Abstract
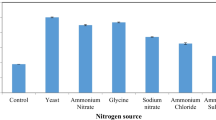
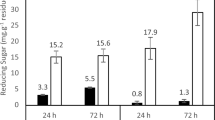
Polygalacturonase is a pectinolytic enzyme with potential applications in the food, chemical, textile, and paper industries. Herein, extracellular Exo-polygalacturonase (Exo-PG) was biosynthesized from Penicillium fellutanum by harnessing the potential of wheat bran as an agricultural substrate via solid-state fermentation (SSF). Optimization protocol revealed the maximum production of the enzyme at 40% moisture level, pH 4.0, temperature 40 °C, and fermentation duration of 96 h. The partially purified enzyme derivative exhibited its maximum biocatalytic activity at pH 4.0 and 40 °C. It retained appreciable stability over a wide temperature range of 30–70 °C and a pH range of 3.0–8.0. Values of Km and Vmax, i.e., Michaelis–Menten constants, were calculated to be 12.5 mg/mL and 22.22 μmol/mL/min, respectively. The activation energy for thermal denaturation was determined to be 128.8 kJ/mol. Exposure to a hydrophobic environment, i.e., urea, considerably suppressed the enzymatic activity. A significant increase in the clarity and viscosity reduction was achieved for all fruit juices after treatment with Exo-PG. Conclusively, the current findings may represent Exo-PG as a prospective and promising candidate for diverse bio-applications in the food and beverage industries.











Similar content being viewed by others
References
Abdulaal WH, Almulaiky YQ (2020) Purification and Thermodynamic Characteristics of an Exo-Polygalacturonase from Trichoderma Pseudokoningii. J Chem Soc Pakistan 42(5)
Adedeji OE, Ezekiel OO (2019) Pretreatment of selected peels for polygalacturonase production by Aspergillus awamori CICC 2040: Purification and application in mango juice. Extraction. Biores Technol Rep 7:100306
Ahmed I, Zia MA, Hussain MA, Akram Z, Naveed MT, Nowrouzi A (2015) Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J Radiat Res Appl Sci 9(2):148–154
Ahmed SA, Mostafa FA (2013) Utilization of Orange Bagasse and Molokhia Stalk for Production of Pectinase Enzyme. Braz J Chem Eng 30(3):449–456
Akhter N, Morshed MA, Uddin A, Begum F, Sultan T, Azad KA (2011) Production of Pectinase by Aspergillus niger Cultured in Solid-state Media. Int J Biosci 1(1):33–42
Amin F, Mohsin A, Nawaz H, Bilal M (2020) Production, thermodynamic characterization, and fruit juice quality improvement characteristics of an Exo- polygalacturonase from Penicillium janczewskii. BBA-Prot Proteom 1868:140379
Amin F, Bhatti HN, Bilal M, Asgher M (2017) Multiple parameter optimizations for enhanced biosynthesis of exo-polygalacturonase enzyme and its application in fruit juice clarification. Int J Food Eng 13(2)
Amin F, Bhatti HN, Bilal M, Asghar M (2017) Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int J Biol Macromol 95:974–984
Anand G, Yadav S, Yadav D (2017) Production, purification, and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotechnology 7:122
Anand G, Yadav S, Yadav D (2017) Purification and biochemical characterization of an exo-polygalacturonase from Aspergillus flavus MTCC 7589. Biocatal Agric Biotechnol 10:264–269
Anisa SK, Ashwini S, Girish K (2013) Isolation and screening of Aspergillus spp For pectinolytic activity. Electron J Biol 9(2):37–41
Antranikian G (2009) Extremophiles and biotechnology. In: Encyclopedia of life sciences (ELS). John Wiley & Sons, Ltd., Chichester
Ázar RLSL, da Morales LM, Alfenas GPM, Falkoski DL, Alfenas RF, Guimarães VM (2020) Apple juice clarification by a purified polygalacturonase from Calonectria pteridis. Food Bioprod Process. 119:238–245
Bhatti HN, Asgher M, Abbas A, Nawaz R, Sheikh MA (2006) Studies on kinetics and thermostability of a novel acid Invertase from Fusarium solani. J Agric Food Chem 54:4617–4623
Bhatti HN, Rashid MH, Nawaz R, Perveen R, Jabbar A, Asgher M (2007) Purification and characterization of a novel glucoamylase from Fusarium solani. Food Chem 103:338–343
Bilal M, Iqbal HM (2020) State-of-the-art strategies and applied perspectives of enzyme biocatalysis in food sector—current status and future trends. Crit Rev Food Sci Nutr 60(12):2052–2066
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castilho LR, Medronho RA, Alves TLM (2000) Production and extraction of pectinases obtained bu solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresour Technol 71:45–50
Chinedu SN, Dayo-Odukoya OP, Iheagwam FN (2017) Partial purification and kinetic properties of polygalacturonase from Solanum macrocarpum L. fruit. Biotechnology 16:27–33
Cipolatti EP, Pinto MCC, Henriques RO, da Silva Pinto JCC, de Castro AM, Freire DMG, Manoel EA (2019) Enzymes in green chemistry: the state of the art in chemical transformations. Adv Enzyme Technol 137–151
de Marco M, Souza RC, da Silva LM, de Bastos G, Dallago RM, Valduga E, ..., Zeni J (2020) In Situ and Contact Immobilization/Adhesion of Penicillium brasilianum in Polyurethane Foam for Production of Exo-Polygalacturonase. Industr Biotechnol 16(4), 241–246
El-Shishtawy RM, Mohamed SA, Asiri AM, Gomaa AM, Ibrahim IH, Al-Talhi HA (2014) Solid Fermentation of Wheat Bran for Hydrolytic Enzymes Production and Saccharification Content by a Local Isolate Bacillus megatherium. BMC Biotechnol 14(29):1–8
Gomes J, Zeni J, Cence K, Toniazzo G, Treichel H, Valduga E (2011) Evaluation of Gummadi, S. N. and T. Panda. 2003. Purification and biochemical properties of microbial pectinases - A review. Process Biochem 38:987–996
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: A review. Process Biochem 40:2931–2944
John J, Kaimal KKS, Smith ML, Rahman PKSM, Chellam PV (2020) Advances in upstream and downstream strategies of pectinase bioprocessing: a review. Int J Biol Macromol 162:1086–1099
Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of pectinases in commercial sector: a review. Biores Technol 77:215–227
Kumar YS, Varakumar S, Reddy OVS (2010) Production and optimization of polygalacturonase from mango (Mangifera indica L.) peel using Fusarium moniliforme in solid state fermentation. World J Microbiol Biotechnol 26:1973–1980
Lu X, Lin J, Wang C, Du X, Cai J (2016) Purification and characterization of exo-polygalacturonase from Zygoascus hellenicus V25 and its potential application in fruit juice clarification. Food Sci Biotechnol 25(5):1379–1385
Majumder K, Paul B, Sundas R (2020) An analysis of exo-polygalacturonase bioprocess in submerged and solid-state fermentation by Pleurotus ostreatus using pomelo peel powder as carbon source. Journal of Genetic Engineering and Biotechnology 18(1):1–8
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Mrudula S, Anithraraj R (2011) Pectinase production in solid-state fermentation by Aspergillus niger using orange peel as substrate. Glob J Biotechnol Biochem 6(2):64–71
Munir N, Asgher M, Tahir IM, Riaz M, Bilal M, Shah SM (2015) Utilization of agro-wastes for production of ligninolytic enzymes in liquid state fermentation by Phanerochaete chrysosporium-IBL-03. Int J Chem Biochem Sci 7:9–4
Naveed M, Nadeem F, Mehmood T, Bilal M, Anwar Z, Amjad F (2020) Protease—A versatile and ecofriendly biocatalyst with multi-industrial applications: an updated review. Catalysis Lett 1–17
Nawaz A, Sameer M, Akram F, Tahir SF, Arshad Y, Haq IU, Mukhtar H (2021) Kinetic and thermodynamic insight of a polygalacturonase: A biocatalyst for industrial fruit juice clarification. Revista Mexicana de Ingeniería Química 20(2):1029–1045
Nazir Z, Ijaz S, Gul R, Saleem M (2019) Purification and characterization of an acidic polygalacturonase from grapes and its potential to juice quality. Pakistan J. Zool 51(4):1387–1402
Niture KS (2008) Comparative biochemical and structural characterizations of fungal Polygalacturonases. Biologia 63:1–19
Pagarra H, Rahman RA, Illias RM, Ramli NA (2019) Screening of factors influencing exo-polygalacturonase production by Aspergillus niger atcc 120120 using two-level fractional factorial design. Jurnal Teknologi (Sciences & Engineering) 81(6):73–80
Patidar MK, Nighojkar S, Kumar A, Nighojkar A (2020) Production of polygalacturonase using Carica papaya peel biowaste and its application for pomegranate juice clarification. Environ Sustainab 3(4):509–520
Patil NP, Patil KP, Chaudhari BL, Chincholkar SB (2012) Production, purification of exo-polygalacturonase from soil isolate Paecilomyces variotii NFCCI 1769 and its application. Indian J Microbiol 52(2):240–246
Prajapati J, Dudhagara P, Patel K (2021) Production of thermal and acid-stable pectinase from Bacillus subtilis strain BK-3: optimization, characterization, and application for fruit juice clarification. Biocatalysis and Agricultural Biotechnology, 102063. production and characterization of polygalacturonase by Aspergillus niger ATCC9642
Qamar SA, Asgher M, Bilal M (2020) Immobilization of alkaline protease from Bacillus brevis using Ca-alginate entrapment strategy for improved catalytic stability, silver recovery, and dehairing potentialities. Catal Lett 150:3572–3583
Rashmi R, Murthy SK, Sneba S, Syama A, Radhika VS (2008) Partial Purification and Biochemical Characterization of Extracellular Pectinase from Aspergillus niger Isolated from Groundnut Seeds. J Appl Biosci 9(1): 378-384. 20093254516
Riaz M, Perveen R, Javed MR, Nadeem HU, Rashid MH (2007) Kinetics and thermodynamics of a novel glucoamylase from Humicola sp. Enzyme Microbiol Technol 41(5):558–564
Roses RP, Guerra NP (2009) Optimization of Amylase Production by Aspergillus niger in Solid-state Fermentation using Sugarcane Bagasse as Solid Support Material. World J Microbiol Biotechnol 25:1929–1939
Saeed S, Bibi I, Mehmood T, Naseer R, Bilal M (2020) Valorization of locally available waste plant leaves for production of tannase and gallic acid by solid-state fermentation. Biom Convers Bioref 1–8
Sandri IG, Lorenzoni CMT, Fontana RC, Silveira MM (2013) Use of pectinases produced by a new strain of Aspergillus niger for the enzymatic treatment of apple and blueberry juice. LWT-Food Sci Technol 51(2):469–475
Sandri IG, Fontana RC, Barfknecht DM, da Silveira MM (2011) Clarification of fruit juices by fungal pectinases. LWT Food Sci Technol 44(10):2217–2222
Sharma DC, Satyanarayana T (2020) Thermostable and alkalistable exopolygalacturonase of Bacillus pumilus dcsr1: characteristics and applicability. Int J Biol Macromol 164:3340–3348
Tu T, Meng K, Bai Y, Shi P, Luo H, Wang Y, Yao B (2013) High-yield production of a low-temperature-active polygalacturonase for papaya juice clarification. Food Chem 141(3):2974–2981
Uzuner S, Cekmecelioglu D (2021) Biochemical characterization and stability of Bacillus subtilis polygalacturonase produced using hazelnut shells by submerged fermentation. Biocatal Biotransform 1–6
Vilar DS, Fernandes CD, Nascimento VR, Torres NH, Leite MS, Bharagava RN, ... Ferreira LF (2020) Hyper-production optimization of fungal oxidative green enzymes using citrus low-cost byproduct. J Environ Chem Eng 105013
Zhan S, Bilal M, Zdarta J, Cui J, Kumar A, Franco M, ... Iqbal HM (2020) Biopolymers and nanostructured materials to develop pectinases-based immobilized nano-biocatalytic systems for biotechnological applications. Food Res Int 109979
Zheng Z, Shetty K (2000) Solid state production of polygalacturonase by Lentinus edodesws using fruit processing wastes. Process Biochem 35:825–830
Acknowledgements
The authors are grateful to the Government College Women University, Faisalabad, Pakistan, for financial support under in-house research grant program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amin, F., Arooj, T., Nazli, ZiH. et al. Exo-polygalacturonase production from agro-waste by Penicillium fellutanum and insight into thermodynamic, kinetic, and fruit juice clarification. Biomass Conv. Bioref. 13, 11141–11151 (2023). https://doi.org/10.1007/s13399-021-01902-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01902-2