Abstract
The expertise of synthetic organic chemistry accumulated over the past century has been instrumental in converting biomass to fuels, chemicals, and materials. Particular emphasis has been attributed to using eco-friendly reagents and reaction conditions by adhering to the principles of green chemistry. Catalysis remains at the heart of organic synthesis and has a ubiquitous presence in the organic chemistry literature. Not surprisingly, catalytic processes are increasingly used in the chemistry of renewables under commercially relevant and environmentally acceptable conditions. In this review, the synthesis of various biofuels and renewable chemicals from biomass-derived furfural and 5-(hydroxymethyl)furfural has been elaborated. Synthetic upgrading of furfurals has been shown in the light of chemical modifications of the reactive sites present in them. This review aims to provide a critical understanding of the influence of synthetic organic chemistry in biomass value addition via the furanic platform. This work will encourage the researchers to improve the existing synthetic pathways, develop new synthetic strategies, and broaden the scope of applications for biorenewable products.
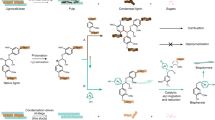
Graphical abstract












Similar content being viewed by others
Change history
22 September 2021
The Graphical Abstract, Schemes 1 and 7 have been replaced with the updated version.
References
Nicolaou KC (2014) Organic synthesis: the art and science of replicating the molecules of living nature and creating others like them in the laboratory. Proc R Soc A 470:20130690. https://doi.org/10.1098/rspa.2013.0690
Wittcoff HA, Reuben BG, Plotkin JS (2012) Chemicals from natural gas and petroleum. In: Industrial Organic Chemicals. John Wiley & Sons, Ltd, pp 93–138. https://doi.org/10.1002/9781118229996.ch4
Roddy DJ (2013) Biomass in a petrochemical world. Interface Focus 3:20120038. https://doi.org/10.1098/rsfs.2012.0038
Lee RP (2019) Alternative carbon feedstock for the chemical industry?-assessing the challenges posed by the human dimension in the carbon transition. J Cleaner Prod 219:786–796. https://doi.org/10.1016/j.jclepro.2019.01.316
Govil T, Wang J, Samanta D, David A, Tripathi A, Rauniyar S, Salem DR, Sani RK (2020) Lignocellulosic feedstock: a review of a sustainable platform for cleaner production of nature’s plastics. J Cleaner Prod 270:122521. https://doi.org/10.1016/j.jclepro.2020.122521
Khoo HH, Ee WL, Isoni V (2016) Bio-chemicals from lignocellulose feedstock: sustainability, LCA and the green conundrum. Green Chem 18:1912–1922. https://doi.org/10.1039/C5GC02065D
Mayer RA (2016) Handbook of petroleum refining processes, 4th ed. McGraw-Hill Education
Corma A, Corresa E, Mathieu Y, Sauvanaud L, Al-Bogami S, Al-Ghrami MS, Bourane A, (2017) Crude oil to chemicals: light olefins from crude oil. Catal Sci Technol 7:12–46. https://doi.org/10.1039/C6CY01886F
Levi PG, Cullen JM (2018) Mapping global flows of chemicals: from fossil fuel feedstocks to chemical products. Environ Sci Technol 52:1725–1734. https://doi.org/10.1021/acs.est.7b04573
Maitlis P, Haynes A (2006) Syntheses based on carbon monoxide. In: Metal-catalysis in Industrial Organic Processes. pp 114–162. https://doi.org/10.1039/9781847555328-00114
Sheldon RA, Arends WCE, Hanefeld U (2007) Introduction: green chemistry and catalysis. In: Green Chemistry and Catalysis. John Wiley & Sons, Ltd, pp 1–47. https://doi.org/10.1002/9783527611003.ch1
de Jong E, Jungmeier G (2015) Biorefinery concepts in comparison to petrochemical refineries. In: Industrial biorefineries & white biotechnology. Elsevier, pp 3–33. https://doi.org/10.1016/B978-0-444-63453-5.00001-X
Yadav VG, Yadav GD, Patankar SC (2020) The production of fuels and chemicals in the new world: critical analysis of the choice between crude oil and biomass vis-à-vis sustainability and the environment. Clean Techn Environ Policy 22:1757–1774. https://doi.org/10.1007/s10098-020-01945-5
Isikgor HF, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559. https://doi.org/10.1039/C5PY00263J
Putro JN, Soetaredjo FE, Lin S-Y, Ju Y-H, Ismadji S (2016) Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv 6:46834–46852. https://doi.org/10.1039/C6RA09851G
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. https://doi.org/10.1021/ie801542g
Delidovich I, Leonhard K, Palkovits R (2014) Cellulose and hemicellulose valorisation: an integrated challenge of catalysis and reaction engineering. Energy Environ Sci 7:2803–2830. https://doi.org/10.1039/C4EE01067A
Mei Q, Shen X, Liu H, Han B (2019) Selectively transform lignin into value-added chemicals. Chin Chem Lett 30:15–24. https://doi.org/10.1016/j.cclet.2018.04.032
Rosatella AA, Simeonov SP, Frade RFM, Afonso CAM (2011) 5-Hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications. Green Chem 13:754–793. https://doi.org/10.1039/C0GC00401D
Mittal A, Pilath HM, Johnson DK (2020) Direct conversion of biomass carbohydrates to platform chemicals: 5-hydroxymethylfurfural (HMF) and furfural. Energy Fuels 34:3284–3293. https://doi.org/10.1021/acs.energyfuels.9b04047
Mascal M, Dutta S (2014) Chemical-catalytic approaches to the production of furfurals and levulinates from biomass. In: Nicholas KM (ed) Selective Catalysis for Renewable Feedstocks and Chemicals. Springer International Publishing, pp 41–83. https://doi.org/10.1007/128_2014_536
Fan W, Verrier C, Queneau Y, Popowycz F (2019) 5-Hydroxymethylfurfural (HMF) in organic synthesis: a review of its recent applications towards fine chemicals. Curr Org Synth 16:583–614. https://doi.org/10.2174/1570179416666190412164738
Menegazzo F, Ghedini E, Signoretto M (2018) 5-Hydroxymethylfurfural (HMF) production from real biomasses. Molecules 23:2201. https://doi.org/10.3390/molecules23092201
Luo Y, Li Z, Li X, Liu X, Fan J, Clark JH, Hu C (2019) The production of furfural directly from hemicellulose in lignocellulosic biomass: a review. Catal Today 319:14–24. https://doi.org/10.1016/j.cattod.2018.06.042
Danon B, Marcotullio G, de Jong W (2014) Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous media employing homogeneous catalysis. Green Chem 16:39–54. https://doi.org/10.1039/C3GC41351A
Xia H, Xu S, Hu H, An J, Li C (2018) Efficient conversion of 5-hydroxymethylfurfural to high-value chemicals by chemo- and bio-catalysis. RSC Adv 8:30875–30886. https://doi.org/10.1039/C8RA05308A
Mariscal R, Maireles-Torres P, Ojeda M, Sádaba I, Granados ML (2016) Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ Sci 9:1144–1189. https://doi.org/10.1039/C5EE02666K
Li S, Dong M, Yang J, Cheng X, Shen X, Liu S, Wang Z-Q, Gong X-Q, Liu H, Han B (2021) Selective hydrogenation of 5-(hydroxymethyl)furfural to 5-methylfurfural over single atomic metals anchored on Nb2O5. Nat Commun 12:584. https://doi.org/10.1038/s41467-020-20878-7
Sun G, An J, Hu H, Li C, Zuo S, Xia H (2019) Green catalytic synthesis of 5-methylfurfural by selective hydrogenolysis of 5-hydroxymethylfurfural over size-controlled Pd nanoparticle catalysts. Catal Sci Technol 9:1238–1244. https://doi.org/10.1039/C9CY00039A
Dutta S, Mascal M (2014) Novel pathways to 2,5-dimethylfuran via biomass-derived 5-(chloromethyl)furfural. ChemSusChem 7:3028–3030. https://doi.org/10.1002/cssc.201402702
Galkin KI, Ananikov VP (2019) Towards improved biorefinery technologies: 5-methylfurfural as a versatile C6 platform for biofuels development. ChemSusChem 12:185–189. https://doi.org/10.1002/cssc.201802126
Dai J (2021) Synthesis of 2,5-diformylfuran from renewable carbohydrates and its applications: a review. Green Energy Environ 6:22–32. https://doi.org/10.1016/j.gee.2020.06.013
Hong M, Min J, Wu S, Cui H, Zhao Y, Li J, Wang S (2019) Metal nitrate catalysis for selective oxidation of 5-hydroxymethylfurfural into 2,5-diformylfuran under oxygen atmosphere. ACS Omega 4:7054–7060. https://doi.org/10.1021/acsomega.9b00391
Lv G, Wang H, Yang Y, Deng T, Chen C, Zhu Y, Hou X (2016) Direct synthesis of 2,5-diformylfuran from fructose with graphene oxide as a bifunctional and metal-free catalyst. Green Chem 18:2302–2307. https://doi.org/10.1039/C5GC02794B
Mittal N, Nisola GM, Malihan LB, Seo JG, Lee S-P, Chung W-J (2014) Metal-free mild oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Korean J Chem Eng 31:1362–1367. https://doi.org/10.1007/s11814-014-0036-0
Zhang W, Meng T, Tang J, Zhuang W, Zhou Y, Wang J (2017) Direct synthesis of 2,5-diformylfuran from carbohydrates using high-silica MOR zeolite-supported isolated vanadium species. ACS Sustainable Chem Eng 5:10029–10037. https://doi.org/10.1021/acssuschemeng.7b02002
Takagaki A, Takahashi M, Nishimura S, Ebitani K (2011) One-pot synthesis of 2,5-diformylfuran from carbohydrate derivatives by sulfonated resin and hydrotalcite-supported ruthenium catalysts. ACS Catal 1:1562–1565. https://doi.org/10.1021/cs200456t
Balakrishnan M, Sacia ER, Bell AT (2012) Etherification and reductive etherification of 5-(hydroxymethyl)furfural : 5-(alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates. Green Chem 14:1626–1634. https://doi.org/10.1039/C2GC35102A
Chen B, Yan G, Chen G, Feng Y, Zeng X, Sun Y, Tang X, Lei T, Lin L (2020) Recent progress in the development of advanced biofuel 5-ethoxymethylfurfural. BMC Energy 2:2. https://doi.org/10.1186/s42500-020-00012-5
Liu X, Wang R (2018) Upgrading of carbohydrates to the biofuel candidate 5-ethoxymethylfurfural (EMF). Int J Chem Eng 2018:e2316939. https://doi.org/10.1155/2018/2316939
Bing L, Zhang Z, Deng K (2012) Efficient one-pot synthesis of 5-(ethoxymethyl)furfural from fructose catalyzed by a novel solid catalyst. Ind Eng Chem Res 51:15331–15336. https://doi.org/10.1021/ie3020445
Alipour S, Omidvarborna H, Kim D-S (2017) A review on synthesis of alkoxymethyl furfural, a biofuel candidate. Renewable Sustainable Energy Rev 71:908–926. https://doi.org/10.1016/j.rser.2016.12.118
Mascal M, Nikitin EB (2008) Direct, high-yield conversion of cellulose into biofuel. Angew Chem 120:8042–8044. https://doi.org/10.1002/ange.200801594
Onkarappa SB, Dutta S (2019) High-yielding synthesis of 5-(alkoxymethyl)furfurals from biomass-derived 5-(halomethyl)furfural (X=Cl, Br). ChemistrySelect 4:5540–5543. https://doi.org/10.1002/slct.201900279
Shen H, Shan H, Liu L (2020) Evolution process and controlled synthesis of humins with 5-hydroxymethylfurfural (HMF) as model molecule. ChemSusChem 13:513–519. https://doi.org/10.1002/cssc.201902799
Portilla-Zuñiga OM, Martínez JJ, Casella M, Lick DI, Sathicq ÁG, Luque R, Romanelli GP (2020) Etherification of 5-hydroxymethylfurfural using a heteropolyacid supported on a silica matrix. Mol Catal 494:111125. https://doi.org/10.1016/j.mcat.2020.111125
Lee Y-K, Woo M-H, Kim C-H, Kim Y, Lee S-H, Jeong B-S, Chang H-W, Son J-K (2007) Two new benzofurans from gastrodia elata and their DNA topoisomerases I and II inhibitory activities. Planta Med 73:1287–1291. https://doi.org/10.1055/s-2007-981619
Quiroz-Florentino H, Hernández-Benitez RI, Aviña JA, Burgueño-Tapia E, Tamariz J (2011) Total synthesis of naturally occurring furan compounds 5-{[(4-hydroxybenzyl)oxy]methyl}-2-furaldehyde and pichiafuran C. Synthesis 2011:1106–1112. https://doi.org/10.1055/s-0030-1258455
Zhou X, Rauchfuss TB (2013) Production of hybrid diesel fuel precursors from carbohydrates and petrochemicals using formic acid as a reactive solvent. ChemSusChem 6:383–388. https://doi.org/10.1002/cssc.201200718
Ryabukhin DS, Zakusilo DN, Kompanets MO, Tarakanov AA, Boyarskaya IA, Artamonova TO, Khohodorkovskiy MA, Opeida IO, Vasilyev AV, (2016) Superelectrophilic activation of 5-hydroxymethylfurfural and 2,5-diformylfuran: organic synthesis based on biomass-derived products. Beilstein J Org Chem 12:2125–2135. https://doi.org/10.3762/bjoc.12.202
Arias KS, Climent MJ, Corma A, Iborra S (2015) Synthesis of high quality alkyl naphthenic kerosene by reacting an oil refinery with a biomass refinery stream. Energy Environ Sci 8:317–331. https://doi.org/10.1039/C4EE03194F
Shinde S, Deval K, Chikate R, Rode C (2018) Cascade synthesis of 5-(acetoxymethyl)furfural from carbohydrates over Sn-Mont catalyst. ChemistrySelect 3:8770–8778. https://doi.org/10.1002/slct.201802040
Zhang Z, Zhou P (2017) Catalytic aerobic oxidation of 5-hydroxymethylfurfural (HMF) into 2,5-furandicarboxylic acid and its derivatives. In: Fang Z, Smith Jr Richard L, Qi X (eds) Production of platform chemicals from sustainable resources. Springer, Singapore, pp 171–206. https://doi.org/10.1007/978-981-10-4172-3_6
Jia H-Y, Zong M-H, Zheng G-W, Li N (2019) One-pot enzyme cascade for controlled synthesis of furancarboxylic acids from 5-hydroxymethylfurfural by H2O2 internal recycling. ChemSusChem 12:4764–4768. https://doi.org/10.1002/cssc.201902199
Mei Z-W, Ma L-J, Kawafuchi H, Okihara T, Inokuchi T (2009) TEMPO-mediated oxidation of primary alcohols to carboxylic acids by exploitation of ethers in an aqueous–organic biphase system. Bull Chem Soc Jpn 82:1000–1002. https://doi.org/10.1246/bcsj.82.1000
Saha B, Dutta S, Abu-Omar MM (2012) Aerobic oxidation of 5-hydroxylmethylfurfural with homogeneous and nanoparticulate catalysts. Catal Sci Technol 2:79–81. https://doi.org/10.1039/C1CY00321F
Kumar K, Dahiya A, Patra T, Upadhyayula S (2018) Upgrading of HMF and biomass-derived acids into HMF esters using bifunctional ionic liquid catalysts under solvent free conditions. ChemistrySelect 3:6242–6248. https://doi.org/10.1002/slct.201800903
Benecke HP, King II JL, Kawczak AW, Zehnder II DW, Hirschl EE (2015) Esters of 5-hydroxymethylfurfural and methods for their preparation. U.S. Patent No. 8,247,582
Kang E-S, Hong Y-W, Chae DW, Kim B, Kim B, Kim YJ, Cho JK, Kim YG (2015) From lignocellulosic biomass to furans via 5-acetoxymethylfurfural as an alternative to 5-hydroxymethylfurfural. ChemSusChem 8:1179–1188. https://doi.org/10.1002/cssc.201403252
De S, Dutta S, Saha B (2012) One-pot conversions of lignocellulosic and algal biomass into liquid fuels. ChemSusChem 5:1826–1833. https://doi.org/10.1002/cssc.201200031
Dutta S (2020) Production of 5-(formyloxymethyl)furfural from biomass-derived sugars using mixed acid catalysts and upgrading into value-added chemicals. Carbohyd Res 497:108140. https://doi.org/10.1016/j.carres.2020.108140
Thananatthanachon T, Rauchfuss TB (2010) Efficient production of the liquid fuel 2,5-dimethylfuran from fructose using formic acid as a reagent. Angew Chem 122:6766–6768. https://doi.org/10.1002/anie.201002267
Anchan HN, Dutta S (2021) Recent advances in the production and value addition of selected hydrophobic analogs of biomass-derived 5-(hydroxymethyl)furfural. Biomass Convers Biorefin 1–23. https://doi.org/10.1007/s13399-021-01315-1
Sanda K, Rigal L, Gaset A (1989) Synthèse du 5-bromométhyl- et du 5-chlorométhyl-2-furannecarboxaldéhyde. Carbohyd Res 187:15–23. https://doi.org/10.1016/0008-6215(89)80052-7
Kumari N, Olesen JK, Pedersen CM, Bols M (2011) Synthesis of 5-bromomethylfurfural from cellulose as a potential intermediate for biofuel. Eur J Org Chem 2011:1266–1270. https://doi.org/10.1002/ejoc.201001539
Mascal M (2019) 5-(Chloromethyl)furfural (CMF): a platform for transforming cellulose into commercial products. ACS Sustainable Chem Eng 7:5588–5601. https://doi.org/10.1021/acssuschemeng.8b06553
Galkin KI, Krivodaeva EA, Romashov LV, Zalesskiy SS, Kachala VV, Burykina JV, Ananikov VP (2016) Critical influence of 5-hydroxymethylfurfural aging and decomposition on the utility of biomass conversion in organic synthesis. Angew Chem 128:8478–8482. https://doi.org/10.1002/ange.201602883
Mascal M, Dutta S (2011) Synthesis of the natural herbicide δ-aminolevulinic acid from cellulose-derived 5-(chloromethyl)furfural. Green Chem 13:40–41. https://doi.org/10.1039/C0GC00548G
Karlinskii BY, Romashov LV, Galkin KI, Kislitsyn PG, Ananikov VP (2019) Synthesis of 2-azidomethyl-5-ethynylfuran: a new bio-derived self-clickable building block. Synthesis 51:1235–1242. https://doi.org/10.1055/s-0037-1610414
Chang F, Hsu W-H, Mascal M (2015) Synthesis of anti-inflammatory furan fatty acids from biomass-derived 5-(chloromethyl)furfural. Sustainable Chem Pharm 1:14–18. https://doi.org/10.1016/j.scp.2015.09.002
Chang F, Dutta S, Becnel JJ, Estep AS, Mascal M (2014) Synthesis of the insecticide prothrin and its analogues from biomass-derived 5-(chloromethyl)furfural. J Agric Food Chem 62:476–480. https://doi.org/10.1021/jf4045843
Mascal M, Dutta S (2011) Synthesis of ranitidine (Zantac) from cellulose-derived 5-(chloromethyl)furfural. Green Chem 13:3101–3102. https://doi.org/10.1039/C1GC15537G
Mascal M (2015) 5-(Chloromethyl)furfural is the new HMF: functionally equivalent but more practical in terms of its production from biomass. ChemSusChem 8:3391–3395. https://doi.org/10.1002/cssc.201500940
Brandolese A, Ragno D, Carmine GD, Bernardi T, Bortolini O, Giovannini PP, Pandoli OG, Altomare A, Massi A (2018) Aerobic oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid and its derivatives by heterogeneous NHC-catalysis. Org Biomol Chem 16:8955–8964. https://doi.org/10.1039/C8OB02425A
Wang F, Zhang Z (2017) Cs-substituted tungstophosphate-supported ruthenium nanoparticles: an effective catalyst for the aerobic oxidation of 5-hydroxymethylfurfural into 5-hydroxymethyl-2-furancarboxylic acid. J Taiwan Inst Chem Eng 70:1–6. https://doi.org/10.1016/j.jtice.2016.10.003
Zhang Z, Liu B, Lv K, Sun J, Deng K (2014) Aerobic oxidation of biomass derived 5-hydroxymethylfurfural into 5-hydroxymethyl-2-furancarboxylic acid catalyzed by a montmorillonite K-10 clay immobilized molybdenum acetylacetonate complex. Green Chem 16:2762–2770. https://doi.org/10.1039/C4GC00062E
Donoeva B, Masoud N, de Jongh PE (2017) Carbon support surface effects in the gold-catalyzed oxidation of 5-hydroxymethylfurfural. ACS Catal 7:4581–4591. https://doi.org/10.1021/acscatal.7b00829
Zhao D, Rodriguez-Padron D, Luque R, Len C (2020) Insights into the selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid using silver oxide. ACS Sustainable Chem Eng 8:8486–8495. https://doi.org/10.1021/acssuschemeng.9b07170
Davis SE, Zope BN, Davis RJ (2012) On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem 14:143–147. https://doi.org/10.1039/C1GC16074E
Subbiah S, Simeonov SP, Esperança JMSS, Rebelo LPN, Afonso CAM (2013) Direct transformation of 5-hydroxymethylfurfural to the building blocks 2,5-dihydroxymethylfurfural (DHMF) and 5-hydroxymethyl furanoic acid (HMFA) via Cannizzaro reaction. Green Chem 15:2849–2853. https://doi.org/10.1039/C3GC40930A
Jiang Y, Woortman AJJ, Alberda van Ekenstein GOR, Petrović DM, Loos K (2014) Enzymatic synthesis of biobased polyesters using 2,5-bis(hydroxymethyl)furan as the building block. Biomacromol 15:2482–2493. https://doi.org/10.1021/bm500340w
Meng J, Zeng Y, Chen P, Zhang J, Yao C, Fang Z, Ouyang P, Guo K (2020) Flame retardancy and mechanical properties of bio-based furan epoxy resins with high crosslink density. Macromol Mater Eng 305:1900587. https://doi.org/10.1002/mame.201900587
Howell BA, Lazar ST (2019) Biobased plasticizers from carbohydrate-derived 2,5-bis(hydroxymethyl)furan. Ind Eng Chem Res 58:1222–1228. https://doi.org/10.1021/acs.iecr.8b05442
Wang T, Wei J, Liu H, Feng Y, Tang X, Zeng X, Sun Y, Lei T, Lin L (2020) Synthesis of renewable monomer 2,5-bishydroxymethylfuran from highly concentrated 5-hydroxymethylfurfural in deep eutectic solvents. J Ind Eng Chem 81:93–98. https://doi.org/10.1016/j.jiec.2019.08.057
Kucherov FA, Galkin KI, Gordeev EG, Ananikov VP (2017) Efficient route for the construction of polycyclic systems from bioderived HMF. Green Chem 19:4858–4864. https://doi.org/10.1039/C7GC02211E
Hu L, Xu J, Zhou S, He A, Tang X, Lin L, Xu J, Zhao Y (2018) Catalytic advances in the production and application of biomass-derived 2,5-dihydroxymethylfuran. ACS Catal 8:2959–2980. https://doi.org/10.1021/acscatal.7b03530
Zhang J, Wang T, Tang X, Peng L, Wei J, Lin L (2018) Methods in the synthesis and conversion of 2,5-bis-(hydroxylmethyl)furan from bio-derived 5-hydroxymethylfurfural and its great potential in polymerization. BioResources 13:7137–7154
Arias KS, Garcia-Ortiz A, Climent MJ, Corma A, Iborra S (2018) Mutual valorization of 5-hydroxymethylfurfural and glycerol into valuable diol monomers with solid acid catalysts. ACS Sustainable Chem Eng 6:4239–4245. https://doi.org/10.1021/acssuschemeng.7b04685
Kumar K, Pathak S, Upadhyayula S (2021) Acetalization of 5-hydroxymethyl furfural into biofuel additive cyclic acetal using protic ionic liquid catalyst- a thermodynamic and kinetic analysis. Renewable Energy 167:282–293. https://doi.org/10.1016/j.renene.2020.11.084
Kirchhecker S, Dell’Acqua A, Angenvoort A, Spannenberg A, Ito K, Tin S, Taden A, de Vries JG (2021) HMF–glycerol acetals as additives for the debonding of polyurethane adhesives. Green Chem 23:957–965. https://doi.org/10.1039/D0GC04093B
Lanzafame P, Papanikolaou G, Perathoner S, Centi G, Migliori M, Catizzone E, Aloise A, Giordano G (2018) Direct versus acetalization routes in the reaction network of catalytic HMF etherification. Catal Sci Technol 8:1304–1313. https://doi.org/10.1039/C7CY02339A
Nakagawa Y, Tamura M, Tomishige K (2019) Recent development of production technology of diesel- and jet-fuel-range hydrocarbons from inedible biomass. Fuel Process Technol 193:404–422. https://doi.org/10.1016/j.fuproc.2019.05.028
Wu L, Moteki T, Gokhale AA, Flaherty DW, Toste FD (2016) Production of fuels and chemicals from biomass: condensation reactions and beyond. Chem 1:32–58. https://doi.org/10.1016/j.chempr.2016.05.002
James OO, Maity S, Usman LA, Ajanaku KO, Ajani OO, Siyanbola TO, Sahu S, Chaubey R (2010) Towards the conversion of carbohydrate biomass feedstocks to biofuels via hydroxylmethylfurfural. Energy Environ Sci 3:1833–1850. https://doi.org/10.1039/B925869H
Yan B, Zang H, Jiang Y, Yu S, Chen EY-X (2016) Recyclable montmorillonite-supported thiazolium ionic liquids for high-yielding and solvent-free upgrading of furfural and 5-hydroxymethylfurfural to C10 and C12 furoins. RSC Adv 6:76707–76715. https://doi.org/10.1039/C6RA14594A
Cywar RM, Wang L, Chen EY-X (2019) Thermally regulated recyclable carbene catalysts for upgrading of biomass furaldehydes. ACS Sustainable Chem Eng 7:1980–1988. https://doi.org/10.1021/acssuschemeng.8b04190
Chang H, Motagamwala AH, Huber GW, Dumesic JA (2019) Synthesis of biomass-derived feedstocks for the polymers and fuels industries from 5-(hydroxymethyl)furfural (HMF) and acetone. Green Chem 21:5532–5540. https://doi.org/10.1039/C9GC01859J
Xia Q-N, Cuan Q, Liu X-H, Gong X-Q, Lu G-Z, Wang Y-Q (2014) Pd/NbOPO4 multifunctional catalyst for the direct production of liquid alkanes from aldol adducts of furans. Angew Chem, Int Ed 53:9755–9760. https://doi.org/10.1002/anie.201403440
Hronec M, Fulajtárova K, Liptaj T, Štolcová M, Prónayová N, Soták T (2014) Cyclopentanone: a raw material for production of C15 and C17 fuel precursors. Biomass Bioenergy 63:291–299. https://doi.org/10.1016/j.biombioe.2014.02.025
Xu Z, Yan P, Xu W, Jia S, Xia Z, Chung B, Zhang ZC (2014) Direct reductive amination of 5-hydroxymethylfurfural with primary/secondary amines via Ru-complex catalyzed hydrogenation. RSC Adv 4:59083–59087. https://doi.org/10.1039/C4RA10349A
Tan J-N, Ahmar M, Queneau Y (2013) HMF derivatives as platform molecules: aqueous Baylis-Hillman reaction of glucosyloxymethyl-furfural towards new biobased acrylates. RSC Adv 3:17649–17653. https://doi.org/10.1039/C3RA43369B
Yu C, Liu B, Hu L (2001) Efficient Baylis−Hillman reaction using stoichiometric base catalyst and an aqueous medium. J Org Chem 66:5413–5418. https://doi.org/10.1021/jo015628m
Huang Q, Yang F, Cao X, Hu Z, Cheng C (2019) Thermally healable polyurethanes based on furfural-derived monomers via Baylis-Hillman reaction. Macromol Res 27:895–904. https://doi.org/10.1007/s13233-019-7123-3
Han M, Liu X, Zhang X, Pang Y, Xu P, Guo J, Liu Y, Zhang S, Ji S (2017) 5-Hydroxymethyl-2-vinylfuran: a biomass-based solvent-free adhesive. Green Chem 19:722–728. https://doi.org/10.1039/C6GC02723G
Xu Z, Yan P, Liu K, Wan L, Xu W, Li H, Liu X, Zhang ZC (2016) Synthesis of bis(hydroxylmethylfurfuryl)amine monomers from 5-hydroxymethylfurfural. ChemSusChem 9:1255–1258. https://doi.org/10.1002/cssc.201600122
Sattler L, Zerban FW, Clark GL, Chu C-C (1951) The reaction of 2-aminobenzenethiol with Al-doses and with hydroxymethylfurfural. J Am Chem Soc 73:5908–5910. https://doi.org/10.1021/ja01156a554
Mitra J, Zhou X, Rauchfuss T (2014) Pd/C-catalyzed reactions of HMF: decarbonylation, hydrogenation, and hydrogenolysis. Green Chem 17:307–313. https://doi.org/10.1039/C4GC01520G
Dai J, Fu X, Zhu L, Tang J, Guo X, Hu C (2016) One-pot deoxygenation of fructose to furfuryl alcohol by sequential dehydration and decarbonylation. ChemCatChem 8:1379–1385. https://doi.org/10.1002/cctc.201501292
Huang Y-B, Yang Z, Chen M-Y, Dai J-J, Guo Q-X, Fu Y (2013) Heterogeneous palladium catalysts for decarbonylation of biomass-derived molecules under mild conditions. ChemSusChem 6:1348–1351. https://doi.org/10.1002/cssc.201300190
Wang H, Zhu C, Li D, Liu Q, Tan J, Wang C, Cai C, Ma L (2019) Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2,5-dimethylfuran. Renewable Sustainable Energy Rev 103:227–247. https://doi.org/10.1016/j.rser.2018.12.010
Xiao T, Liu X, Xu G, Zhang Y (2021) Phase tuning of ZrO2 supported cobalt catalysts for hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran under mild conditions. Appl Catal, B 295:120270. https://doi.org/10.1016/j.apcatb.2021.120270
Xu H, Wang C (2016) A comprehensive review of 2,5-dimethylfuran as a biofuel candidate. In: Biofuels from Lignocellulosic Biomass. John Wiley & Sons, Ltd, pp 105–129. https://doi.org/10.1002/9783527685318.ch5
Sajid M, Zhao X, Liu D (2018) Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): recent progress focusing on the chemical-catalytic routes. Green Chem 20:5427–5453. https://doi.org/10.1039/C8GC02680G
Sousa AF, Vilela C, Fonseca AC, Matos M, Freire CSR, Gruter G-JM, Coelho JFJ, Silvestre AJD (2015) Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: a tribute to furan excellency. Polym Chem 6:5961–5983. https://doi.org/10.1039/C5PY00686D
Zhang Z, Deng K (2015) Recent advances in the catalytic synthesis of 2,5-furandicarboxylic acid and its derivatives. ACS Catal 5:6529–6544. https://doi.org/10.1021/acscatal.5b01491
Masoud N, Donoeva B, de Jongh PE (2018) Stability of gold nanocatalysts supported on mesoporous silica for the oxidation of 5-hydroxymethyl furfural to furan-2,5-dicarboxylic acid. Appl Catal, A 561:150–157. https://doi.org/10.1016/j.apcata.2018.05.027
Wojcieszak R, Itabaiana I (2020) Engineering the future: perspectives in the 2,5-furandicarboxylic acid synthesis. Catal Today 354:211–217. https://doi.org/10.1016/j.cattod.2019.05.071
Komanoya T, Kinemura T, Kita Y, Kamata K, Hara M (2017) Electronic effect of ruthenium nanoparticles on efficient reductive amination of carbonyl compounds. J Am Chem Soc 139:11493–11499. https://doi.org/10.1021/jacs.7b04481
Pingen D, Schwaderer JB, Walter J, Wen J, Murray G, Vogt D, Mecking S (2018) Diamines for polymer materials via direct amination of lipid- and lignocellulose-based alcohols with NH3. ChemCatChem 10:3027–3033. https://doi.org/10.1002/cctc.201800365
Xu Y, Jia X, Ma J, Gao J, Xia F, Li X, Xu J (2018) Efficient synthesis of 2,5-dicyanofuran from biomass-derived 2,5-diformylfuran via an oximation–dehydration strategy. ACS Sustainable Chem Eng 6:2888–2892. https://doi.org/10.1021/acssuschemeng.7b03913
Jia X, Ma J, Xia F, Xu Y, Gao J, Xu J (2018) Carboxylic acid-modified metal oxide catalyst for selectivity-tunable aerobic ammoxidation. Nat Commun 9:933. https://doi.org/10.1038/s41467-018-03358-x
Lancien A, Wojcieszak R, Cuvelier E, Duban M, Dhulster P, Paul S, Dumeignil F, Froidevaux R, Houson E (2021) Hybrid conversion of 5-hydroxymethylfurfural to 5-aminomethyl-2-furancarboxylic acid: toward new bio-sourced polymers. ChemCatChem 13:247–259. https://doi.org/10.1002/cctc.202001446
Kraus GA, Lee JJ (2013) A direct synthesis of renewable sulfonate-based surfactants. J Surfact Deterg 16:317–320. https://doi.org/10.1007/s11743-012-1408-2
Dutta S, Bhat NS (2020) Catalytic synthesis of renewable p-xylene from biomass-derived 2,5-dimethylfuran: a mini review. Biomass Convers Biorefin https://doi.org/10.1007/s13399-020-01042-z
Tomás RAF, Bordado JCM, Gomes JFP (2013) p-Xylene oxidation to terephthalic acid: a literature review oriented toward process optimization and development. Chem Rev 113:7421–7469. https://doi.org/10.1021/cr300298j
Fei X, Wang J, Zhu J, Wang X, Liu X (2020) Biobased poly(ethylene 2,5-furancoate): no longer an alternative, but an irreplaceable polyester in the polymer industry. ACS Sustainable Chem Eng 8:8471–8485. https://doi.org/10.1021/acssuschemeng.0c01862
Nicklaus CM, Minnaard AJ, Feringa BL, de Vries JG (2013) Synthesis of renewable fine-chemical building blocks by reductive coupling between furfural derivatives and terpenes. ChemSusChem 6:1631–1635. https://doi.org/10.1002/cssc.201300179
Yan L, Greenwood AA, Hossain A, Yang B (2014) A comprehensive mechanistic kinetic model for dilute acid hydrolysis of switchgrass cellulose to glucose, 5-HMF and levulinic acid. RSC Adv 4:23492–23504. https://doi.org/10.1039/C4RA01631A
Liu C, Lu X, Yu Z, Xiong J, Bai H, Zhang R (2020) Production of levulinic acid from cellulose and cellulosic biomass in different catalytic systems. Catalysts 10:1006. https://doi.org/10.3390/catal10091006
Antonetti C, Licursi D, Fulignati S, Valentini G, Raspolli Galletti AM (2016) New frontiers in the catalytic synthesis of levulinic acid: from sugars to raw and waste biomass as starting feedstock. Catalysts 6:196. https://doi.org/10.3390/catal6120196
Pyo S-H, Glaser SJ, Rehnberg N, Hatti-Kaul R (2020) Clean production of levulinic acid from fructose and glucose in salt water by heterogeneous catalytic dehydration. ACS Omega 5:14275–14282. https://doi.org/10.1021/acsomega.9b04406
Dutta S, Bhat NS (2021) Recent advances in the value addition of biomass-derived levulinic acid: a review focusing on its chemical reactivity patterns. ChemCatChem 13:3202–3222. https://doi.org/10.1002/cctc.202100032
Pileidis FD, Titirici M-M (2016) Levulinic acid biorefineries: new challenges for efficient utilization of biomass. ChemSusChem 9:562–582. https://doi.org/10.1002/cssc.201501405
Girisuta B, Heeres HJ (2017) Levulinic acid from biomass: synthesis and applications. In: Fang Z, Smith Jr. RL (ed). Production of Platform Chemicals from Sustainable Resources. Springer, Singapore, pp 143–169. https://doi.org/10.1007/978-981-10-4172-3_5
Fulignati S, Antonetti C, Licursi D, Pieraccioni M, Wilbers E, Heeres HJ, Raspolli Galletti AM (2019) Insight into the hydrogenation of pure and crude HMF to furan diols using Ru/C as catalyst. Appl Catal, A 578:122–133. https://doi.org/10.1016/j.apcata.2019.04.007
Kong X, Zhu Y, Zheng H, Dong F, Zhu Y, Li Y-W (2014) Switchable synthesis of 2,5-dimethylfuran and 2,5-dihydroxymethyltetrahydrofuran from 5-hydroxymethylfurfural over Raney Ni catalyst. RSC Adv 4:60467–60472. https://doi.org/10.1039/C4RA09550B
Pellis A, Byrne FP, Sherwood J, Vastano M, Comerford JW, Farmer TJ (2019) Safer bio-based solvents to replace toluene and tetrahydrofuran for the biocatalyzed synthesis of polyesters. Green Chem 21:1686–1694. https://doi.org/10.1039/C8GC03567A
Yuan Q, Hiemstra K, Meinds TG, Chaabane I, Tang Z, Rohrbach L, Vrijburg W, Verhoeven T, Hensen EJM, van der Veer S, Pescarmona PP, Heeres HJ, Deuss PJ (2019) Bio-based chemicals: selective aerobic oxidation of tetrahydrofuran-2,5-dimethanol to tetrahydrofuran-2,5-dicarboxylic acid using hydrotalcite-supported gold catalysts. ACS Sustainable Chem Eng 7:4647–4656. https://doi.org/10.1021/acssuschemeng.8b03821
Cavani F, Centi G, Perathoner S, Triffiro F (2009) Sustainable industrial chemistry: principles, tools and industrial examples. Wiley
Gilkey MJ, Mironenko AV, Vlachos DG, Xu B (2017) Adipic acid production via metal-free selective hydrogenolysis of biomass-derived tetrahydrofuran-2,5-dicarboxylic acid. ACS Catal 7:6619–6634. https://doi.org/10.1021/acscatal.7b01753
Tuteja J, Choudhary H, Nishimura S, Ebitani K (2014) Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source. ChemSusChem 7:96–100. https://doi.org/10.1002/cssc.201300832
Ohyama J, Kanao R, Esaki A, Satsuma A (2014) Conversion of 5-hydroxymethylfurfural to a cyclopentanone derivative by ring rearrangement over supported Au nanoparticles. Chem Commun 50:5633–5636. https://doi.org/10.1039/C3CC49591D
Mliki K, Trabelsi M (2016) Efficient mild oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2(5H)-furanone, a versatile chemical intermediate. Res Chem Intermed 42:8253–8260. https://doi.org/10.1007/s11164-016-2593-9
Pischetsrieder M, Severin T (1994) Maillard reaction of maltose-Isolation of 4-(glucopyranosyloxy)-5-(hydroxymethyl)-2-methyl-3(2H)-furanone. J Agric Food Chem 42:890–892. https://doi.org/10.1021/jf00040a010
Cottier L, Descotes G, Eymard L, Rapp K (1995) Syntheses of γ-oxo acids or γ-oxo esters by photooxygenation of furanic compounds and reduction under ultrasound: application to the synthesis of 5-aminolevulinic acid hydrochloride. Synthesis 1995:303–306. https://doi.org/10.1055/s-1995-3897
Heugebaert TSA, Stevens CV, Kappe CO (2015) Singlet-oxygen oxidation of 5-hydroxymethylfurfural in continuous flow. ChemSusChem 8:1648–1651. https://doi.org/10.1002/cssc.201403182
Wang Y, Zhao D, Rodríguez-Padrón D, Len C (2019) Recent advances in catalytic hydrogenation of furfural. Catalysts 9:796. https://doi.org/10.3390/catal9100796
Iroegbu AO, Hlangothi SP (2019) Furfuryl alcohol a versatile, eco-sustainable compound in perspective. Chemistry Africa 2:223–239. https://doi.org/10.1007/s42250-018-00036-9
Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Rev 107:2411–2502. https://doi.org/10.1021/cr050989d
Adkins H, Connor R (1931) The catalytic hydrogenation of organic compounds over copper chromate. J Am Chem Soc 53:1091–1095. https://doi.org/10.1021/ja01354a041
Gong W, Chen C, Zhang Y, Zhou H, Wang H, Zhang H, Zhang Y, Wang G, Zhao H (2017) Efficient synthesis of furfuryl alcohol from H2-hydrogenation/transfer hydrogenation of furfural using sulfonate group modified Cu catalyst. ACS Sustainable Chem Eng 5:2172–2180. https://doi.org/10.1021/acssuschemeng.6b02343
Iqbal S, Liu X, Aldosari OF, Miedziak PJ, Edwards JK, Brett GL, Akram A, King GM, Davies TE, Morgan DJ, Knight DK, Hutchings GJ (2014) Conversion of furfuryl alcohol into 2-methylfuran at room temperature using Pd/TiO2 catalyst. Catal Sci Technol 4:2280–2286. https://doi.org/10.1039/C4CY00184B
Date NS, Hengne AM, Huang K-W, Chikate RC, Rode CV (2018) Single pot selective hydrogenation of furfural to 2-methylfuran over carbon supported iridium catalysts. Green Chem 20:2027–2037. https://doi.org/10.1039/C8GC00284C
Yan K, Chen A (2013) Efficient hydrogenation of biomass-derived furfural and levulinic acid on the facilely synthesized noble-metal-free Cu–Cr catalyst. Energy 58:357–363. https://doi.org/10.1016/j.energy.2013.05.035
Green SK, Patet RE, Nikbin N, Williams CL, Chang C-C, Yu J, Gorte RJ, Caratzoulas S, Fan W, Vlachos DG, Dauenhauer PJ (2016) Diels-Alder cycloaddition of 2-methylfuran and ethylene for renewable toluene. Appl Catal, B 180:487–496. https://doi.org/10.1016/j.apcatb.2015.06.044
Yan K, Chen A (2014) Selective hydrogenation of furfural and levulinic acid to biofuels on the ecofriendly Cu–Fe catalyst. Fuel 115:101–108. https://doi.org/10.1016/j.fuel.2013.06.042
Liu S, Saha B, Vlachos DG (2019) Catalytic production of renewable lubricant base oils from bio-based 2-alkylfurans and enals. Green Chem 21:3606–3614. https://doi.org/10.1039/C9GC01044K
Eldeeb MA, Akih-Kumgeh B (2018) Recent trends in the production, combustion and modeling of furan-based fuels. Energies 11:512. https://doi.org/10.3390/en11030512
Pace V, Hoyos P, Castoldi L, Domínguez de María P, Alcántara AR (2012) 2-Methyltetrahydrofuran (2-MeTHF): a biomass-derived solvent with broad application in organic chemistry. ChemSusChem 5:1369–1379. https://doi.org/10.1002/cssc.201100780
Lejemble P, Gaset A, Kalck P (1984) From biomass to furan through decarbonylation of furfural under mild conditions. Biomass 4:263–274. https://doi.org/10.1016/0144-4565(84)90039-8
Dau DX (2019) Recent progress in the synthesis of furan. Mini-Rev Org Chem 16:422–452. https://doi.org/10.2174/1570193X15666180608084557
Yuan Q, Pang J, Yu W, Zheng M (2020) Vapor-phase furfural decarbonylation over a high-performance catalyst of 1%Pt/SBA-15. Catalysts 10:1304. https://doi.org/10.3390/catal10111304
Jiménez-Gómez CP, Cecilia JA, García-Sancho C, Moreno-Tost R, Maireles-Torres P (2019) Selective production of furan from gas-phase furfural decarbonylation on Ni-MgO catalysts. ACS Sustainable Chem Eng 7:7676–7685. https://doi.org/10.1021/acssuschemeng.8b06155
Ishida T, Kume K, Kinjo K, Honma T, Nakada K, Ohashi H, Yokoyama T, Hamasaki A, Murayama H, Izawa Y, Utsunomiya M, Tokunaga M (2016) Efficient decarbonylation of furfural to furan catalyzed by zirconia-supported palladium clusters with low atomicity. ChemSusChem 9:3441–3447. https://doi.org/10.1002/cssc.201601232
Goossen LJ, Manjolinho F, Khan BA, Rodríguez N (2009) Microwave-assisted Cu-catalyzed protodecarboxylation of aromatic carboxylic acids. J Org Chem 74:2620–2623. https://doi.org/10.1021/jo802628z
Dupuy S, Nolan SP (2013) Gold(I)-catalyzed protodecarboxylation of (hetero)aromatic carboxylic acids. Chemistry–A European Journal 19:14034–14038. https://doi.org/10.1002/chem.201303200
Toy XY, Roslan IIB, Chuah GK, Jaenicke S (2014) Protodecarboxylation of carboxylic acids over heterogeneous silver catalysts. Catal Sci Technol 4:516–523. https://doi.org/10.1039/C3CY00580A
Li X, Jia P, Wang T (2016) Furfural: a promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal 6:7621–7640. https://doi.org/10.1021/acscatal.6b01838
Drault F, Snoussi Y, Paul S, Itabaiana Junoir I, Wojcieszak R (2020) Recent advances in carboxylation of furoic acid into 2,5-furandicarboxylic acid: pathways towards bio-based polymers. ChemSusChem 13:5164–5172. https://doi.org/10.1002/cssc.202001393
Moore JA, Partain III EM, (1985) Oxidation of furfuraldehydes with sodium chlorite. Org Prep Proced Int 17:203–205. https://doi.org/10.1080/00304948509355502
Douthwaite M, Huang X, Iqbal S, Miedziak PJ, Brett GL, Kondrat SA, Edwards JK, Sankar M, Knight DW, Bethell D, Hutchings GJ (2017) The controlled catalytic oxidation of furfural to furoic acid using AuPd/Mg(OH)2. Catal Sci Technol 7:5284–5293. https://doi.org/10.1039/C7CY01025G
Yu H, Ru S, Dai G, Zhai Y, Lin H, Han S, Wei Y (2017) An efficient iron(III)-catalyzed aerobic oxidation of aldehydes in water for the green preparation of carboxylic acids. Angew Chem, Int Ed 56:3867–3871. https://doi.org/10.1002/anie.201612225
Hajimohammadi M, Safari N, Mofakham H, Shaabani A (2010) A new and efficient aerobic oxidation of aldehydes to carboxylic acids with singlet oxygen in the presence of porphyrin sensitizers and visible light. Tetrahedron Lett 51:4061–4065. https://doi.org/10.1016/j.tetlet.2010.05.124
Khatana AK, Singh V, Gupta MK, Tiwari B (2018) A highly efficient NHC-catalyzed aerobic oxidation of aldehydes to carboxylic acids. Synthesis 50:4290–4294. https://doi.org/10.1055/s-0037-1610069
Zhang Y, Cheng Y, Cai H, He S, Shan Q, Zhao H, Chen Y, Wang B (2017) Catalyst-free aerobic oxidation of aldehydes into acids in water under mild conditions. Green Chem 19:5708–5713. https://doi.org/10.1039/C7GC02983G
Wu X-F, Darcel C (2009) Iron-catalyzed one-pot oxidative esterification of aldehydes. Eur J Org Chem 2009:1144–1147. https://doi.org/10.1002/ejoc.200801176
Gopinath R, Barkakaty B, Talukdar B, Patel BK (2003) Peroxovanadium-catalyzed oxidative esterification of aldehydes. J Org Chem 68:2944–2947. https://doi.org/10.1021/jo0266902
Patil A, Shinde S, Kamble S, Rode CV (2019) Two-step sequence of acetalization and hydrogenation for synthesis of diesel fuel additives from furfural and diols. Energy Fuels 33:7466–7472. https://doi.org/10.1021/acs.energyfuels.9b01640
Pinheiro DLJ, Nielsen M (2021) Base-free synthesis of furfurylamines from biomass furans using Ru pincer complexes. Catalysts 11:558. https://doi.org/10.3390/catal11050558
Chatterjee M, Ishizaka T, Kawanami H (2016) Reductive amination of furfural to furfurylamine using aqueous ammonia solution and molecular hydrogen: an environmentally friendly approach. Green Chem 18:487–496. https://doi.org/10.1039/C5GC01352F
Yuan H, Li J-P, Su F, Yan Z, Kusema BT, Streiff S, Huang Y, Pera-Titus M, Shi F (2019) Reductive amination of furanic aldehydes in aqueous solution over versatile NiyAlOx catalysts. ACS Omega 4:2510–2516. https://doi.org/10.1021/acsomega.8b03516
Wang P, Jing Y, Guo Y, Cui Y, Dai S, Liu X, Wang Y (2020) Highly efficient alloyed NiC/Nb2O5 catalyst for the hydrodeoxygenation of biofuel precursors into liquid alkanes. Catal Sci Technol 10:4256–4263. https://doi.org/10.1039/D0CY00684J
Strohmann M, Bordet A, Vorholt JA, Leitner W (2019) Tailor-made biofuel 2-butyltetrahydrofuran from the continuous flow hydrogenation and deoxygenation of furfuralacetone. Green Chem 21:6299–6306. https://doi.org/10.1039/C9GC02555C
Mahfud A, Ulfa SM, Utomo EP (2016) Hydrogenation/deoxygenation (H/D) reaction of furfural-acetone condensation product using Ni/Al2O3-ZrO2 catalyst. J Pure App Chem Res 5:108–115. https://doi.org/10.21776/ub.jpacr.2016.005.02.263
Kumar A, Srivastava R (2020) Bi-functional magnesium silicate catalyzed glucose and furfural transformations to renewable chemicals. ChemCatChem 12:4807–4816. https://doi.org/10.1002/cctc.202000711
Taimoory SM, Sadraei SI, Fayoumi RA, Nasri S, Revington M, Trant JF (2018) Preparation and characterization of a small library of thermally-labile end-caps for variable-temperature triggering of self-immolative polymers. J Org Chem 83:4427–4440. https://doi.org/10.1021/acs.joc.8b00135
Antonioletti R, D’Auria M, de Mico A, Piancatelli G, Scettri A (1985) Photochemical synthesis of 3- and 5-aryl-2-furyl derivatives. J Chem Soc, Perkin Trans 1:1285–1288. https://doi.org/10.1039/P19850001285
Nishimura S, Shibata A (2019) Hydroxymethylation of furfural to HMF with aqueous formaldehyde over zeolite beta catalyst. Catalysts 9:314. https://doi.org/10.3390/catal9040314
Wang C, Wang A, Yu Z, Wang Y, Sun Z, Kogan VM, Liu Y-Y (2021) Aqueous phase hydrogenation of furfural to tetrahydrofurfuryl alcohol over Pd/UiO-66. Catal Commun 148:106178. https://doi.org/10.1016/j.catcom.2020.106178
Gavilà L, Lähde A, Jokiniemi J, Constanti M, Medina F, del Río E, Tichit D, Álvarez MG (2019) Insights on the one-pot formation of 1,5-pentanediol from furfural with Co−Al spinel-based nanoparticles as an alternative to noble metal catalysts. ChemCatChem 11:4944–4953. https://doi.org/10.1002/cctc.201901078
Montagnon T, Kalaitzakis D, Triantafyllakis M, Stratakis M, Vassilikogiannakis G (2014) Furans and singlet oxygen–why there is more to come from this powerful partnership. Chem Commun 50:15480–15498. https://doi.org/10.1039/C4CC02083A
Trost BM, Toste FD (2003) Palladium catalyzed kinetic and dynamic kinetic asymmetric transformations of γ-acyloxybutenolides. Enantioselective total synthesis of (+)-aflatoxin B1 and B2a. J Am Chem Soc 125:3090–3100. https://doi.org/10.1021/ja020988s
Marinković S, Brulé C, Hoffmann N, Prost E, Nuzillard J-M, Bulach V (2004) Origin of chiral induction in radical reactions with the diastereoisomers (5R)- and (5S)-5-l-menthyloxyfuran-2[5H]-one. J Org Chem 69:1646–1651. https://doi.org/10.1021/jo030292x
Stockton KP, May JP, Taylor DK, Greatrex BW (2014) Practical evaluation of compact fluorescent lamps for dye-sensitized photooxidation reactions. Synlett 25:1168–1172. https://doi.org/10.1055/s-0033-1340976
Moradei OM, Paquette LA (2003) (5S)-(d-Menthyloxy)-2(5H)-Furanone. Org Synth 80:66. https://doi.org/10.15227/orgsyn.080.0066
Chen B, Li F, Yuan G (2017) Selective hydrodeoxygenation of 5-hydroxy-2(5H)-furanone to γ-butyrolactone over Pt/mesoporous solid acid bifunctional catalyst. RSC Adv 7:21145–21152. https://doi.org/10.1039/C7RA03205F
de Jong JC, van Bolhuis F, Feringa BL (1991) Asymmetric Diels-Alder reactions with 5-menthyloxy-2-(5H)-furanone. Tetrahedron: Asymmetry 2:1247–1262. https://doi.org/10.1016/S0957-4166(00)80024-5
Krow GR (2004) The Baeyer–Villiger oxidation of ketones and aldehydes. In: Organic Reactions. Wiley-VCH, pp 251–798. https://doi.org/10.1002/0471264180.or043.03
Li X, Lan X, Wang T (2016) Highly selective catalytic conversion of furfural to γ-butyrolactone. Green Chem 18:638–642. https://doi.org/10.1039/C5GC02411K
Li X, Lan X, Wang T (2016) Selective oxidation of furfural in a bi-phasic system with homogeneous acid catalyst. Catal Today 276:97–104. https://doi.org/10.1016/j.cattod.2015.11.036
Näsman J-AH, Pensar KG (1985) An improved one-pot preparation of 2-furanones. Synthesis 1985:786–788. https://doi.org/10.1055/s-1985-31349
Zvarych V, Nakonechna A, Marchenko M, Khudyi O, Lubenets V, Khuda L, Kushniryk O, Novikov V (2019) Hydrogen peroxide oxygenation of furan-2-carbaldehyde via an easy, green method. J Agric Food Chem 67:3114–3117. https://doi.org/10.1021/acs.jafc.8b06284
Bhat NS, Kumar R, Jana A, Mal SS, Dutta S (2021) Selective oxidation of biomass-derived furfural to 2(5H)-furanone using trifluoroacetic acid as the catalyst and hydrogen peroxide as a green oxidant. Biomass Convers Biorefin https://doi.org/10.1007/s13399-021-01297-0
Li X, Li Y, Wang T (2019) Effect of oxide supports on Pt-Ni bimetallic catalysts for the selective hydrogenation of biomass-derived 2(5H)-furanone. Catal Today 319:93–99. https://doi.org/10.1016/j.cattod.2018.03.053
Gassama A, Ernenwein C, Hoffmann N (2010) Synthesis of surfactants from furfural derived 2[5H]-furanone and fatty amines. Green Chem 12:859–865. https://doi.org/10.1039/B924187F
Silva Ferreira AC, Barbe J-C, Bertrand A (2003) 3-Hydroxy-4,5-dimethyl-2(5H)-furanone: a key odorant of the typical aroma of oxidative aged port wine. J Agric Food Chem 51:4356–4363. https://doi.org/10.1021/jf0342932
Hjelmgaard T, Persson T, Rasmussen TB, Givskov M, Nielsen J (2003) Synthesis of furanone-based natural product analogues with quorum sensing antagonist activity. Bioorg Med Chem 11:3261–3271. https://doi.org/10.1016/S0968-0896(03)00295-5
Alonso-Fagúndez N, Granados ML, Mariscal R, Ojeda M (2012) Selective conversion of furfural to maleic anhydride and furan with VOx/Al2O3 catalysts. ChemSusChem 5:1984–1990. https://doi.org/10.1002/cssc.201200167
Hronec M, Fulajtarová K (2012) Selective transformation of furfural to cyclopentanone. Catal Commun 24:100–104. https://doi.org/10.1016/j.catcom.2012.03.020
Panten J, Surburg H (2015) Flavors and fragrances, 2. aliphatic compounds. In: Ullmann’s Encyclopedia of Industrial Chemistry. Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, pp 1–55. https://doi.org/10.1002/14356007.t11_t01
Shen T, Hu R, Zhu C, Li M, Zhuang W, Tang C, Ying H (2018) Production of cyclopentanone from furfural over Ru/C with Al11.6PO23.7 and application in the synthesis of diesel range alkanes. RSC Adv 8:37993–38001. https://doi.org/10.1039/C8RA08757A
Zhang Y, Fan G, Yang L, Li F (2018) Efficient conversion of furfural into cyclopentanone over high performing and stable Cu/ZrO2 catalysts. Appl Catal, A 561:117–126. https://doi.org/10.1016/j.apcata.2018.05.030
Hronec M, Fulajtárová K, Vávra I, Soták T, Dobročka E, Mičušík M (2016) Carbon supported Pd–Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl Catal, B 181:210–219. https://doi.org/10.1016/j.apcatb.2015.07.046
Li X-L, Deng J, Shi J, Pan T, Yu C-G, Xu H-J, Fu Y (2015) Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu–Co catalysts. Green Chem 17:1038–1046. https://doi.org/10.1039/C4GC01601G
Hronec M, Fulajtárova K, Soták T (2014) Highly selective rearrangement of furfuryl alcohol to cyclopentanone. Appl Catal, B 154–155:294–300. https://doi.org/10.1016/j.apcatb.2014.02.029
Fang C, Liu Y, Wu W, Li H, Wang Z, Zhao W, Yang T, Yang S (2019) One pot cascade conversion of bio-based furfural to levulinic acid with Cu-doped niobium phosphate catalysts. Waste Biomass Valor 10:1141–1150. https://doi.org/10.1007/s12649-017-0131-7
Kim H, Lee S, Ahn Y, Lee J, Won W (2020) Sustainable production of bioplastics from lignocellulosic biomass: technoeconomic analysis and life-cycle assessment. ACS Sustainable Chem Eng 8:12419–12429. https://doi.org/10.1021/acssuschemeng.0c02872
Li L, Barnett KJ, McClelland DJ, Zhao D, Liu G, Huber GW (2019) Gas-phase dehydration of tetrahydrofurfuryl alcohol to dihydropyran over γ-Al2O3. Appl Catal, B 245:62–70. https://doi.org/10.1016/j.apcatb.2018.12.039
Chang X, Liu A-F, Cai B, Luo J-Y, Pan H, Huang Y-B (2016) Catalytic transfer hydrogenation of furfural to 2-methylfuran and 2-methyltetrahydrofuran over bimetallic copper–palladium catalysts. ChemSusChem 9:3330–3337. https://doi.org/10.1002/cssc.201601122
Liu B, Zhang Z (2016) One-pot conversion of carbohydrates into furan derivatives via furfural and 5-hydroxylmethylfurfural as intermediates. ChemSusChem 9:2015–2036. https://doi.org/10.1002/cssc.201600507
Démolis A, Essayem N, Rataboul F (2014) Synthesis and applications of alkyl levulinates. ACS Sustainable Chem Eng 2:1338–1352. https://doi.org/10.1021/sc500082n
Hu X, Song Y, Wu L, Gholizadeh M, Li C-Z (2013) One-pot synthesis of levulinic acid/ester from C5 carbohydrates in a methanol medium. ACS Sustainable Chem Eng 1:1593–1599. https://doi.org/10.1021/sc400229w
Wang M, Peng L, Gao X, He L, Zhang J (2020) Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process. Sustainable Energy Fuels 4:1383–1395. https://doi.org/10.1039/C9SE00982E
Morales G, Osatiashtiani A, Hernández B, Iglesias J, Melero JA, Paniagua M, Brown DR, Granollers M, Lee AF, Wilson K (2014) Conformal sulfated zirconia monolayer catalysts for the one-pot synthesis of ethyl levulinate from glucose. Chem Commun 50:11742–11745. https://doi.org/10.1039/C4CC04594G
Pinheiro PF, Chaves DM, da Silva MJ (2019) One-pot synthesis of alkyl levulinates from biomass derivative carbohydrates in tin(II) exchanged silicotungstates-catalyzed reactions. Cellulose 26:7953–7969. https://doi.org/10.1007/s10570-019-02665-w
Upare PP, Hwang YK, Hwang DW (2018) An integrated process for the production of 2,5-dihydroxymethylfuran and its polymer from fructose. Green Chem 20:879–885. https://doi.org/10.1039/C7GC03597G
Zhao W, Wu W, Li H, Fang C, Yang T, Wang Z, He C, Yang S (2018) Quantitative synthesis of 2,5-bis(hydroxymethyl)furan from biomass-derived 5-hydroxymethylfurfural and sugars over reusable solid catalysts at low temperatures. Fuel 217:365–369. https://doi.org/10.1016/j.fuel.2017.12.069
Yang W, Sen A (2011) Direct catalytic synthesis of 5-methylfurfural from biomass-derived carbohydrates. ChemSusChem 4:349–352. https://doi.org/10.1002/cssc.201000369
Peng Y, Li X, Gao T, Li T, Yang W (2019) Preparation of 5-methylfurfural from starch in one step by iodide mediated metal-free hydrogenolysis. Green Chem 21:4169–4177. https://doi.org/10.1039/C9GC01645G
Feng Y, Li Z, Long S, Sun Y, Tang X, Zeng X, Lin L (2020) Direct conversion of biomass derived l-rhamnose to 5-methylfurfural in water in high yield. Green Chem 22:5984–5988. https://doi.org/10.1039/D0GC02105A
Insyani R, Verma D, Kim SM, Kim J (2017) Direct one-pot conversion of monosaccharides into high-yield 2,5-dimethylfuran over a multifunctional Pd/Zr-based metal–organic framework@sulfonated graphene oxide catalyst. Green Chem 19:2482–2490. https://doi.org/10.1039/C7GC00269F
Xiang X, Cui J, Ding G, Zheng H, Zhu Y, Li Y (2016) One-step continuous conversion of fructose to 2,5-dihydroxymethylfuran and 2,5-dimethylfuran. ACS Sustainable Chem Eng 4:4506–4510. https://doi.org/10.1021/acssuschemeng.6b01411
Nguyen XP, Hoang AT, Ölçer AI, Engel D, Pham VV, Nayak SK (2021) Biomass-derived 2,5-dimethylfuran as a promising alternative fuel: an application review on the compression and spark ignition engine. Fuel Process Technol 214:106687. https://doi.org/10.1016/j.fuproc.2020.106687
Zhou C, Zhao J, Sun H, Song Y, Wan X, Lin H, Yang Y (2019) One-step approach to 2,5-diformylfuran from fructose over molybdenum oxides supported on carbon spheres. ACS Sustainable Chem Eng 7:315–323. https://doi.org/10.1021/acssuschemeng.8b03470
Yang Z, Qi W, Su R, He Z (2017) Selective synthesis of 2,5-diformylfuran and 2,5-furandicarboxylic acid from 5-hydroxymethylfurfural and fructose catalyzed by magnetically separable catalysts. Energy Fuels 31:533–541. https://doi.org/10.1021/acs.energyfuels.6b02012
Yan D, Wang G, Gao K, Lu X, Xin J, Zhang S (2018) One-pot synthesis of 2,5-furandicarboxylic acid from fructose in ionic liquids. Ind Eng Chem Res 57:1851–1858. https://doi.org/10.1021/acs.iecr.7b04947
Yangyang J, Zhou F, Ma H, Li X, Yuan X, Liang F, Zhang J (2020) One step synthesis of 2,5-furandicarboxylic acid from fructose catalyzed by Ce modified Ru/HAP. J Fuel Chem Technol 48:942–948. https://doi.org/10.1016/S1872-5813(20)30066-9
Cui J, Tan J, Cui X, Zhu Y, Deng T, Ding G, Li Y (2016) Conversion of xylose to furfuryl alcohol and 2-methylfuran in a continuous fixed-bed reactor. ChemSusChem 9:1259–1262. https://doi.org/10.1002/cssc.201600116
Gómez Millán G, Sixta H (2020) Towards the green synthesis of furfuryl alcohol in a one-pot system from xylose: a review. Catalysts 10:1101. https://doi.org/10.3390/catal10101101
Cueto J, Faba L, Díaz E, Ordóñez S (2017) Performance of basic mixed oxides for aqueous-phase 5-hydroxymethylfurfural-acetone aldol condensation. Appl Catal, B 201:221–231. https://doi.org/10.1016/j.apcatb.2016.08.013
Arias KS, Climent MJ, Corma A, Iborra S (2016) Chemicals from biomass: synthesis of biologically active furanochalcones by claisen–schmidt condensation of biomass-derived 5-hydroxymethylfurfural (HMF) with acetophenones. Top Catal 59:1257–1265. https://doi.org/10.1007/s11244-016-0646-3
Lin L, Shi Q, Nyarko AK, Bastow KF, Wu C-C, Su C-Y, Shih CC-Y, Lee K-H (2006) Antitumor agents. 250. design and synthesis of new curcumin analogues as potential anti-prostate cancer agents. J Med Chem 49:3963–3972. https://doi.org/10.1021/jm051043z
Lockman JW, Reeder MD, Suzuki K, Ostanin K, Hoff R, Bhoite L, Austin H, Baichwal V, Willardsen AJ (2010) Inhibition of eEF2-K by thieno[2,3-b]pyridine analogues. Bioorg Med Chem Lett 20:2283–2286. https://doi.org/10.1016/j.bmcl.2010.02.005
Zang H, Chen EYX (2015) Organocatalytic upgrading of furfural and 5-hydroxymethyl furfural to C10 and C12 furoins with quantitative yield and atom-efficiency. Int J Mol Sci 16:7143–7158. https://doi.org/10.3390/ijms16047143
Saikachi H, Ogawa H, Minami Y, Sato K (1970) Synthesis of furan derivatives. L. The Wittig reaction of 2, 5-furandialdehyde with Furan-2, 5-bis (2-cis-4-trans-2, 4-dimethyl-2, 4-pentadienylidene triphenylphos-phorane). Chem Pharm Bull 18:465–473. https://doi.org/10.1248/cpb.18.465
McDermott PJ, Stockman RA (2005) Combining two-directional synthesis and tandem reactions: synthesis of trioxadispiroketals. Org Lett 7:27–29. https://doi.org/10.1021/ol0479702
Mouloungui Z, Delmas M, Gaset A (1984) Synthesis of α,β-unsaturated esters using a solid-liquid phase transfer in a slightly hydrated aprotic medium. Synth Commun 14:701–706. https://doi.org/10.1080/00397918408059583
Li J, Stephanopoulos MF, Xia Y (2020) Introduction: Heterogeneous single-atom catalysis. Chem Rev 120:11699–11702. https://doi.org/10.1021/acs.chemrev.0c01097
Suchorski Y, Rupprechter G (2018) Heterogeneous surfaces as structure and particle size libraries of model catalysts. Catal Lett 148:2947–2956. https://doi.org/10.1007/s10562-018-2506-1
Zhang L, Ren Y, Liu W, Wang A, Zhang T (2018) Single-atom catalyst: a rising star for green synthesis of fine chemicals. Natl Sci Rev 5:653–672. https://doi.org/10.1093/nsr/nwy077
Sudarsanam P, Peeters E, Makshina EV, Parvulescu VI, Sels BF (2019) Advances in porous and nanoscale catalysts for viable biomass conversion. Chem Soc Rev 48:2366–2421. https://doi.org/10.1039/C8CS00452H
Lu Y, Zhang Z, Wang H, Wang Y (2021) Toward efficient single-atom catalysts for renewable fuels and chemicals production from biomass and CO2. Appl Catal, B 292:120162. https://doi.org/10.1016/j.apcatb.2021.120162
Mondelli C, Gözaydın G, Yan N, Pérez-Ramírez J (2020) Biomass valorisation over metal-based solid catalysts from nanoparticles to single atoms. Chem Soc Rev 49:3764–3782. https://doi.org/10.1039/D0CS00130A
Vono LLR, Damasceno CC, Matos JR, Jardim RF, Landers R, Masunaga SH, Rossi LM (2018) Separation technology meets green chemistry: development of magnetically recoverable catalyst supports containing silica, ceria, and titania. Pure Appl Chem 90:133–141. https://doi.org/10.1515/pac-2017-0504
Rajkumari K, Changmai B, Meher AK, Vanlalveni C, Sudarsanam P, Wheatley AEH, Rokhum SL (2021) A reusable magnetic nanocatalyst for bio-fuel additives: the ultrasound-assisted synthesis of solketal. Sustainable Energy Fuels 5:2362–2372. https://doi.org/10.1039/D0SE01900C
Acknowledgements
SD thanks NITK, Surathkal, for the research facilities.
Funding
The work was partially funded by the Council of Scientific & Industrial Research (CSIR), India, under the grant number (02)0301/17/EMR-II.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dutta, S. Valorization of biomass-derived furfurals: reactivity patterns, synthetic strategies, and applications. Biomass Conv. Bioref. 13, 10361–10386 (2023). https://doi.org/10.1007/s13399-021-01924-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01924-w