Abstract
Fructans are carbohydrates consisting of fructose monomers linked by β-2,1- and/or β-2,6-glycosidic bonds with linear or branched structure. These carbohydrates belong to the group of prebiotic dietary fibre with health-promoting potential for humans and mammals due to their indigestibility and selective stimulation of microorganisms in the gastrointestinal tract. This makes fructans interesting mainly for healthy food as well as animal feed applications. As a consequence of a growing public awareness for animal welfare, dietary fibre and thus fructans move into the focus as a fibre-rich feeding improving not only animals’ health but also their well-being. Against this background, this paper summarises the known effects of fructans focusing on pigs and highlights the state of the art in fructan production processes from plant material as well as selected current research lines. Additionally, an attempt is made to assess the potential of European fructan production for an application as animal feed. Based on this, challenges in the field of fructan production are addressed and alternative substrates for fructans are discussed and pointed out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fructans, also referred to as polyfructoses or fructosans, are oligo- and polysaccharides naturally occurring in specific plants (e.g., chicory, wheat), where they serve as storage carbohydrates. Additionally, fructans play a role in the metabolism of bacteria and fungi (e.g., as an outer protection layer). After starch and sucrose, fructans are the most abundant non-structural polysaccharides in nature.
Carbohydrates containing almost exclusively fructose monomers are referred to as fructans, while short-chain fructans with a degree of polymerisation (DP) lower than 10 are often called fructooligosaccharides (FOS) or oligofructose. Depending on their type of bond, fructans with only β-2,1 glycosidic bonds are called inulin-type showing a linear chemical structure. This unbranched fructan type plays the most important role in plant-based fructan production and thus is preferentially required.
Due to the lack of appropriate digestive enzymes, fructans are non-digestible for humans as well as for some farm animals (e.g., pigs). In contrast, these carbohydrates are metabolised by the intestinal microbiome. Consequently, fructans belong to the group of dietary fibre, i.e. they are neither digested nor absorbed in the mouth, stomach or small intestine of humans and specific mammals as these are not able to hydrolyse the glycosidic bonds within these fructans. Hence, the amount of fructan, which enters the large intestine, is almost the same as initially ingested (> 90% in case of humans [1]). As fructans are soluble, they are easily metabolised by the located microbiota in the large intestine and hence can be classified as prebiotics, i.e. they are positively affecting the gastrointestinal microbiota by inducing their growth or activity and thus increase the welfare and health of the host. Besides the type of glycosidic bond, the DP influences the health-promoting effects. This is especially true for the prebiotic effects being particularly enhanced by long-chain inulin [2,3,4].
As many properties of fructans are similar to sucrose, their handling is comparable in terms of processing, and their colour and odour are food compatible. Consequently, fructans are well suited for food applications. The highly soluble short-chain FOS taste sweet, similar to sucrose (about 30 to 60% sweetness of sucrose), while fructans with a higher DP are tasteless and moderately soluble in water (at room temperature). Sheared in water or milk, long-chain fructans create a smooth creamy texture and create a fat-like mouthfeel in food applications due to higher water-binding capacities compared to sucrose. As a result, fructans with a DP above 10 can be used as a fat replacer. Simultaneously, fructans show very low caloric values making them an interesting sweetener (in case of FOS) or a tasteless filler (in case of long-chain fructans). Fructans are stable at temperatures typically used for food processing (below 100 °C) and are stable in a pH range of 5 to 10 [1, 5,6,7].
Functional foods (e.g., desserts, yoghurt, dietary products, meat substitutes) are the main application for industrially produced fructans, where they function as fat or sugar substitutes or enrich the fibre content. Here fructans achieve prices of about 3 €/kg for inulin-type fructans [8], about 35 €/kg for pure inulin [9] and up to 150 €/kg for FOS [10]. The most important markets for functional food are found in the USA and in Japan [6, 10, 11].
All these characteristics outlined above make fructans attractive not only for food applications, but also for animal feed in terms of fibre-enrichment. The latter is especially true with regard to an increasing interest in animal welfare due to numerous complaints of NGOs and the resulting growing public awareness for this topic, mainly within the EU. This results not only in a public demand for better conditions in animal husbandry (e.g. fibre-rich feeding by fructans [12]), but also in an increasing demand for meat substitutes (e.g. in Germany [13]). For all these reasons, the demand for fructans and their application in feed and functional foods is expected to rise in the coming years [14].
Against this background, this paper envisages to give an overview of fructan effects in monogastric animals focusing on pigs, showing their importance for animal welfare and production. Additionally, conventional fructan production options and the different industrial scale production pathways are highlighted focusing on the provision from plant material. Furthermore, the current status in the field of production is presented. In this light, the potential of conventional fructan production for animal feed application is estimated and assessed, as it is shown that fructans can decisively contribute to improve the current situation in animal husbandry. Based on these considerations, open research challenges in alternative fructan production are presented. Together with alternative raw materials for fructans, these challenges are discussed, providing a possible solution for meeting the increasing demand for fructans [13].
2 Prebiotics in monogastric animals, especially in pigs
In the public debate about livestock production, some overriding issues become increasingly urgent in recent years. Especially for pig production, there are topics like natural living conditions, outdoor access and some others that can be assigned mainly to the overarching aspect “animal welfare” [15, 16]. Besides, many consumers demand products characterised by high food safety and quality [16]. Therefore, animal health and consumer protection [17, 18] as well as food quality such as avoidance of boar taint are further challenges [19, 20]. For some of these challenges mentioned above, the use of specific carbohydrates, namely prebiotics, can be particularly interesting.
2.1 General aspects of prebiotics
Since the end of the last millennium, a class of compounds called prebiotics has been recognised for its ability to manipulate the microbiota in the intestinal tract to the benefit of the host [21]. At that time, prebiotics were first defined as indigestible food components that favourably affect the host by selectively stimulating the growth and activity of one or a limited number of colon bacterial species to improve the host’s health [22]. Only a few years ago, the International Scientific Association on Probiotics and Prebiotics (ISAPP) has updated this definition [21]: a substrate that is selectively utilised by microorganisms conferring a health benefit for the host. This definition differs from the original version. Prebiotics thus also include potentially non-carbohydrate substances. Prebiotics are supposed to induce targeted metabolic processes and thus bring health benefits to the host’s ecosystem [23]. Some of the benefits have already been scientifically tested.
In this context, the use of indigestible oligosaccharides such as fructans and galactans should be highlighted [24]. This is due to the specific structure of FOS and galactooligosaccharides (GOS). As mammal intestinal enzymes act specifically on certain α-glycosidic bonds (e.g. in starch), they are not able to hydrolyse the β-configuration in fructans or galactans. However, these are easily degraded by certain enzymes such as β-fructanosidase and β-galactosidase, which are frequently found in bacteria of the genus Bifidobacterium. Nowadays, FOS, GOS and inulin, but also isomaltooligosaccharides (IMO), xylooligosaccharides (XOS), lacticol, lactulose, cereal fibre, are commonly used in livestock [23].
Symbiotic microorganisms extend the digestive physiology of mammals by forming an armamentarium of various polysaccharide-degrading enzymes that are largely absent in the genomes of mammals. Therefore, the ability to adapt to different carbohydrate nutrients in a very short time is possible, possibly within hours [25]. It is even argued that the start inoculum has a greater effect on the fermentation of these substances than the polysaccharide structure itself and determines more or less a distinct fermentation characteristics [26]. Research on the human microbiome has led to the conclusion that symbiotic microorganisms have the ability to pick up new traits by lateral gene transfer and therefore gut microbes enable adaption over time periods as long as centuries and millennia by adjusting their relevant gene content for degrading enzymes to reflect cultural dietary trends [25].
Fermentations of dietary polysaccharides by microbes in the large intestine of mammals produces biologically active short-chain fatty acids (SCFA) as major metabolites [26]. Acetate is a common endpoint of fermentation in the hindgut, the succinate pathway is the most important route for propionate production from most hexoses and pentoses. The formation of the SCFA butyrate can be catalysed directly from hexose fermentation or via the utilisation of exogenous sources of acetate [26]. Fermentation of prebiotic dietary fibres and thus SCFA production promotes many beneficial health outcomes to the host [27].
The fermentation of twelve different fibre sources has been examined using pig and human faecal inoculum [28]. These fibres (or carbohydrates) were grouped into mannans (guar gum, konjac glucomannan), homoglucans (cellulose, retrograded tapioca starch, retrograded maize starch, oat β-glucan), fructans (inulin, FOS), polyuronides (high methyl esterified citrus pectin, alginate) and complex heteroglycans (xanthan gum, soy pectin), based on their sugar composition, regardless of the glycosidic bond. Soy pectin and xanthan gum were grouped as complex heteroglycans because they contained both uronic acids and neutral sugars. Gas production is a result of the microbial fermentation differing depending on the substrate; for example, a maximum cumulative gas production has been shown for oat β-glucan at 15.3 h and for cellulose at 64.0 h [28]. In the early stages of fermentation, most of the fibres are present as polymers, which are not readily available as an energy source. Therefore, the microbiota tends to produce acetate but also lactate can be detected in the early stage of fermentation [28]. As the fermentation continues, the proportion of acetate decreases while the propionate proportion generally increases. Lactate is an intermediate fermentation product, which can be converted to propionate and butyrate. The kind of inoculum (human or pig) also determines the composition of fermentation products for defined fibre sources. On the one hand, there is a comparable composition of the fermentation products (acetate:propionate:butyrate) for fast degrading FOS in case of humans (51:39:10) and pigs (53:35:12). On the other hand, there is a huge difference reported for cellulose and the respective fermentation products (human: 63:21:15; pig: 50:44:6) [28]. In general, this experiment has shown that sugar and linkage composition as well as the DP affect fibre degradation and the composition of the resulting fermentation products [28]. XOS composed of xylose units are another potential candidate group for the production of beneficial SCFA. In a study [27] on potential prebiotic effects and fermentability of five commonly consumed fibres, inter alia, XOS, pure inulin and pure β-glucan have been investigated. In so doing, an in vitro fermentation system has been used for measuring changes in faecal microbiota, total gas production and formation of SCFA. The results show that XOS fermentation results in less gas production than inulin, and more gas production than β-glucan. In vivo, a high gas production potential can result in mild negative gastrointestinal symptoms. The inulin samples showed the highest average production of butyrate, and were similar to the ones from XOS [27].
Also depending on the fibre source, the time required for fibre degradation may differ and might be too high in monogastric animals (in comparison to the passage rate of the intestinal content). Therefore, one approach is to process raw materials in order to improve their fermentability and to produce desired fermentation products such as butyrate [29].
2.2 Effects of plant-based fructans in pig’s gut
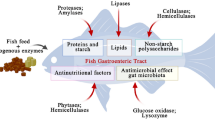
Fructans affect the conditions in the gastrointestinal tract of pigs including the microbiota, their metabolites, the morphological structures and also indirect mechanisms on the behaviour [30,31,32,33].
Cereals with a high content of specific dietary fibres not digested in the small intestine are specifically interesting in terms of animal health and welfare due to direct effects in the gut and indirect effects of the metabolites. For example, rye contains 3.6 to 6.6% fructans based on dry matter (DM), whereas fructan concentrations in wheat grains, more often used for feed, are lower varying between 0.7 and 2.9% (DM). Depending on their nature, the fructans are partly or totally decomposed and metabolised by microorganisms in the hind gut only [32].
In pig production, leaving the sow, accompanied by nutritional, emotional and environmental stress factors, is a critical stage with an enhanced susceptibility to intestinal pathogens for young piglets [34, 35]. This is induced by a disrupted state of the microbiota, or a dysbiosis [34] opening up possibilities for dietetic concepts [18, 34]. Thus, adding cereal grains with high contents of fermentable carbohydrates to the feed, is a sustainable option to increase microbial diversity and beneficial microbes [18] and therefore can help to prevent incidence of post-weaning diarrhoea, and decrease subtherapeutic antibiotic use.
By fermentation of the dietary fibres, the hind gut’s microorganisms produce beneficial substances mainly acetic, propionic and butyric acid (i.e. SCFA) [35, 36]. Also in pigs, the amount of fermentation products depends on the raw material and its composition; for example, chicory root and pulp produce lower amounts of total SCFA and especially butyrate compared to purified FOS and inulin-type fructans as substrates [35]. To maintain the gut mucous membrane healthy, the intestinal epithelium must be supplied constantly with energy for regeneration, which is achieved by the major symbiotic function of the gut microbiota through their ability to provide energy to the intestinal epithelium as SCFA. The SCFA butyrate is the preferred energy substrate for intestinal cells, promoting normal proliferation and differentiation [18, 37].
There are also positive effects of butyrate apart from the intestine wall, for example, positive effects on satiety, activity and the social behaviour of pigs. Fermentable fibres, particularly if resulting in the production of high butyrate amounts, enhance satiety in adult pigs, which may affect long-term energy intake and body weight development [36]. Ethopathies in pregnant sows have been known for decades and are still present if sows are only provided with concentrated feed in accordance with their relatively low energy and nutrient requirements and only quantities of 2.5 to 3.0 kg per animal and day are used [38]. The following section addresses two common challenges in pig production against the background of prebiotics:
Salmonella
Salmonella is one of the main causes of food-borne diseases in humans, especially caused by the serovars Salmonella Enteritidis and Salmonella Typhimurium [39, 40]. Most salmonella infections in pigs are subclinical and prevalent among all age groups and in different production stages, i.e. identifying infected pigs on farms can be difficult and costly [41]. Correlations between salmonella prevalence on pig farms and carcass contamination demonstrate the major importance of implementing control mechanisms at the farm level [42, 43].
It has been demonstrated that the concentrations of certain volatile fatty acids in the surrounding milieu act as a signal for salmonella in terms of their adhesion and reproduction/metabolic activity [44]. Higher butyrate contents in the chyme tend to have an inhibiting or reducing effect on adhesion and proliferation and seem to act in lowering salmonella prevalence in practice [43, 44]. As the caecum is the main refuge for salmonella [45], dietetics which act in this location are of special importance.
Inulin-type fructans are reported to be beneficial for butyrate production in the gut [37]. Strategic feeding of sodium butyrate to finishing pigs for a relatively short period of time (below 30 days) immediately prior to slaughter was effective in reducing salmonella shedding in a trial without other complication (Lawsonia intracellularis infection) [46]. It has been supposed that inulin, lactulose, exopolysaccharide from probiotic bacteria or dietary fibre such as wheat bran or locust bean are efficient against Salmonella species [47]. However, there are still no studies testing this on a larger scale in practice.
Boar taint
The “boar taint” is caused by two components, namely by androstenone (pheromone formed in the testicles) and skatole [48].
-
Common methods to prevent the androstenone-related boar taint are surgical or immunologically induced castration. Surgical castration of nearly all male suckling piglets is done up to now in most European countries [49]. However, this procedure is highly controversial discussed in public [19].
-
Skatole is a product formed in the intestinal tract by microbial decomposition of the amino acid tryptophan [48]. The skatole production can significantly be influenced by the feeding strategy. These effects are based on carbohydrates (e.g. resistant starch) not digested in the small intestine but in the hind gut [48, 50, 51].
Most of the studies which were effective in reducing skatole formation and deposition in adipose tissue increased energy availability and shifted microbial metabolism from proteolytic to saccharolytic [48]. Among others, this has been confirmed by trials with potato starch [48, 50, 52]. By using 30% of crude potato starch in the compound feed 7 days prior slaughter, the skatole tissue concentrations were significantly reduced (p = 0.04, 0.22 μg/g lipid versus 0.85 μg/g lipid) [52]. The addition of fermentable carbohydrates to diets for pigs has also shown to raise SCFA production in the hind gut [50], thus, also butyric acid arises. These higher concentrations of butyric acid in the chyme are supposed to promote the supply of the mucosa. This applies in particular in such a way that the epithelial losses (endogenous protein) already decrease (possibly also as a result of a relatively changed apoptosis rate) and thus less tryptophan is produced [50] resulting in less skatole production. As a consequence, less skatole is absorbed and can be stored in the body fat tissue. It is still completely open whether this is really only about the butyrate content or not about very specific changes within the colon flora. Whereas many bacteria are able to metabolise tryptophan to indole and indole acetic acid, the key precursor of skatole, only a few specialised gut bacteria, mainly from the Clostridium and Bacteroides genera, can catalyse the steps from indole acetic acid (IAA) to skatole [48]. For example, declining germ counts of clostridia were observed with increasing inulin contents in the feed [51], therefore the mechanism named above [48].
3 Fructan production
In the following section, conventional fructan production is described. The different production pathways (Fig. 1) for fructans can be distinguished with regard to the basic substrate:
-
Fructans are extracted from plant material and, if desired, are partially hydrolysed to FOS by enzymes (Fig. 1, pathways A to C).
-
Fructans, mainly short-chain FOS, are systematically built up from sucrose by enzymes (Fig. 1, pathway D).
-
Fructans are chemically synthesised from saccharide units, i.e. fructose and glucose (Fig. 1, pathway E).
Fructan production pathways, modified from [3] (FOS: Fructooligosaccharides)
Chemical synthesis of fructans (Fig. 1, pathway E) is mentioned for the sake of completeness. However, this chemical glycosylation pathway has hardly played a role in literature and research up to now and thus is considered to be irrelevant (for fructans) at this time. In brief, this complex multi-stage process starts from a glycosyl donor and a glycosyl acceptor. The glycosyl donors is a sugar with a specific leaving group (e.g. halides, thioalkyl groups) being activated prior to glycosylation (i.e. elimination of the leaving group). The glycosyl acceptors are sugars with partially protected hydroxyl groups (e.g. by acetyl groups). The protection groups are necessary in order to stereo selectively couple the saccharides either with a β-2,1- or a β-2,6-glycosidic bond (in case of fructans). As a result, oligo- or polysaccharides can be obtained after removal of the protective groups. In contrast to the biochemical pathways (Fig. 1, pathways A to D), a chemical synthesis of fructans and FOS has been graded as being not economically feasible for the moment [53,54,55,56].
Below, the conventional fructan production and its different process steps are described in detail and the corresponding research activities are discussed.
3.1 Production from plant material by extraction
In case of plant-based fructans, the production process usually comprises three to four steps: (1) extraction of fructans from the raw plant material, (2) subsequent purification, (3) depolymerisation in case of FOS and finally (4) a spray-drying step (Fig. 1, pathways A to C) [8].
Artichoke (mainly Helianthus tuberosus) and chicory (mainly Chichorium intybus var. sativum) but also agave are the most important raw materials for the industrial scale production of fructans [57]. Besides plants of natural origin, genetically modified plants are subject of research [58, 59].
The obtained fructan type, its average degree of polymerisation (DPav) and the respective distribution depend on the raw material. Table 1 gives an overview of the properties of some industrially relevant fructan-containing plants; for example linear inuline-type fructans can be extracted from artichoke. However, only about 20% of these fructans show a DP above 10. Therefore, these artichoke fructans do not have the desired properties (e.g. fat-like behaviour) and thus are not suitable for many food applications. Highly branched fructans can be obtained from agave commonly used for Tequila production. Apart from these fructan sources, there are several other mentionable plants such as cereals, garlic and onions with high fructan contents [60, 61, 65,66,67].
Within the EU, the roots of chicory are more or less exclusively processed for fructan provision. However, the fructan production process using other plants (e.g. agave) is analogous to that of chicory. The chicory plant is native to this latitude and shows only a limited sensitivity to coldness. The shape of the chicory root is quite similar to that of sugar beets and comprises for about 30% of the whole chicory plant with a fructan content above 70% related to dry mass (DM) or 15 to 20% related to the fresh mass (FM). In contrast to the chicory salad production (Chichorium intybus var. foliosum), the chicory roots intended for fructan production are not forced (a specific growing method) prior to processing. During forcing, typically in a cool and dark environment, the targeted long-chain fructans are degraded and the fructan mono- and dimers (glucose, fructose, sucrose) accumulate within the chicory roots. Thus, forced chicory roots are poorly suited for fructan production [3, 68,69,70].
3.1.1 Fructan extraction
Industrial scale process (Fig. 2, stage 1)
The industrial scale production of fructans from plants closely resembles the sucrose production from sugar beets. The corresponding process (Orafti-process, Fig. 2) is split into two stages starting on the fields with harvesting the roots of the chicory plant and their transportation to the manufacturing site. Harvesting, transportation and storage have to be done quickly (below 7 days) in order to minimise fructan loses due to natural degradation within the plant. In practice, losses of 0.05 up to 0.1% (w/w)/day occur during storage under a controlled atmosphere at temperatures of about 10 °C [60, 71].
Orafti-process, modified from [68] (1) extraction and (2) downstreaming
In the production plant, stones, sand and other impurities are removed and the cleaned roots are sliced. Then the fructans are extracted with hot water in a counter current process (e.g. tower extractor) with yields of inuline-type fructans up to 92% [69]. The provided dark liquid contains about 10% of interfering sugars (mono-, di- and small oligosaccharides). The remaining extracted plant material is pressed, dried and used as animal feed afterwards [60, 69, 72].
Laboratory research
In general, the fructan-rich parts of the plant (mostly tubers or roots) are cleaned, cut into pieces and grounded in order to increase their specific surface area and to break the plant cells prior to extraction. Typically, hot water is used for extraction in laboratory scale as well, since fructan solubility increases significantly with the temperature; for example, the solubility of native chicory fructans (inulin-type) in water improves from 60 g/L at 10 °C up to 330 g/L at 90 °C [73]. Hot water extraction processes are usually performed at temperatures from 70 to almost 100 °C using extraction times of 1 to 2 h with fructan recoveries ranging from 88 to 93% [70, 74].
Moreover, multistage extraction has been investigated on a laboratory scale; for example, a hot water treatment is carried out in order to inactivate degrading enzymes initially (e.g. polyphenol oxidases responsible for browning of the plant material [75]). Higher fructan yields and less fructan degradation as well as being water-saving and energy efficient can be clear advantages of such multistage extraction processes. However, polar solvents favour the (co-)extraction of monosaccharides and low molecular oligosaccharides resulting in a higher purification effort.
The usage of non-polar solvents (e.g. ethanol) leads to a decreased solubility of higher-molecular fructans while low molecular saccharides remain dissolved. This effect can also be used in multistage extraction in order to remove mono-, di- and lower-molecular saccharides first (e.g. ethanol 80% (v/v)) simplifying the purification step [76]. Subsequently, fructans are extracted with hot water. Besides the reduced effort for low-molecular saccharide removal, the usage of heated aqueous alcoholic solutions (mostly ethanol or methanol) at 80 to 90 °C promotes the deactivation of the plant-based enzymes and thus reduces degradation of the fructans.
On a laboratory scale, several modifications like ultrasound-, microwave-, or enzyme-assisted extraction (e.g. cellulases, hemicellulases) have been investigated [68, 74, 77, 78]. All these methods have in common that plants’ cell walls are destroyed to improve fructan extractability. Moreover, supercritical fluid extraction and pulsed-electric field-assisted extraction have been studied finding pulsed-electric field extraction as one of the most promising alternatives in comparison to conventional hot water extraction [74, 79].
Important factors determining the fructan extraction yield are extraction time, type of solvent as well as the solvent-to-solid ratio and the pH value. The solvent-to-solid ratio is typically varied between 1:1 and 10:1 (w/v) in laboratory scale, and the pH is usually adjusted in a range between 6 and 7 in order to avoid fructan hydrolysis. Besides, fructan-degrading enzymes partly being present in samples may lower fructan yields. Elevated temperatures and alcohols can be used for enzyme deactivation [68, 70, 74, 80].
3.1.2 Fructan downstreaming
Industrial scale process (Fig. 2, stage 2)
Subsequent to extraction (analogous to sucrose production from sugar beet), the fructan raw juice is filtered and refined. The first purification step uses lime (Ca(OH)2) and carbon dioxide (CO2) leading to the formation of calcium carbonate (CaCO3). The formed microcrystalline calcium carbonate particles precipitate, while impurities like proteins, anions (e.g. phosphate) and insoluble material are adsorbed or trapped in the flocks and co-precipitated. Subsequent filtration separates these impurities together with the CaCO3 from the fructan juice. This side-product can be used for soil improvement due to its high calcium and organic matter content [60].
Remaining dry matter, dissolved colour and odour compounds, organic acids and salts are removed in the second purification step by means of cationic and anionic ion-exchange, adsorption, filtration and/or active carbon treatment. The resulting juice has a purity of > 99.5% carbohydrates. Accrued material streams, resulting from the regeneration of the purification process (e.g. ion-exchange) by means of sulphuric acid (H2SO4) and ammonia (NH3), contain ammonium sulphate (NH4)2SO4 and potassium sulphate (K2SO4) being a valuable resource for fertiliser production [60].
The obtained enriched and purified fructan solution is then sterilised by ultrafiltration (< 0.2 μm), evaporated and spray-dried to stabilise the final product in the form of a powder (Fig. 1, pathway A). The chain-length of these fructans is in the range of the naturally occurring fructans in the underlying plant material (e.g. in the case of chicory roots the DPav ranges from 10 to 12 [60]). Obtained fructans have purities of 92 to 99.5% with mainly smaller saccharides as impurities [69, 74].
For the industrial production of short-chain FOS enzymes of Aspergillus niger and Aspergillus fumigatus are mainly used (Fig. 1, pathway C). Visa versa, physical separation (e.g. filtration) is used for the aimed production of long-chain inulin (high-performance inulin) by separating low molecular FOS (Fig. 1, pathway B).
Finally, the provided product inulin typically has a composition of 93% inulin (DP > 3), 5% dimeric saccharides and 2% monomeric saccharides. The majority of global plant-based fructan production takes place in Belgium (e.g. Beneo-Orafti), and most of the products are inuline-type fructans forming a white, odourless powder. Commercial fructan producers comprise Beneo-Orafti (Südzucker AG), Cosucra SA, Sensus (Cosun UA) or GTC Nutrition, producing fructans as functional food ingredients [3, 10, 68, 69, 71, 74, 81,82,83].
Laboratory research
After extraction, the subsequent process steps aim to concentrate and purify the extracted fructans by removal of impurities such as proteins, monosaccharides, lipids and salts. For the production of highly pure fructans, interfering oligo- and polysaccharides (e.g. hemicellulose) need to be removed as well. The selected processes depend on the desired properties (mainly chain-length) of the target product and its desired purity, which in turn depends on the aimed application (e.g. tasteless fibre enrichment, sweetener).
As a first step, usually insoluble but suspended material is removed by solid-liquid separation processes (e.g. centrifugation, filtration). For the subsequent purification of the resulting fructan extract, available processes are presented below. The major points of concern are again the pH value, the process temperature and the residence time under fructan-degrading conditions.
-
Precipitation is a commonly performed process step for isolation of saccharides and thus fructans. Generally, precipitation (crystallisation from solution) can be achieved by temperature reduction (cooling crystallisation [84, 85]), solvent evaporation (evaporation crystallisation) and/or use of different solvents (displacement crystallisation [68, 72, 77, 86]).
However, for the (selective) precipitation of fructans from an aqueous solution, organic solvents like methanol, ethanol, propanol, acetone and acetonitrile are typically used. Thereby, acetone followed by ethanol and methanol shows the best results with respect to the preservation of the initial DP in the precipitate [77, 87]. Keeping the subsequent solvent separation and recycling in mind, acetone is technically and energetically easier to recover due to its comparatively low boiling point. Nevertheless, ethanol is the most appropriate solvent for precipitation among others for safety reasons and to enable food-grade processes. Precipitation is especially interesting for fructans with a high DP as their solubility is lower (in comparison to low-molecular fructans) in organic solvents. Thus, long-chain fructans precipitate easier while the low-molecular and monomeric saccharides remain dissolved. In case of ethanol, 80% (v/v) are typically adjusted for the removal of low-molecular saccharides. After the precipitation step, a solid-liquid-separation (e.g. centrifugation, decantation) is conducted in order to separate the precipitated, crystallised fructans from the extract. Subsequently, the solvent is removed and can be recycled. Analogous to industry, liming may be used for purification in order to remove impurities by the formed and precipitated calcium carbonate (CaCO3). Additionally in laboratory scale precipitation of proteins with trichloroacetic acid and subsequent centrifugation is commonly utilised for fructan purification as well [68, 74, 75, 77, 88].
-
Selective fermentation is a microbial treatment for purifying fructan-containing solutions by means of microorganisms. Co-cultivation of microorganisms (e.g. Pichia p. X-33 [63], Aspergillus p. [89]) in the fructan-containing extract selectively eliminates interfering mono- and disaccharides (e.g. glucose, fructose, sucrose). These mono- and disaccharides are process-related co-extracted and co-precipitated [89]. However, the used microorganisms obviously should not be able to degrade fructans. After an appropriate incubation time, the microorganisms are removed by centrifugation and/or filtration [63]. It has been shown that microbial treatment increases the fructan content by removing interfering saccharides [90]. Nevertheless, an additional separation step is needed in order to remove the microorganisms and their fermentation products (e.g., ethanol) after selective fermentation.
-
Membrane processes like ultrafiltration (molecular weight cut-off: 10 to 100 kDa) and nanofiltration (molecular weight cut-off: 300 to 1,000 Da) are basically a cost-effective option. Filtration can be used for the removal of interfering substances (e.g. monosaccharides) as well as for the fractionation of fructans according to their chain-length [91]. Both, different membranes as well as different operation modes (dead-end or cross-flow process) have been used successfully on a laboratory and pilot scale for oligosaccharide separation (mainly FOS from model sugar solutions containing glucose, fructose and sucrose) [92,93,94,95,96,97,98]. Choosing the appropriate membrane depends on the respective task as well as the sample matrix, fouling potential, etc.
-
Adsorption and chromatography. Adsorption on activated charcoal is another possibility of fructan purification commonly used in sugar processing for pre-purified media [53, 99]. With increasing saccharide-chain-length, the hydrophobic character increases and hence the adsorption of long-chain fructans on the charcoal surface is increased in comparison to small sugar molecules or salts. Therefore, activated charcoal can be used for demineralisation and removal of mono- and disaccharides. Using an ethanol gradient for fructan desorption allows for a fractionation of fructans according to their chain-length [90]. By means of an ethanol gradient, FOS have been separated using carbon-celite columns [100].
Ion exchange chromatography is also a common technique in the field of sugar separation for impurity removal being investigated intensively in a small scale (analytical chromatography). In this context, diethylaminoethyl cellulose with positively charged groups in the stationary phase is a frequently used and commercially available resin [77]. Nonetheless, usually strong acidic cation resins (calcium, sodium, etc.) are applied. However, size exclusion is an important separation mechanism in the context of sugar chromatography as well [101, 102]. Typically, chromatography like the use of activated charcoal is process-related not operated continuously, as both processes require a regeneration step.
Simulated moving bed (SMB) chromatography is one of the most complex and sophisticated separation techniques investigated in the context of fructan purification. This process allows for a continuous chromatographic separation and has been used successfully for sugar separation on an industrial scale [103]. This is in particular true for the separation of glucose and fructose mixtures in order to obtain high fructose corn syrup. SMB chromatography was used for the purification of short-chain FOS by a Japanese company firstly, but was found to be not applicable for industrial scale back in the 1980s [6]. However, research on SMB chromatography for fructan separation continued and has been investigated via simulation by designing and modelling a SMB chromatography process for the separation of FOS from a model sugar mixture [104, 105]. Thereby, using a cation exchange resin has led to separation yields of 95% and FOS with less than 5% mono- and disaccharide impurities. Moreover, upstream purification prior to SMB chromatography has been investigated in order to improve its efficiency [89].
-
Fructooligosaccharide (FOS) production by targeted hydrolysis. Fructan production from plant material usually envisages to obtain high-molecular fructans. Depending on their designated application (e.g. short-chain FOS as a sweetener), an additional depolymerisation step might be required in order to shorten the fructan chains. As the obtained natural plant-based fructans differ in chain-length and partially in type of linkage, it might be necessary to create a more uniform DP distribution. This can be achieved by targeted hydrolysis, i.e. the glycosidic bonds and thus the fructan chains are partly cleaved (Fig. 1, pathway C).
By means of chemical (mild acidic hydrolysis) or enzymatic (from microorganisms) hydrolysis, fructans are systematically broken down with the purpose of obtaining short-chain FOS. For this controlled partial hydrolysis, enzymes are usually preferred allowing to avoid undesired parallel and consecutive reactions. Especially in the case of linear fructans (e.g., from artichoke), inulinases have been found to be suitable, while in the case of branched fructans (e.g. from agave), the partial enzymatic hydrolysis has been inefficient. Also for cost reasons, thermal acidic hydrolysis is an alternative to such an enzyme application, which can be performed by addition of hydrochloric or sulphuric acid or by the usage of acidic cation-exchange resins. During acidic hydrolysis, unwanted hydroxymethylfurfural (HMF) formation is a crucial point, especially in the case of food applications. Autohydrolysis at temperatures between 130 and 230 °C, as it is mainly used for hemicellulose liquefaction, might be an alternative for short-chain FOS production not requiring any additional chemicals [63, 68, 106, 107].
As a result of such a partial hydrolysis, a mixture of FOS is obtained containing GFn-type FOS (fructans with a terminal glucose (G) unit) as well as Fn-type FOS (fructans only comprising fructose (F) units). In contrast to this mixture comprising pure fructose fragments (Fn), natural plant-based fructans are exclusively of GFn-type.
-
Drying. Depending on the desired product (syrup or powder), a concentration step for fructan enrichment may be needed using evaporation analogously to sucrose production providing a syrup. A dried product might be obtained by spray-drying or freeze-drying resulting in a fructan powder allowing for a long-term storage [102]. Spray-drying has been optimised for inulin-concentrates from chicory in laboratory scale [108]. For sterilisation of the product either heat, ultraviolet radiation or sterile filtration is used [6].
3.2 Production from sucrose by enzymatic synthesis
The FOS production path via enzymes yields FOS with a DP of 2 to 5. In contrast to the direct production of fructans from plant material, discussed above, the production pathway by means of enzymes starts indirectly from plants (Fig. 1, pathway D). The raw material is already isolated sucrose from sugar factories originating from sugar cane or sugar beet. With the help of food-grade microbial, mostly fungal, enzymes fructose molecules from the precursor sucrose are linked together. Simultaneously, the by-product glucose is produced. In the process, transfructosylation enzymes cleave the β-2,1-linkage of the substrate sucrose and transfer the released fructosyl group to an acceptor (here: sucrose) while eliminating glucose. Thus, enzyme type and synthesis conditions allow to control the chain-length of the resulting FOS.
Figure 3 gives a schematic overview of enzymatic FOS production. The respective production pathways can be differentiated in batch processes (Fig. 3, pathway A and B) and continuous processes using immobilised enzymes or immobilised microorganisms (Fig. 3, pathway C and D) [10, 100, 109]. Below, industrially relevant fructan production processes using enzymes and their different process steps are elucidated as well as corresponding research activities.
Schematic overview of enzymatic fructooligosaccharide (FOS) synthesis, modified from [6].
3.2.1 Enzyme production and enzymatic synthesis of fructans
Industrial scale process
Besides the plant-based production, short-chain FOS are also produced industrially based on sucrose by means of enzymes. This is especially true in Japan due to a large market for prebiotic food ingredients. Even most of the short-chain oligosaccharides (not only FOS) used as prebiotic ingredients are produced via enzymatic technologies. Commercially, the β-2,1-type FOS are mainly produced, whereas the production of high-molecular branched fructans is not economically feasible and thus is commonly realised by extraction from plant material [6, 59].
-
Batch processes (Fig. 3, pathway A and B) can be further differentiated regarding their process steps. Firstly, a one-stage process without enzyme purification is performed, i.e. the whole microorganism is directly used for synthesis (Fig. 3, pathway A). Therefore, lower temperatures (25 to 35 °C) are adjusted with starting sucrose concentrations of approx. 15% (w/w) and residence times of 16 to 48 h [110]. Secondly, a two-stage process is performed comprising one step for enzyme production and subsequent enzyme purification prior to the synthesis step (Fig. 3, pathway B). Thereby, the step of enzyme production starts with high sucrose concentrations (30 to 40% (w/w)) at 50 to 60 °C and pH values of 5 to 7 with residence times of 24 to 120 h. The most appropriate enzymes are originated from fungi such as Aspergillus niger or Aureobasidium pullulans [6, 110].
In the subsequent synthesis, purified enzymes (or the microorganisms) are filled into a stirred tank reactor with a solution of 50 to 70% (w/w) sucrose. The process operates at pH 5.5 to 6.0 and at temperatures between 50 and 60 °C. The reaction is stopped by enzyme deactivation (heating to 90 °C for 30 min). The resulting solution, containing FOS, glucose and non-converted sucrose, is cooled, clarified by filtration, deionised by ion-exchange and evaporated in order to obtain a FOS concentrate (75% (w/w)). Further purification is realised by subsequent chromatographic methods with the purpose of removing residual saccharides.
-
Continuous process (Figure 3, pathway C and D). Apart from batch operation, continuous industrial processes exist using immobilised enzymes or entire immobilised cells [6]. Such an industrial scale continuous production process for FOS has already been developed for the first time in the early 1980s. Thereby, a packed bed reactor is used with an immobilised Aspergillus niger cultivation (producing the needed enzymes) fixed in calcium alginate gel. Later, a similar process has been designed with Aureobasidium pullulans operating since 1990. Such conventional fixed bed reactors in fructan industry have volumes ranging from 1 to 2 m3 operating at temperatures of about 50 °C with flow rates of 0.15 to 0.3 BV/h (bed volumes per hour) in case of fixed cells and 1 to 2 BV/h in case of immobilised enzymes [6, 111].
Laboratory research
Several fungi like Aspergillus species and Penicillium species as well as bacteria such as Zymomonas mobilis and Arthrobacter species and yeasts like Saccharomyces cerevisiae produce fructosyl-transferase-enzymes for the production of FOS. Many microorganisms with transfructosylation activity suited for the synthesis of FOS are known and assessed [112]. The cultivation of these microorganisms is usually realised in an aerobic submerged environment, within a fluid-bed cultivation or simply in a broth. Temperatures during cultivation are typically adjusted to around 30 °C with a pH value around 6 in order to create optimal conditions.
-
Batch processes (Fig. 3, pathway A and B). After cultivation, the microbial cells are separated by centrifugation or harvested with a basket either for direct use in synthesis (Fig. 3, pathway A) or previous enzyme extraction (Fig. 3, pathway B). In case of enzyme extraction, collected microbial cells are washed, and their structure is disrupted by either ultrasonic treatment, use of lysozymes and/or grinding. Afterwards, insoluble material is removed by centrifugation or filtration. Subsequent ultrafiltration may be used for further purification resulting in a crude enzyme solution [6, 113]. Reported FOS yields range from 30 to 60% (gFOS/gSucrose). These yields strongly depend on the microorganisms used for enzyme production [109]. In batch operation, the maximum FOS yield is limited due to the formation of the inevitable by-product glucose inhibiting the transfructosylation reaction. A maximum theoretical FOS yield of 55 to 60% (gFOS/gSucrose) for batch processing has been reported, limited by the inevitably co-produced glucose [59, 109].
-
Continuous process (Fig. 3, pathway C and D). Besides batch operation, cell immobilisation (Fig. 3, pathway D) by entrapping cells with calcium alginate has been studied intensively [6] as well as solid-state fermentation [10] allowing for a continuous process.
Solid-state fermentation is a common immobilisation concept for microorganisms intending their growth on moist solid material (in the absence of a free aqueous phase). Solid-state fermentation conditions correspond to the natural living conditions of many fungi and thus offer well-suited growth conditions. Further advantages of solid-state fermentation are a comparatively low water and energy consumption. Additionally, high volumetric productivity and a high product concentration can be achieved resulting in a simplified downstreaming [10, 110]. The main disadvantages, however, are the scale-up of such solid-state fermentation processes due to heat and mass transfer gradients occurring in such heterogeneous systems and the danger of irreversible dehydration. Thus, solid-state fermentation has not yet found a broad use on an industrial scale and is interesting only for small scale operation primarily in research. Reported FOS overall yields are in the range of 60 to 70% (gFOS/gSucrose) [10].
Enzyme immobilisation has been assessed as it has some advantages over cell immobilisation like a higher volumetric activity and less mass transport limitation. Enzyme immobilisation may be achieved by anion exchange resins with amines as functional groups by mixing previously purified enzymes with the corresponding resin [6]. Immobilisation with chitosan is another possibility for continuous FOS production [114]. However, all these concepts have in common to be complex and laborious.
3.2.2 Fructan downstreaming
After enzymatic FOS synthesis, subsequent downstreaming processes are comparable with and analogous to the fructan processing from plant material as described above [4, 71, 90, 105, 109, 115]. Additionally, with the intention of increasing production yields, a continuous removal of the co-product glucose and/or the product FOS from the fermentation is envisaged to avoid both substrate and product inhibition [10, 89].
A continuous removal of glucose (inevitable by-product) is thus desirable in order to increase the fructan yield. Several methods like simultaneous selective fermentation by microorganisms (e.g. by Saccharomyces cerevisiae), enzyme usage (e.g. glucose oxidase), membrane techniques or activated charcoal exist in order to remove the inhibiting substances within the process [116]. Moreover, continuous glucose removal from enzymatic FOS production in a membrane reactor using nanofiltration has been investigated [96]. As a result, a significant increase of the FOS yield was found (90% in comparison to 55 to 60% in conventional batch mode) [117]. Another possibility of inhibitor removal is the use of glucose oxidase in the fermentation media leading to the formation of gluconic acid and thus a reduction of the glucose content [10].
The obtained sucrose-based FOS are very similar to those produced from long-chain fructans by partial hydrolysis. However, all sucrose-based FOS contain a terminal glucose monomer (GFn-type), while FOS obtained from hydrolysis (and thus long-chain fructans) additionally comprise fructans built only from fructose (Fn-type) [10]. Both plant-based and sucrose-based FOS products are usually provided with purity levels ranging between 80 and 99%.
4 Potential of current fructan production capacities for European animal production
4.1 Assessment of the conventional production from chicory
Fructans can play an important role in animal nutrition. However, the respective contribution has not been completely investigated nor fully understood yet [23, 118, 119]. The growing public awareness for animal welfare results in a call for better husbandry conditions comprising a species-appropriate feeding and thus shifting away from a performance-orientated feeding regime [120]. Therefore, the usage of prebiotics in animal feeding and thus fructans as a prebiotic additive might become an interesting or rather promising field of application especially within the EU on the background of the growing public demand related to such aspects.
Below, the current potential of the fructan production volume inside the EU is estimated in order to assess whether conventional fructan production (as described above) could fulfil such an increased demand or not. As chicory is the main fructan resource within the EU so far, the current fructan production capacities are supposed to be estimated based on the current chicory production and assessed with regard to a potential usage as a feed additive. Figure 4 gives an overview of this estimation.
The total amount of chicory produced within the EU sums up to about 1.2 Mt/a (average of 2016 to 2018) comprising both chicory for food and for processing [121]. For a first rough estimation, it is assumed that this total amount is used exclusively for fructan production and the whole chicory plant is fully processed. The overall yield of such an industrial fructan production from this plant is calculated based on sugar beet processing (in Germany) achieving approx. 85% overall yield (sugar beet to sucrose) [122]. Based thereupon and with a fructan content of 15 to 20% (FM) [60], the maximum amount of producible fructans within the EU sums up to about 160 to 210 kt/a.
As outlined above, fructan production strongly resembles sucrose production. However, as fructans are more likely degraded during transportation, storage and subsequent processing, the overall yield presented above is most likely overestimated. This means that the overall fructan yield is expected to be lower in reality due to the described effects. Additionally, any other use of chicory (e.g. salad production) is neglected here; however, about 50% of the produced chicory is used for fresh consumption [121]. This adds up to the assumption that the figures outlined above are clearly an overestimation.
With about 245 million slaughtered animals per year (average of 2016 to 2018), pigs are the largest population of livestock (except poultry) within the EU [123] and thus being the target market for fructan-containing feed additives. Sticking to the (most likely overestimated) figures above, up to 0.9 kg (fructan) per pig would be available at most. This neglects that there is already today a (high-price) market for fructans especially for human nutrition on the one side and pigs are not the only commercially kept animals within the EU on the other side. Therefore, it is unlikely that the available amount of fructans accessible on the market today might be available as an additive to fodder for animals.
According to current knowledge at least 20 to 80 g/kg (per fodder) of inulin-type fructans are required in order to obtain positive effects by feeding (here: microbial SCFA production analogous to the discussion above) [124]. However, in this context, reliable figures for pig diet are rare and respective figures strongly differ depending on the examined effect in the pigs (e.g., SCFA production, growth of the intestinal microorganisms, colon cancer prevention) [124,125,126].
Depending on their phase of life, pigs require feed amounts of about 1 to 3 kg/day and thus overall about 260 kg fodder per fattening pig [127]. Assuming the outlined consumption per pig, there would be a demand for fructans (only for pigs) of about 1200 to 5100 kt/a (respectively 5 to 20 kg/pig) within the EU. In comparison to the estimated maximum fructan production capacity of 160 to 210 kt/a (respectively up to 0.9 kg/pig) based on chicory (neglecting the fact that these amounts are not available for this purpose for the time being), this results in a significant gap. Together with the clear decline in arable land throughout the EU [128], this gap between supply (fructans based on chicory) and demand (animal feed, food) is expected to be even larger than shown in Fig. 4. This is especially true since other farm animals such as poultry (if the attempt to increase animal welfare is treated seriously) have a potential demand for fructans as well. And last but not the least, the need of many other fructan applications especially for human nutrition (e.g. functional foods) should neither be forgotten nor neglected [119, 129].
4.2 Prospective need for research concerning fructan production
Obviously, fructans are not the only prebiotic substance available in nature, but this group of biopolymers is comparatively well studied. Thus, a major contribution of fructans to fibre-enrichment of food and animal feed is clearly foreseeable. And, as outlined above, a significantly increasing fructan demand could only be partly satisfied by the currently existing fructan production capacity. Based on this, below the need for research in the field of fructan production and especially alternative fructan substrates is addressed and discussed.
Alternative fructan sources
The public discussion about animal welfare is a current topic and thus improving animals’ lives as well as a resulting, growing demand for fibre-enrichment in feed are expected to become an increasingly tackled issue [130, 131]. One consequence might be a strongly increasing demand for (low-priced) fructans from the animal feed industry. Simultaneously, the already existing and most likely also growing fructan demand for direct use in foods has to be covered and kept in mind. For these reasons, additional (low-priced) fructan resources have to be exploited, if the EU wants to improve the current situation in animal breeding (e.g. pig production) by providing sufficient amounts of prebiotic dietary fibre (mainly fructans) for feeding, without imports from third countries. The estimated fructan production capacities (based on chicory) as outlined above indicate that currently available fructan processes and resources are not able to fulfil a significant growth in demand (Fig. 4).
Since most relevant fructan-rich plants are already known and their use is limited due to cultivation, harvest and storage conditions [132], one of the main topics in current fructan research (from a production point of view) is the search for alternative (new) raw materials (i.e. substitutes for chicory) being ideally more sustainable. Especially in the context of animal feed, low-priced fructans are required and thus expensive enzymatic pathways towards fructans are probably not economically feasible. Potential sources like residues from the food and agricultural sector (e.g. cereal brans) or (bio)ethanol industry (e.g., stillage) are available in huge quantities at very low prices. But these have not or hardly been researched with respect to fructans or rather fructan production especially in a large scale yet. Next to economic benefits, the utilisation of residues promises clear environmental advantages like reducing waste disposal problems and saving arable land. On the other hand, for some of these residue streams already strongly established competitive utilisation pathways have been developed and implemented within the market. Here, the thermal or energetic utilisation (e.g., cereal bran) as well as a direct use as low value animal feed (e.g., dried distiller’s grains with solubles (DDGS)) are only some typical examples. Nonetheless, fructans offer the potential of a higher-value utilisation and open the option to diversify the product portfolio of the respective processes (e.g., bioethanol production).
Potential residue streams for fructan production are shown in Table 2 together with an estimation of the potential fructan production volume thereof. This first and certainly rough estimation shows that cereal bran, especially from wheat, has high potential for fructan production with regard to the respective production volumes. Fructan separation (for analytical purposes) from wheat bran has been investigated in laboratory scale obtaining a fructan extract with a purity of about 75% and a yield based on used bran of 3.7% (w/w) [133]. Nevertheless, cereal bran is a product obtained from milling, typically a strict dry process and thus an additional wet processing chain would be necessary in order to extract the fructans. However, further research is needed in this context for a further assessment.
Moreover, Table 2 shows the fructan content of stillage, the main by-product of bioethanol production [136]. The shown amounts are comparatively low. However, from the process engineering point of view, the bioethanol production process offers the great advantage that the fructans are already dissolved and accessible for separation. By this, an additional pretreatment (e.g., milling) and extraction step is unnecessary. In contrast, fructan separation might be impeded as bioethanol stillage, a fermentation broth, typically comprises microorganisms and partly their metabolites from alcoholic fermentation and thus further impurities. Such a novel process for fructan production and especially the downstreaming can be oriented towards the established fructan processing. So far, no investigation or assessment of fructan production from ethanol stillages has been published.
The presented figures (Fig. 4 and Table 2) indicate that residues, especially bran, can make an important contribution to the outlined lack of fructans in pig production. However, this would still not be sufficient. Therefore, additional fructan-containing sources or rather residues originating from food processing are needed; for example, artichoke with a global production quantity of ca. 1.6 Mt in 2018 [139] and its residues. Usually about 80% (w/w) of the whole artichoke plant is waste comprising 5 to 28% (DM) fructans making this residue also a potential source of an increased fructan production [140, 141]. However, the utilisation of such “new” resources requires further research activities. This is also true for the types of fructan (originating from cereals) and their suitability for the discussed applications.
Apart from the exploitation of natural sources, genetically modified plants are investigated as they offer the potential of synthesising tailor-made fructans [58]. However, genetically engineered plans as well as all genetically modified organisms (GMO) are highly controversial in public being particularly true for the EU characterised by strong NGOs.
Outlook for further research topics
Besides new raw materials, novel prebiotics other than fructans are in the focus of research and have the potential to help closing the shown gap between supply and probably growing demand. As outlined above, XOS and GOS are potential alternatives [23]. In this context, lignocellulose-based prebiotics like XOS (e.g. from straw) are particularly interesting as their origin does not compete with food. In general, the pentose-containing (mainly xylose, arabinose) oligo- and polysaccharides (pentosans) are promising prebiotics. According to current knowledge, the required daily dose for achieving a prebiotic effect is lower for XOS than for fructans [142]. In this context, targeted modification of prebiotics is also an interesting field allowing for an improved prebiotic effect; for example, enzymatically modified arabinoxylans from bran have been investigated and found to be promoting the growth of specific butyrate-producing bacteria in the human gut [29]. As outlined above, the extent to which the fibrous material can be fermented in the intestinal tract depends on the microbiota but also on the passage rate, i.e. how long the intestinal content remains in the large intestine. This is a limiting factor for some substrates. Therefore, corresponding (biotechnological) pre-processing of fibrous material could be an option enabling the availability of alternative substances with significant prebiotic effects.
Besides the development of appropriate production processes and their approval for food and feed (e.g. by the European Food Safety Authority (EFSA)), it is especially important to fully understand the mechanisms of action of prebiotic substances in humans and animals in order to permit a clear declaration as a prebiotic substance [113, 142, 143].
The search for novel food-grade enzymes selectively hydrolysing or synthesising fructans is also a research area tackled at the moment. Hereby, open research questions address the reaction mechanisms of the corresponding enzymes as well as their control and regulation (e.g. by temperature) [144, 145]. However, enzymatic fructan synthesis is more likely suited for high-value food applications rather than for the supply of the feed market requiring huge quantities of low-priced fructans.
5 Conclusion
Besides the description of the state of knowledge on fructans in pig diet, the aim of this paper was to give an overview of fructan production, the corresponding state of technology and research as well as a rough assessment of a potential fructan utilisation in European pig production.
-
Fructans in pig diet. Fructans are classified as prebiotics and thus are an attractive ingredient for animal feed and human food offering great potential for health and well-being. Due to their structure and their resulting indigestibility for pigs, fructans enhance the production of short-chain fatty acids (SCFA) by the intestinal microbiota. As outlined above, SCFA positively influence the boar taint and promote salmonella reduction in pigs.
-
Fructan production. Industrial fructan production is state of the art mainly using two pathways differing with regard to the product. On the one side, extraction from plant material (typically chicory) leads to comparatively long-chain fructans. On the other hand, targeted enzymatic synthesis from sucrose results in short-chain fructooligosaccharides (FOS). Subsequently, there are several feasible downstream processes available for fructans differing in complexity and efficiency depending on the desired purity of the resulting fructans.
-
Fructan production for pig feed in the EU. Based on the presented estimation of the fructan production within the EU using conventional production methods and raw materials (here: chicory), the provision of sufficient amounts of fructans for humans and especially farm animals is unfeasible. However, fructans are comparatively well investigated prebiotics with established applications and thus have an advantage over alternative prebiotics.
Larger quantities of fructans can be produced theoretically by expanding chicory cultivation or other plants with a high fructan content. However, this competes not only with other chicory utilisation but also for arable land. Therefore, alternative resources for fructans, especially for the low-price application in animal feed, are expected to be required in the future due to the growing awareness for animal welfare in animal production. This goes hand in hand with the need for the development of appropriate production process requiring further R&D activities.
-
Alternatives to conventional fructans from chicory. The residues cereal bran and bioethanol stillage (Table 2) are potential substrates for fructan production occurring in large quantities. Even though, the utilisation of both substrates (cereal bran and stillage) is partly in competition with an energetic utilisation and to some extent also with direct use in animal feed. Nonetheless, both residue streams are assessed to be a promising fructan source and thus for thorough research. This is especially true as a higher-value utilisation is desirable not only from an economic point of view (e.g. in comparison to the thermal utilisation of cereal bran) but also against the background of expanding the product portfolio (mainly bioethanol production / biorefinery).
As shown, despite the currently occurring quantities of bran and stillage, further fructan-containing substrates or rather preferably residues are required in order to fulfil the potential need for low-priced fructans, exemplified by the EU pig production. In order to close this gap between demand and supply, additional alternative low-price prebiotics with similar properties are of particular interest, promising representatives are xylooligosaccharides (XOS) and comparable pentosans.
References
Bosscher D (2009) Fructan prebiotics derived from Inulin. In: Charalampopoulos D, Rastall RA (eds) Prebiotics and probiotics science and technology, Springer New York, vol 151. New York, NY, pp 163–205
van de Wiele T, Boon N, Possemiers S et al (2007) Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J Appl Microbiol 102:452–460. https://doi.org/10.1111/j.1365-2672.2006.03084.x
Franck A (2002) Technological functionality of inulin and oligofructose. Br J Nutr 87(Suppl 2):S287–S291. https://doi.org/10.1079/BJNBJN/2002550
Bornet FRJ (2000) Fructo-oligosaccharides and other fructans: chemistry, structure and nutritional effects. In: McCleary BV, Prosky L (eds) Advanced Dietary Fibre Technology. Blackwell Science Ltd, Oxford, UK, pp 480–493
Niness KR (1999) Inulin and oligofructose: what are they? J Nutr 129:1402S–1406S. https://doi.org/10.1093/jn/129.7.1402S
Monsan PF, Ouarné F (2009) Oligosaccharides derived from sucrose. In: Charalampopoulos D, Rastall RA (eds) Prebiotics and probiotics science and technology, Springer New York, vol 51. New York, NY, pp 293–336
Sumiyanto J, Dayan FE, Cerdeira AL et al (2012) Oligofructans content and yield of yacon (Smallanthus sonchifolius) cultivated in Mississippi. Sci Hortic 148:83–88. https://doi.org/10.1016/j.scienta.2012.09.020
Kays SJ, Nottingham S (2008) Biology and chemistry of Jerusalem artichoke: Helianthus tuberosus L. Taylor & Francis, Boca Raton
Kriukova Y, Jakubiak-Augustyn A, Ilyinska N et al (2017) Chain length distribution of inulin from dahlia tubers as influenced by the extraction method. Int J Food Prop 20:S3112–S3122. https://doi.org/10.1080/10942912.2017.1357043
Dominguez AL, Rodrigues LR, Lima NM et al (2014) An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Technol 7:324–337. https://doi.org/10.1007/s11947-013-1221-6
Ulber R, Sell D (2007) White biotechnology, vol 105. Springer, Berlin Heidelberg, Berlin, Heidelberg
KWS SAAT SE & Co. KGaA Feldstudie zur optimierten Schwergetreidefütterung beim Mastschwein in Hinblick auf Salmonellenstatus und Ebergeruchbefunddaten. https://www.kws.com/de/de/produkte/getreide/roggen/feldstudie-zur-roggenfuetterung/. Accessed 05 Dec 2019
Agriculture and Agri-Food Canada Entwicklung des Umsatzes mit Fleischersatzprodukten in Deutschland in den Jahren 2010 bis 2020 (in Millionen US-Dollar). https://de.statista.com/statistik/daten/studie/769415/umfrage/entwicklung-verkaufter-fleischersatzprodukte-in-deutschland/. Accessed 07 Aug 2019
Ni D, Xu W, Zhu Y et al (2019) Inulin and its enzymatic production by inulosucrase: characteristics, structural features, molecular modifications and applications. Biotechnol Adv 37:306–318. https://doi.org/10.1016/j.biotechadv.2019.01.002
Caracciolo F, Cicia G, Del Giudice T et al (2016) Human values and preferences for cleaner livestock production. J Clean Prod 112:121–130. https://doi.org/10.1016/j.jclepro.2015.06.045
Sonntag WI, Kiehas MT, Spiller A et al (2019) Consumer evaluation of intra-sustainable trade-offs in pig production – a mixed-method approach to analyze different consumer segments. Livest Sci 224:102–113. https://doi.org/10.1016/j.livsci.2019.04.010
Bonardi S (2017) Salmonella in the pork production chain and its impact on human health in the European Union. Epidemiol Infect 145:1513–1526. https://doi.org/10.1017/S095026881700036X
Fouhse JM, Zijlstra RT, Willing BP (2016) The role of gut microbiota in the health and disease of pigs. Animal Frontiers 6:30–36. https://doi.org/10.2527/af.2016-0031
Meier-Dinkel L, Strack M, Höinghaus K et al (2016) Consumers dislike boar taint related off-flavours in pork chops regardless of a meal context. Meat Sci 122:119–124. https://doi.org/10.1016/j.meatsci.2016.07.014
Miller R (2020) Drivers of consumer liking for beef, pork, and lamb: a review. Foods 9. https://doi.org/10.3390/foods9040428
Gibson GR, Hutkins R, Sanders ME et al (2017) Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14:491–502. https://doi.org/10.1038/nrgastro.2017.75
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125:1401–1412. https://doi.org/10.1093/jn/125.6.1401
Markowiak P, Śliżewska K (2018) The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog 10:21. https://doi.org/10.1186/s13099-018-0250-0
Rastall RA, Gibson GR (2015) Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol 32:42–46. https://doi.org/10.1016/j.copbio.2014.11.002
Martens EC, Kelly AG, Tauzin AS et al (2014) The devil lies in the details: how variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J Mol Biol 426:3851–3865. https://doi.org/10.1016/j.jmb.2014.06.022
Feng G, Mikkelsen D, Hoedt EC et al (2020) In vitro fermentation outcomes of arabinoxylan and galactoxyloglucan depend on fecal inoculum more than substrate chemistry. Food Funct 11:7892–7904. https://doi.org/10.1039/D0FO01103G
Carlson JL, Erickson JM, Hess JM et al (2017) Prebiotic dietary fiber and gut health: comparing the in vitro fermentations of beta-glucan, inulin and xylooligosaccharide. Nutrients 9. https://doi.org/10.3390/nu9121361
Jonathan MC, van den Borne JJGC, van Wiechen P et al (2012) In vitro fermentation of 12 dietary fibres by faecal inoculum from pigs and humans. Food Chem 133:889–897. https://doi.org/10.1016/j.foodchem.2012.01.110
Zhang X, Chen T, Lim J et al (2019) Fabrication of a soluble crosslinked corn bran arabinoxylan matrix supports a shift to butyrogenic gut bacteria. Food Funct 10:4497–4504. https://doi.org/10.1039/c8fo02575d
Barszcz M, Taciak M, Skomiał J (2016) The effects of inulin, dried Jerusalem artichoke tuber and a multispecies probiotic preparation on microbiota ecology and immune status of the large intestine in young pigs. Arch Anim Nutr 70:278–292. https://doi.org/10.1080/1745039X.2016.1184368
Ceppa F, Mancini A, Tuohy K (2019) Current evidence linking diet to gut microbiota and brain development and function. Int J Food Sci Nutr 70:1–19. https://doi.org/10.1080/09637486.2018.1462309
Verspreet J, Dornez E, van den Ende W et al (2015) Cereal grain fructans: structure, variability and potential health effects. Trends Food Sci Technol 43:32–42. https://doi.org/10.1016/j.tifs.2015.01.006
Wu W, Zhang L, Xia B et al. (2020) Bioregional alterations in gut microbiome contribute to the plasma metabolomic changes in pigs fed with inulin. Microorganisms 8. https://doi.org/10.3390/microorganisms8010111
Pluske JR, Turpin DL, Kim J-C (2018) Gastrointestinal tract (gut) health in the young pig. Animal Nutrition 4:187–196. https://doi.org/10.1016/j.aninu.2017.12.004
Uerlings J, Bindelle J, Schroyen M et al (2019) Fermentation capacities of fructan- and pectin-rich by-products and purified fractions via an in vitro piglet faecal model. J Sci Food Agric 99:5720–5733. https://doi.org/10.1002/jsfa.9837
Souza da Silva C, Bolhuis JE, Gerrits WJJ et al (2013) Effects of dietary fibers with different fermentation characteristics on feeding motivation in adult female pigs. Physiol Behav 110-111:148–157. https://doi.org/10.1016/j.physbeh.2013.01.006
Fu X, Liu Z, Zhu C et al (2019) Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit Rev Food Sci Nutr 59:S130–S152. https://doi.org/10.1080/10408398.2018.1542587
Priester M, Visscher C, Fels M et al (2020) Fibre supply for breeding sows and its effects on social behaviour in group-housed sows and performance during lactation. Porcine Health Manag 6:15. https://doi.org/10.1186/s40813-020-00153-3
Robert Koch-Institut (2018) Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2017. RKI-Bib1 (Robert Koch-Institut)
The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. (2018) EFSA J 16. 10.2903/j.efsa.2018.5500
Smith RP, Andres V, Cheney TE et al (2018) How do pig farms maintain low Salmonella prevalence: a case-control study. Epidemiol Infect 146:1909–1915. https://doi.org/10.1017/S0950268818002248
Andres VM, Davies RH (2015) Biosecurity measures to control Salmonella and other infectious agents in pig farms: a review. Compr Rev Food Sci Food Saf 14:317–335. https://doi.org/10.1111/1541-4337.12137
Visscher CF, Winter P, Verspohl J et al (2009) Effects of feed particle size at dietary presence of added organic acids on caecal parameters and the prevalence of Salmonella in fattening pigs on farm and at slaughter. J Anim Physiol Anim Nutr (Berl) 93:423–430. https://doi.org/10.1111/j.1439-0396.2008.00821.x
Lawhon SD, Maurer R, Suyemoto M et al (2002) Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol 46:1451–1464. https://doi.org/10.1046/j.1365-2958.2002.03268.x
Visscher CF, Klein G, Verspohl J et al (2011) Serodiversity and serological as well as cultural distribution of Salmonella on farms and in abattoirs in Lower Saxony, Germany. Int J Food Microbiol 146:44–51. https://doi.org/10.1016/j.ijfoodmicro.2011.01.038
Walia K, Argüello H, Lynch H et al (2016) Effect of feeding sodium butyrate in the late finishing period on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev Vet Med 131:79–86. https://doi.org/10.1016/j.prevetmed.2016.07.009
Tran THT, Everaert N, Bindelle J (2018) Review on the effects of potential prebiotics on controlling intestinal enteropathogens Salmonella and Escherichia coli in pig production. J Anim Physiol Anim Nutr (Berl) 102:17–32. https://doi.org/10.1111/jpn.12666
Wesoly R, Weiler U (2012) Nutritional influences on skatole formation and skatole metabolism in the pig. Animals (Basel) 2:221–242. https://doi.org/10.3390/ani2020221
Backus G, Higuera M, Juul N et al. Second progress report 2015 –2017 on the European declaration on alternatives to surgical castration of pigs. https://www.boarsontheway.com/wp-content/uploads/2018/08/Second-progress-report-2015-2017-final-1.pdf. Accessed 21 Jul 2020
Claus R, Lösel D, Lacorn M et al (2003) Effects of butyrate on apoptosis in the pig colon and its consequences for skatole formation and tissue accumulation. J Anim Sci 81:239–248. https://doi.org/10.2527/2003.811239x
Vhile SG, Kjos NP, Sørum H et al (2012) Feeding Jerusalem artichoke reduced skatole level and changed intestinal microbiota in the gut of entire male pigs. Animal 6:807–814. https://doi.org/10.1017/S1751731111002138
Pauly C, Spring P, O'Doherty JV et al (2008) Performances, meat quality and boar taint of castrates and entire male pigs fed a standard and a raw potato starch-enriched diet. Animal 2:1707–1715. https://doi.org/10.1017/S1751731108002826
Nobre C, Teixeira JA, Rodrigues LR (2012) Fructo-oligosaccharides purification from a fermentative broth using an activated charcoal column. New Biotechnol 29:395–401. https://doi.org/10.1016/j.nbt.2011.11.006
Palcic MM (1999) Biocatalytic synthesis of oligosaccharides. Curr Opin Biotechnol 10:616–624. https://doi.org/10.1016/S0958-1669(99)00044-0
Barreteau H, Delattre C, Michaud P (2006) Production of oligosaccharides as promising new food additive generation. Food Technol Biotechnol 3:323–333
Demchenko AV (2008) Handbook of Chemical Glycosylation. Wiley
Flamm G, Glinsmann W, Kritchevsky D et al (2001) Inulin and oligofructose as dietary fiber: a review of the evidence. Crit Rev Food Sci Nutr 41:353–362. https://doi.org/10.1080/20014091091841
van Arkel J, Sévenier R, Hakkert JC et al (2013) Tailor-made fructan synthesis in plants: a review. Carbohydr Polym 93:48–56. https://doi.org/10.1016/j.carbpol.2012.02.001
Banguela A, Hernández L (2006) Fructans: from natural sources to transgenic plants. Biotecnol Apl:202–210
Ullmann F, Elvers B (2016 // 2017) Ullmann’s food and feed, 3 volume set // Ullmann’s food and feed. Wiley-VCH; Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Kaur N, Gupta AK (2000) Carbohydrate reserves in plants: Synthesis and regulation, 1st ed. Developments in crop science, vol 26. Elsevier, Amsterdam, New York
Davis SC, Dohleman FG, Long SP (2011) The global potential for Agave as a biofuel feedstock. GCB Bioenergy 3:68–78. https://doi.org/10.1111/j.1757-1707.2010.01077.x
Ávila-Fernández Á, Galicia-Lagunas N, Rodríguez-Alegría ME et al (2011) Production of functional oligosaccharides through limited acid hydrolysis of agave fructans. Food Chem 129:380–386. https://doi.org/10.1016/j.foodchem.2011.04.088
Agricultural output. (2017) OECD
Barta J (1993) Jerusalem artichoke as a multipurpose raw material for food products of high fructose or inulin content. In: Fuchs A (ed) Inulin and inulin-containing crops, vol 3. Elsevier, pp 323–339
Karppinen S, Myllymäki O, Forssell P et al (2003) Fructan content of rye and rye products. Cereal Chemistry Journal 80:168–171. https://doi.org/10.1094/CCHEM.2003.80.2.168
Tomasik P (ed) (2004) Chemical and functional properties of food saccharides. Chemical and functional properties of food components series. CRC Press, Boca Raton
Moreno FJ, Sanz ML (eds) (2014) Food oligosaccharides: production, analysis, and bioactivity. IFT press series. John Wiley & Sons Inc, Chichester, West Sussex, Hoboken, NJ
Nollet LML, Toldra F (2012) Handbook of analysis of active compounds in functional foods. CRC Press, Hoboken
Stökle K, Kruse A (2019) Extraction of sugars from forced chicory roots. Biomass Conv Bioref 9:699–708. https://doi.org/10.1007/s13399-019-00374-9
de Leenheer L (1996) Production and use of inulin: industrial reality with a promising future. In: van Bekkum H, Rper H, Voragen F (eds) Carbohydrates as organic raw materials III. Wiley-VCH Verlag GmbH, Weinheim, Germany, pp 67–92
Mensink MA, Frijlink HW, van der Voort MK et al (2015) Inulin, a flexible oligosaccharide I: review of its physicochemical characteristics. Carbohydr Polym 130:405–419. https://doi.org/10.1016/j.carbpol.2015.05.026
Singh RS, Singh RP, Kennedy JF (2016) Recent insights in enzymatic synthesis of fructooligosaccharides from inulin. Int J Biol Macromol 85:565–572. https://doi.org/10.1016/j.ijbiomac.2016.01.026
Zhu Z, He J, Liu G et al (2016) Recent insights for the green recovery of inulin from plant food materials using non-conventional extraction technologies: a review. Innovative Food Sci Emerg Technol 33:1–9. https://doi.org/10.1016/j.ifset.2015.12.023
Li H, Zhu H, Qiao J et al (2012) Optimization of the main liming process for inulin crude extract from Jerusalem artichoke tubers. Front Chem Sci Eng 6:348–355. https://doi.org/10.1007/s11705-012-1295-0
Weiß K, Alt M (2017) Determination of single sugars, including inulin, in plants and feed materials by high-performance liquid chromatography and refraction index detection. fermentation 3:36. https://doi.org/10.3390/fermentation3030036
Apolinário AC, de Lima Damasceno BPG, de Macêdo Beltrão NE et al (2014) Inulin-type fructans: a review on different aspects of biochemical and pharmaceutical technology. Carbohydr Polym 101:368–378. https://doi.org/10.1016/j.carbpol.2013.09.081
Pourfarzad A, Habibi Najafi MB, Haddad Khodaparast MH et al (2015) Characterization of fructan extracted from Eremurus spectabilis tubers: a comparative study on different technical conditions. J Food Sci Technol 52:2657–2667. https://doi.org/10.1007/s13197-014-1310-1
Belwal T, Ezzat SM, Rastrelli L et al (2018) A critical analysis of extraction techniques used for botanicals: trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal Chem 100:82–102. https://doi.org/10.1016/j.trac.2017.12.018
Carranza CO, Fernandez AÁ, Bustillo Armendáriz GR et al (2015) Processing of fructans and oligosaccharides from agave plants. Processing and impact on active components in food. Elsevier, In, pp 121–129
Südzucker AG (2017) Stark in die Zukunft: Geschäftsbericht 2016/17. http://www.suedzucker.de/de/Downloads/Download_Daten/Finanzberichte/2016_17/Geschaeftsberichte_2016_17/GB_2016_17/SZ_GB_2016_17_de_WEB_1.pdf
Blakeney AB, McCleary BV, Mugford DC (1997) Fructans – analytical approaches to a fibre that ferments
Roberfroid MB (2007) Inulin-type fructans: functional food ingredients. J Nutr 137:2493S–2502S. https://doi.org/10.1093/jn/137.11.2493S
Ronkart SN, Blecker CS, Fourmanoir H et al (2007) Isolation and identification of inulooligosaccharides resulting from inulin hydrolysis. Anal Chim Acta 604:81–87. https://doi.org/10.1016/j.aca.2007.07.073
Moerman FT, van Leeuwen MB, Delcour JA (2004) Enrichment of higher molecular weight fractions in inulin. J Agric Food Chem 52:3780–3783. https://doi.org/10.1021/jf030590v
Livingston DP (1990) Fructan precipitation from a water/ethanol extract of oats and barley. Plant Physiol 92:767–769. https://doi.org/10.1104/pp.92.3.767
Ku Y, Jansen O, Oles CJ et al (2003) Precipitation of inulins and oligoglucoses by ethanol and other solvents. Food Chem 81:125–132. https://doi.org/10.1016/S0308-8146(02)00393-X
Paseephol T, Small D, Sherkat F (2007) Process optimisation for fractionating Jerusalem artichoke fructans with ethanol using response surface methodology. Food Chem 104:73–80. https://doi.org/10.1016/j.foodchem.2006.10.078
Nobre C, Castro CC, Hantson AL et al (2016) Strategies for the production of high-content fructo-oligosaccharides through the removal of small saccharides by co-culture or successive fermentation with yeast. Carbohydr Polym 136:274–281. https://doi.org/10.1016/j.carbpol.2015.08.088
Nobre C, Teixeira JA, Rodrigues LR (2015) New trends and technological challenges in the industrial production and purification of fructo-oligosaccharides. Crit Rev Food Sci Nutr 55:1444–1455. https://doi.org/10.1080/10408398.2012.697082
Novalin S, Zweckmair T (2009) Renewable resources - green biorefinery: separation of valuable substances from fluid-fractions by means of membrane technology. Biofuels Bioprod Biorefin 3:20–27. https://doi.org/10.1002/bbb.118
Kuhn RC, Palacio L, Prádanos P et al (2011) Selection of membranes for purification of fructooligosaccharides. Desalin Water Treat 27:18–24. https://doi.org/10.5004/dwt.2011.2038
Moreno-Vilet L, Moscosa-Santillán M, Grajales-Lagunes A et al (2013) Sugars and fructans separation by nanofiltration from model sugar solution and comparative study with natural agave juice. Sep Sci Technol 48:1768–1776. https://doi.org/10.1080/01496395.2013.786729
Kamada T, Nakajima M, Nabetani H et al (2002) Availability of membrane technology for purifying and concentrating oligosaccharides. Eur Food Res Technol 214:435–440. https://doi.org/10.1007/s00217-001-0486-6
Alles MJL, Tessaro IC, Noreña CPZ (2015) Concentration and purification of yacon (Smallanthus sonchifolius) root fructooligosaccharides using membrane technology. Food Technol. Biotechnol 53:190–200. https://doi.org/10.17113/ftb.53.02.15.3766
Pinelo M, Jonsson G, Meyer AS (2009) Membrane technology for purification of enzymatically produced oligosaccharides: Molecular and operational features affecting performance. Sep Purif Technol 70:1–11. https://doi.org/10.1016/j.seppur.2009.08.010
Moreno-Vilet L, Bonnin-Paris J, Bostyn S et al (2014) Assessment of sugars separation from a model carbohydrates solution by nanofiltration using a design of experiments (DoE) methodology. Sep Purif Technol 131:84–93. https://doi.org/10.1016/j.seppur.2014.04.040
Ortiz-Cerda IE, Bonnin J, Bostyn S et al (2014) Experimental and CFD modeling study of inulin-type fructan purification from a model solution by diafiltration on a pilot-scale unit. Sep Sci Technol 49:1125–1134. https://doi.org/10.1080/01496395.2014.880929
Kuhn RC, Mazutti MA, Albertini LB et al (2014) Evaluation of fructooligosaccharides separation using a fixed-bed column packed with activated charcoal. New Biotechnol 31:237–241. https://doi.org/10.1016/j.nbt.2014.02.005
Yun JW (1996) Fructooligosaccharides—Occurrence, preparation, and application. Enzym Microb Technol 19:107–117. https://doi.org/10.1016/0141-0229(95)00188-3
Hincha DK, Livingston DP, Premakumar R et al (2007) Fructans from oat and rye: composition and effects on membrane stability during drying. Biochim Biophys Acta 1768:1611–1619. https://doi.org/10.1016/j.bbamem.2007.03.011
Ronkart SN, Deroanne C, Paquot M et al (2007) Characterization of the physical state of spray-dried inulin. Food Biophysics 2:83–92. https://doi.org/10.1007/s11483-007-9034-7
Flickinger MC (ed) (2013) Downstream industrial biotechnology: recovery and purification. Wiley, Hoboken, New Jersey
Vaňková K, Polakovič M (2012) Design of fructooligosaccharide separation using simulated moving-bed chromatography. Chem Eng Technol 35:161–168. https://doi.org/10.1002/ceat.201100254
Vaňková K, Onderková Z, Antošová M et al (2008) Design and economics of industrial production of fructooligosaccharides. Chem Pap 62:303386. https://doi.org/10.2478/s11696-008-0034-y
Moura FAd, Macagnan FT, da Silva LP (2015) Oligosaccharide production by hydrolysis of polysaccharides: a review. Int J Food Sci Technol 50:275–281. https://doi.org/10.1111/ijfs.12681
Ricca E, Calabrò V, Curcio S et al (2007) The state of the art in the production of fructose from inulin enzymatic hydrolysis. Crit Rev Biotechnol 27:129–145. https://doi.org/10.1080/07388550701503477
Toneli J, Park K, Negreiros A et al (2010) Spray-drying process optimization of chicory root inulin. Dry Technol 28:369–379. https://doi.org/10.1080/07373931003645017
Sangeetha PT, Ramesh MN, Prapulla SG (2005) Recent trends in the microbial production, analysis and application of fructooligosaccharides. Trends Food Sci Technol 16:442–457. https://doi.org/10.1016/j.tifs.2005.05.003
Nobre C, Cerqueira MÂ, Rodrigues LR et al. (2015) Production and extraction of polysaccharides and oligosaccharides and their use as new food additives. In: Industrial biorefineries & white biotechnology. Elsevier, pp 653–679
Kovács Z, Benjamins E, Grau K et al (2014) Recent developments in manufacturing oligosaccharides with prebiotic functions. Adv Biochem Eng Biotechnol 143:257–295. https://doi.org/10.1007/10_2013_237
Ganaie MA, Lateef A, Gupta US (2014) Enzymatic trends of fructooligosaccharides production by microorganisms. Appl Biochem Biotechnol 172:2143–2159. https://doi.org/10.1007/s12010-013-0661-9
Farias DP, Araújo FF, Neri-Numa IA et al (2019) Prebiotics: trends in food, health and technological applications. Trends Food Sci Technol 93:23–35. https://doi.org/10.1016/j.tifs.2019.09.004
Lorenzoni ASG, Aydos LF, Klein MP et al (2015) Continuous production of fructooligosaccharides and invert sugar by chitosan immobilized enzymes: comparison between in fluidized and packed bed reactors. J Mol Catal B Enzym 111:51–55. https://doi.org/10.1016/j.molcatb.2014.11.002
Sangeetha PT, Ramesh MN, Prapulla SG (2005) Maximization of fructooligosaccharide production by two stage continuous process and its scale up. J Food Eng 68:57–64. https://doi.org/10.1016/j.jfoodeng.2004.05.022
Castro CC, Nobre C, de Weireld G et al (2019) Microbial co-culturing strategies for fructo-oligosaccharide production. New Biotechnol 51:1–7. https://doi.org/10.1016/j.nbt.2019.01.009
Nishizawa K, Nakajima M, Nabetani H (2001) Kinetic study on transfructosylation by .BETA.-Fructofuranosidase from Aspergillus niger ATCC 20611 and availability of a membrane reactor for ructooligosaccharide production. FSTR 7:39–44. https://doi.org/10.3136/fstr.7.39
Birmani MW, Nawab A, Ghani MW et al (2019) A review: role of inulin in animal nutrition. Journal of Food Technology. Research 6:18–27. https://doi.org/10.18488/journal.58.2019.61.18.27
Verdonk JMAJ, Shim SB, van Leeuwen P et al (2005) Application of inulin-type fructans in animal feed and pet food. Br J Nutr 93(Suppl 1):S125–S138. https://doi.org/10.1079/BJN20041355
Potthast C (2018) “Fibre”: necessary paradigm shift in pig feeding. https://en.engormix.com/pig-industry/articles/fibre-necessary-paradigm-shift-t41685.htm. Accessed 09 Apr 2020
Eurostat. https://ec.europa.eu/eurostat/de/data/database. Accessed 26 Aug 2020
Marlander B, Hoffmann C, Koch HJ et al (2003) Environmental situation and yield performance of the sugar beet crop in Germany: heading for sustainable development. J Agron Crop Sci 189:201–226. https://doi.org/10.1046/j.1439-037X.2003.00035.x
Cook E (2018) Agriculture, forestry and fishery statistics: 2018 edition, 2018 edition. Agriculture, forestry and fishery statistics, vol 2018. Publications Office of the European Union, Luxembourg
Jha R, Fouhse JM, Tiwari UP et al (2019) Dietary fiber and intestinal health of monogastric animals. Front Vet Sci 6:48. https://doi.org/10.3389/fvets.2019.00048
Flickinger EA, Fahey GC (2002) Pet food and feed applications of inulin, oligofructose and other oligosaccharides. Br J Nutr 87:S297–S300. https://doi.org/10.1079/BJN/2002552
Zhao PY, Wang JP, Kim IH (2013) Evaluation of dietary fructan supplementation on growth performance, nutrient digestibility, meat quality, fecal microbial flora, and fecal noxious gas emission in finishing pigs. J Anim Sci 91:5280–5286. https://doi.org/10.2527/jas.2012-5393
Rohlmann C, Verhaagh M (2019) Efken J. Steckbriefe zur Tierhaltung in Deutschland, Ferkelerzeugung und Schweinemast
Agriculture and Rural Development (2017) EU agricultural outlook: arable land area to continue its decline. https://ec.europa.eu/info/news/eu-agricultural-outlook-arable-land-area-continue-its-decline_en. Accessed 15 Feb 2021
Agriculture and Rural Development (2017) EU agricultural outlook: arable land area to continue its decline. https://ec.europa.eu/info/news/eu-agricultural-outlook-arable-land-area-continue-its-decline_en. Accessed 05 Dec 2019
Zühlsdorf A, Spiller A (2016) Kaufbereitschaft bei verpackten Schweinefleischprodukten im Lebensmitteleinzelhandel: Wie wichtig ist Verbrauchern das Thema Tierschutz? https://www.vzbv.de/sites/default/files/downloads/Tierschutz-Umfrage-Ergebnisbericht-vzbv-2016-01.pdf. Accessed 05 Dec 2019
European Court of Auditors (2018) Animal welfare in the EU: closing the gap between ambitious goals and practical implementation: Special Report. https://op.europa.eu/webpub/eca/special-reports/animal-welfare-31-2018/en/. Accessed 05 Dec 2019
Vijn I, Smeekens S (1999) Fructan: more than a reserve carbohydrate? Plant Physiol 120:351–360. https://doi.org/10.1104/pp.120.2.351
Verspreet J, Dornez E, Delcour JA et al (2015) Purification of wheat grain fructans from wheat bran. J Cereal Sci 65:57–59. https://doi.org/10.1016/j.jcs.2015.06.013
Uno Y (2019) The Japanese low Fodmap diet manual. CAMBRIDGE SCHOLARS Publishing, Newcastle upon Tyne
Johnson J, Wallace T (2019) Whole grains and their bioactives. Wiley
Lamp A, Kaltschmitt M, Lüdtke O (2020) Protein recovery from bioethanol stillage by liquid hot water treatment. J Supercrit Fluids 155:104624. https://doi.org/10.1016/j.supflu.2019.104624
Thomann R (2014) Weizen, Roggen & Co. Aktuel Ernahrungsmed 39:S13–S16. https://doi.org/10.1055/s-0033-1360024
McCleary BV, Charmier LMJ, McKie VA et al (2019) Determination of fructan (Inulin, FOS, Levan, and Branched Fructan) in animal food (Animal Feed, Pet Food, and Ingredients): Single-Laboratory Validation, First Action 2018.07. J AOAC Int 102:883–892. https://doi.org/10.5740/jaoacint.18-0330
FAOSTAT (2020) Artichokes: World production quantity. http://www.fao.org/faostat/en/#data/QC. Accessed 15 Feb 2021
Lavecchia R, Maffei G, Paccassoni F et al (2019) Artichoke waste as a source of phenolic antioxidants and bioenergy. Waste Biomass Valor 10:2975–2984. https://doi.org/10.1007/s12649-018-0305-y
Zeaiter Z, Regonesi ME, Cavini S et al (2019) Extraction and characterization of inulin-type fructans from artichoke wastes and their effect on the growth of intestinal bacteria associated with health. Biomed Res Int 2019:1083952. https://doi.org/10.1155/2019/1083952
Amorim C, Silvério SC, Prather KLJ et al (2019) From lignocellulosic residues to market: production and commercial potential of xylooligosaccharides. Biotechnol Adv 37:107397. https://doi.org/10.1016/j.biotechadv.2019.05.003
Samanta AK, Jayapal N, Jayaram C et al (2015) Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioactive Carbohydrates and Dietary Fibre 5:62–71. https://doi.org/10.1016/j.bcdf.2014.12.003
van den Ende W (2018) Novel fructan exohydrolase: unique properties and applications for human health. J Exp Bot 69:4227–4231. https://doi.org/10.1093/jxb/ery268
Xu W, Ni D, Zhang W et al (2019) Recent advances in levansucrase and inulosucrase: evolution, characteristics, and application. Crit Rev Food Sci Nutr 59:3630–3647. https://doi.org/10.1080/10408398.2018.1506421
Acknowledgements
We would like to thank the reviewers for their valuable assistance. Additionally, the authors want to thank Anne Lamp for proofreading and useful comments.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.Z., M.K.; Writing of the original draft: A.Z., C.V., M.K.; Writing, review and editing: A.Z., C.V., M.K.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zimmermann, A., Visscher, C. & Kaltschmitt, M. Plant-based fructans for increased animal welfare: provision processes and remaining challenges. Biomass Conv. Bioref. 13, 2667–2685 (2023). https://doi.org/10.1007/s13399-021-01473-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01473-2