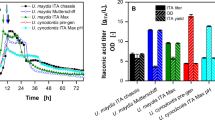
This study reports the biorefinery potential of a newly isolated yeast, Clavispora lusitaniae JARR-1, for production of erythritol as a sweet low-calorie food additive, along with co-production of ethanol as a biofuel candidate. The co-production process for synthesis of ethanol and erythritol was optimized using two-stage response surface methodology employing Placket-Burman (PB) and Central Composite Rotatable (CCR) designs. PB design indicated that the medium components, glucose, peptone, urea, and MgSO4, significantly affected (p < 0.05) erythritol production. Furthermore, optimization of erythritol and ethanol co-production process by CCR design revealed the significant (p < 0.05) interactive effects of glucose and peptone on erythritol production and glucose, peptone, and MgSO4 on ethanol production. Under optimized co-production process, maximum titers of erythritol and ethanol were 22.02 ± 0.04 g/L and 68.00 ± 0.02 g/L, respectively. The optimized process resulted in fivefold increase in erythritol production. Thus, C. lusitaniae JARR-1 has potential for sustainable co-production of erythritol and ethanol using biorefinery approach.
Graphical abstract

Highlights
• Erythritol production by Clavispora lusitaniae JARR-1
• Erythritol and ethanol co-production optimization by RSM
• fivefold erythritol production (from 4.2 ± 0.19 to 22.02 ± 0.04 g/L) after optimization
• Glucose (20% wt.) & peptone (3% wt.) significantly affected erythritol production
• Maximum ethanol co-production of 68.00 ± 0.02 g/L






Similar content being viewed by others
References
Billaux MS, Flourie B, Jacquemin C, Messing B (1991). Sugar alcohols. In: Marie S, Piggott JR (eds) Handbook of sweeteners, Springer, Boston, MA, pp 72–103. https://doi.org/10.1007/978-1-4757-5380-6
Nakagawa Y, Kasumi T, Ogihara J, Tamura M, Arai T, Tomishige K (2020) Erythritol: another C4 platform chemical in biomass refinery. ACS Omega 5:2520–2530. https://doi.org/10.1021/acsomega.9b04046
Savergave LS, Gadre RV, Vaidya BK, Narayanan K (2011) Strain improvement and statistical media optimization for enhanced erythritol production with minimal by-products from Candida magnoliae mutant R23. Biochem Eng J 55:92–100. https://doi.org/10.1016/j.bej.2011.03.009
Yang LB, Zhan XB, Zhu L, Gao MJ, Lin CC (2016) Optimization of a low-cost hyperosmotic medium and establishing the fermentation kinetics of erythritol production by Yarrowia lipolytica from crude glycerol. Prep Biochem Biotechnol 46:376–383. https://doi.org/10.1080/10826068.2015.1045604
Xiaoyan L, Yu X, Lv J, Xu J, Xia J, Wu Z, Zhang T, Deng Y (2017) A cost-effective process for the coproduction of erythritol and lipase with Yarrowia lipolytica M53 from waste cooking oil. Food Bioprod Process 103:86–94. https://doi.org/10.1016/j.fbp.2017.03.002
Rakicka-Pustułka M, Mirończuk AM, Celińska E, Białas W, Rymowicz W (2020) Scale-up of the erythritol production technology–process simulation and techno-economic analysis. J Clean Prod 257:120533. https://doi.org/10.1016/j.jclepro.2020.120533
Yu JH, Lee DH, Oh YJ, Han KC, Ryu YW, Seo JH (2006) Selective utilization of fructose to glucose by Candida magnoliae, an erythritol producer. Appl Biochem Biotechnol 131:870–879. https://doi.org/10.1385/ABAB:131:1:870
Veiga-Da-Cunha M, Firme P, San Romão MV, Santos H (1992) Application of 13C nuclear magnetic resonance to elucidate the unexpected biosynthesis of erythritol by Leuconostoc oenos. Appl Environ Microbiol 58:2271–2279. https://doi.org/10.1128/AEM.58.7.2271-2279.1992
Lee KJ, Lim JY (2003) Optimized conditions for high erythritol production by Penicillium sp. KJ-UV29, mutant of Penicillium sp. KJ81. Biotechnol Bioprocess Eng 8:173–178. https://doi.org/10.1007/BF02935892
Lee JK, Ha SJ, Kim SY, Oh DK (2000) Increased erythritol production in Torula sp. by Mn2+ and Cu2+. Biotechnol Lett 22:983–986. https://doi.org/10.1023/A:1005672801826
Jeya M, Lee KM, Tiwari MK, Kim JS, Gunasekaran P, Kim SY, Kim IW, Lee JK (2009) Isolation of a novel high erythritol-producing Pseudozyma tsukubaensis and scale-up of erythritol fermentation to industrial level. Appl Microbiol Biotechnol 83:225–231. https://doi.org/10.1007/s00253-009-1871-5
Ishizuka H, Wako K, Kasumi T, Sasaki T (1989) Breeding of a mutant of Aureobasidium sp. with high erythritol production. J. Ferment. Bioeng. 68(5) 310–314. https://doi.org/10.1016/0922-338X(89)90003-2
Kim KA, Noh BS, Lee JK, Kim SY, Park YC, Oh DK (2000) Optimization of culture conditions for erythritol production by Torula sp. J Microbiol Biotech 10:69–74
Kobayashi Y, Sahara T, Suzuki T, Kamachi S, Matsushika A, Hoshino T, Ohgiya S, Kamagata Y, Fujimori KE (2017) Genetic improvement of xylose metabolism by enhancing the expression of pentose phosphate pathway genes in Saccharomyces cerevisiae IR-2 for high-temperature ethanol production. J Ind Microbiol Biotechnol 44(6):879–891. https://doi.org/10.1007/s10295-017-1912-5
Lin SJ, Wen CY, Wang PM, Huang JC, Wei CL, Chang JW, Chu WS (2010) High-level production of erythritol by mutants of osmophilic Moniliella sp. Process Biochem 45:973–979. https://doi.org/10.1016/j.procbio.2010.03.003
Li L, Yang T, Guo W et al (2015) Construction of an efficient mutant strain of Trichosporonoides oedocephalis with HOG1 gene deletion for production of erythritol. J Microbiol Biotechnol 26:700–709
Carly F, Gamboa-Melendez H, Vandermies M, Damblon C, Nicaud JM, Fickers P (2017) Identification and characterization of EYK1, a key gene for erythritol catabolism in Yarrowia lipolytica. Appl Microbiol Biotechnol 101:6587–6596. https://doi.org/10.1007/s00253-017-8361-y
Mirończuk AM, Biegalska A, Dobrowolski A (2017) Functional overexpression of genes involved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotechno Biofuels 10:1–12. https://doi.org/10.1186/s13068-017-0772-6
Janek T, Dobrowolski A, Biegalska A, Mirończuk AM (2017) Characterization of erythrose reductase from Yarrowia lipolytica and its influence on erythritol synthesis. Microb Cell Factories 16:1–13. https://doi.org/10.1186/s12934-017-0733-6
Dargahi A, Samarghandi MR, Shabanloo A, et al. (2021) Statistical modeling of phenolic compounds adsorption onto low-cost adsorbent prepared from aloe vera leaves wastes using CCD-RSM optimization: effect of parameters, isotherm, and kinetic studies. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-021-01601-y
Rymowicz W, Rywińska A, Gładkowski W (2008) Simultaneous production of citric acid and erythritol from crude glycerol by Yarrowia lipolytica Wratislavia K1. Chem Pap 62:239–246. https://doi.org/10.2478/s11696-008-0018-y
Dey, P., Rangarajan, V., Singh, J., Nayak, J., & Dilip, K. J. (2021). Current perspective on improved fermentative production and purification of fungal cellulases for successful biorefinery applications: a brief review. Biomass Convvers Biorefin, 1–29.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Jadhav AS, Raut PD (2014) Evaluation of microbiological quality of ice creams marketed in Kolhapur city, Maharashtra, India. Int J Curr Microbiol App Sci 3:78–84
Lima GB, Lucas MR, Rosa CA, Gomes FC (2016) Analysis of the microbial quality of commercialized tropical fruit ice cream in Belo Horizonte, Brazil. J Agroaliment Processes Technol 22:79–86
Maciel NO, Piló FB, Freitas LF, Gomes FC, Johann S, Nardi RM, Lachance MA, Rosa CA (2013) The diversity and antifungal susceptibility of the yeasts isolated from coconut water and reconstituted fruit juices in Brazil. Int J Food Microbiol 160:201–205. https://doi.org/10.1016/j.ijfoodmicro.2012.10.012
Lachance MA, Clavispora Rodrigues de Miranda (1979), In: The Yeasts, Elsevier, pp. 349–353.
Park JB, Seo BC, Kim JR, Park YK (1998) Production of erythritol in fed-batch cultures of Trichosporon sp. J Ferment Bioeng 86:577–580
Ishizuka H, Wako K, Kasumi T, Sasaki T (1989) Breeding of a mutant of Aureobasidium sp. with high erythritol production. J Ferment Bioeng 68:310–314
Tomaszewska L (2012) Peptone as a nitrogen source for erythritol production from glycerol by Yarrowia lipolytica yeast. Challenges of Modern Technology 3.
Tomaszewska L, Rymowicz W, Rywińska A (2014) Mineral supplementation increases erythrose reductase activity in erythritol biosynthesis from glycerol by Yarrowia lipolytica. Appl Biochem Biotechnol 172:3069–3078. https://doi.org/10.1007/s12010-014-0745-1
Li L, Kang P, Ju X, Chen J, Zou H, Hu C, Yan L (2018) Enhancement of erythritol production by Trichosporonoides oedocephalis ATCC 16958 through regulating key enzyme activity and the NADPH/NADP ratio with metal ion supplementation. Preparative Biochem Biotechnol 48:257–263. https://doi.org/10.1080/10826068.2018.1425712
Guo J, Li J, Chen Y, Guo X, Xiao D (2016) Improving erythritol production of Aureobasidium pullulans from xylose by mutagenesis and medium optimization. Appl Biochem Biotechnol 180:717–727. https://doi.org/10.1007/s12010-016-2127-3
Kim SY, Lee KH, Kim JH, Oh DK (1997) Erythritol production by controlling osmotic pressure in Trigonopsis variabilis. Biotechnol Lett 19:727–729. https://doi.org/10.1023/A:1018371722456
Nigam JN, Margaritis A, Lachance MA (1985) Aerobic fermentation of D-xylose to ethanol by Clavispora sp. Appl Environ Microbiol 50:763–766
Liu ZL, Cotta MA (2015) Technical assessment of cellulosic ethanol production using β-glucosidase producing yeast Clavispora NRRL Y-50464. BioEnerg Res 8(3):1203–1211
Ryu YW, Park CY, Park JB, Kim SY, Seo JH (2000) Optimization of erythritol production by Candida magnoliae in fed-batch culture. J Ind Microbiol Biotechnol 25:100–103. https://doi.org/10.1038/sj.jim.7000039
Freer SN, Greene RV (1990) Transport of glucose and cellobiose by Candida wickerhamii and Clavispora lusitaniae. J Biol Chem 265:12864–12868. https://doi.org/10.1016/S0021-9258(19)38239-0
Jovanović B, Mach RL, Mach-Aigner AR (2014) Erythritol production on wheat straw using Trichoderma reesei. AMB Expr 4:34. https://doi.org/10.1186/s13568-014-0034-y
Mirończuk AM, Rakicka M, Biegalska A, Rymowicz W, Dobrowolski A (2015) A two-stage fermentation process of erythritol production by yeast Y. lipolytica from molasses and glycerol. Bioresour Technol 198:445–455. https://doi.org/10.1016/j.biortech.2015.09.008
Acknowledgements
The authors thank the University Grants Commission (UGC), Govt. of India, for providing financial support under Dr. D.S. Kothari post-doctoral fellowship scheme and the Central University of Haryana, Mahendergarh, for infrastructural support. JKS acknowledges financial support from SERB-DST (grant no. ECR/2016/000929/LS). H acknowledges CSIR-UGC for her NET-junior research fellowship. Authors also acknowledge Dr. Rishi Gupta for providing his inputs in designing the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shukla, R., Hemansi, Kumar, G. et al. Biorefinery potential of newly isolated yeast Clavispora lusitaniae for co-production of erythritol and ethanol. Biomass Conv. Bioref. 13, 8061–8073 (2023). https://doi.org/10.1007/s13399-021-02073-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02073-w