Abstract
Oligosaccharide analysis is commonly done by acid hydrolysis and following HPLC analysis. A major problem is the incomplete hydrolysis of oligosaccharides and disaccharides and the increasing formation of volatile furfural from pentose monomers and hydroxymethylfurfural (HMF) from hexose monomers. This paper optimizes the conditions of hydrolysis approaches and proposes a method for oligosaccharide quantification. The optimal condition for hydrolysis of model xylan from corn cob was found to be for 100 °C hydrolysis temperature, 120 min hydrolysis time, and 2 wt% sulfuric acid concentration. Under these conditions, the total free and bound xylose yield was 77.4% and hemicellulose conversion 87.4% respectively; no degradation products were found. The optimal conditions for hydrolysis of model xylan from beech wood were found to be for 120 °C hydrolysis temperature, 120 min hydrolysis time, and 2 wt% sulfuric acid concentration. Under these conditions, the total free and bound xylose yield was 65.1% and hemicellulose conversion 70.5% respectively; no degradation products were found. For pentosan hydrolysate, conditions were further optimized (110 °C, 60 min, 2 wt% H2SO4). Standard addition of xylan from the corn cob for hydrolysation showed similar conversion rates (< 2% deviation); no matrix effects were detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbohydrates represent a major portion of biomass samples and consist mainly of polysaccharides constructed primarily of glucose, xylose, arabinose, galactose, and mannose monomeric subunits. In general, carbohydrates with a degree of polymerization (DP) lower than 10 are called oligosaccharides, while polysaccharides show a DP above 10 [1].
During hydrothermal pretreatment of lignocellulosic biomass, a certain share of these polysaccharides is partly hydrolyzed to water-soluble oligomers and monomers. They are hereafter referred to as sugar monomers being mainly xylose and glucose [2]. HPLC with refractive index detection enables the quantification of these soluble sugars as well as the subsequent carbohydrate degradation products (e.g., HMF and furfural). If the sugars are present in an oligomeric structure, an additional hydrolysis to separate the monomeric units is required.
Important parameters affecting the hydrolysis of pentose oligomers are temperature, time, and acid concentration. The challenge is that a complete hydrolysis of oligomeric structures is aimed for, while the further degradation of the released saccharides should be avoided as much as possible. This is true because typically, the combination of extreme temperatures (140° C) and long treatment time periods (> 3 h) during hemicellulose and cellulose hydrolysis leads to the degradation of the released monomeric sugars into insoluble humin. This final degradation product is typically challenging to quantify [3].
Most studies investigating lignocellulose pretreatment follow the NREL method for the quantification of overall C5 sugar content [4]. The problem with this measurement method is that the optimum conditions for the recovery of C5 and C6 sugars are not the same, since cellulose requires temperatures higher than hemicellulose for a complete hydrolysis. Thus, these higher temperature level results in more frequent degradation of C5 sugars. Furthermore, the interaction effects of temperature and time during the hydrolysis of C5 and C6 oligomers are hardly fully understood.
In this work, two model xylans as well as an unknown C5 sample from liquid hot water hydrolysis are investigated and optimized in terms of temperature, time, and acid concentration using HPLC-RI detection and response surface methodology with Box-Behnken design. The aim is to find the optimal hydrolysis conditions for a maximum recovery of total (free and bound) xylose and glucose from the different oligomers, respectively. The additional inclusion of the degradation products HMF and furfural enables full monomeric recovery. Furthermore, this paper aims to adjust the calculation of the total xylose recovery by adding a correction factor considering monomeric losses and degrading product formation, following the method of Lamp et al. [5]. Solid-phase analysis of products was not considered.
1.1 Pentosan structure
Pentosans are polymeric anhydrides of pentoses, mainly consisting of xylans, with an occurrence in wooden biomass [6]. Xylan itself is synthesized by enzymes in the Golgi apparatus. Xylan synthesis thus requires the coordinated action and regulation of these enzymes as well as others that synthesize and transport substrates [7].
Pentosans from plant tissues and cereals are carbohydrates consisting of a linear β-1.4-d-xylopyranosyl backbone structure linked with side chains of α-l-arabinofuranosyl at 2 and/or 3 position of the xylose chain (Fig. 1) [8].
Partial chemical structure of an arabinoxylan pentosan a linear β-1.4-d-xylopyranosyl backbone structure linked with randomly attached side chains of α-l-arabinofuranosyl at 2 and/or 3 position of the xylose chain [8]
Lignocellulosic biomass mainly consists of lignin, cellulose, and hemicellulose. During acid-catalyzed thermal hydrolysis, polymeric saccharides are depolymerized into their monomeric forms. Figure 2 illustrates the conversion paths of lignocellulosic biomass from polymeric structure via monomeric sugars—glucose from cellulose and hemicellulose, xylose from hemicellulose—towards 5-hydroxymethylfurfural from glucose and furfural from xylose. A prominent example of the conversion process is the acid-catalyzed thermal hydrolysis of the polymeric saccharides, releasing C5 and C6 saccharides. For example, harsh reaction conditions like temperatures above 140 °C lead to the formation of undesired humin byproducts [9].
Conversion paths of cellulose and hemicellulose during acid hydrolysis [9]
The formation of aqueous xylose monomers from solid xylan is described in a simple model of reversible reactions. However, the decomposition of xylan to xylose is in fact a multistep process. The xylan chain is first decomposed to xylo-oligosaccharides, then decomposition proceeds to xylose monomer, followed by the further decomposition into furfural [10].
1.2 Pentosan quantification methods
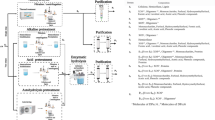
Quantitative pentosan analysis is mainly done by the quantification of the sugar monomers within the pentosan molecules and a subsequent recalculation step [11]. Generally, pentosan analysis includes the following fundamental steps:
-
Extraction of pentosans from sample matrix,
-
Purification of pentosan extract,
-
Hydrolysis of pentosan chains,
-
Determination and quantification of the released monomeric sugars xylose and arabinose for pentosans, and
-
Calculation of the original pentosan amount.
A widely spread procedure for sugar determination was established by Sluiter et al. [12], covering sugar, byproducts, and degradation products in liquid fraction process. The subsequent calculation of oligosaccharide concentration after acid hydrolysis is based on the method described by Huynh et al. [13] and was extended by Verspreet et al. [14] (Eq. (1)).
Equation (1) describes the concentration of the monomeric saccharides released from the oligomeric structure, where
- Ms (g/mol):
-
is the molecular weight of the saccharide monomer,
- Ve (mL):
-
is the volume of the extract,
- ms (g):
-
is the sample mass,
- Sa and Sb (g/L):
-
are the saccharide monomer concentrations in the non-hydrolyzed and the hydrolyzed sample, respectively, and
- Sx:
-
is the concentration of disaccharides containing the searched monomer.
This calculation does not account for a monomer yield loss due to the formation of HMF and furfural during oligomer hydrolysis. An alternative method for pentosan quantification is the colorimetric UV/Vis detection after acidic hydrolysis [15].
2 Experimental methods
2.1 Materials
Monomeric and oligosaccharide standards were purchased from Carl Roth GmbH. Beech wood and corn cob xylans (132 g/mol) were provided with a 95% purity and used without further purification. Wheat straw extract from liquid hot water treatment (LHW) was used as an unknown sample material [16].
2.2 Hydrolysis
The optimization of the hydrolysis conditions has been executed in two steps. First, standard samples of beech wood xylan and corn cob xylan were investigated to find the optimal hydrolysis conditions for maximum total sugar recovery. Second, these conditions have been applied to an unknown sample of a liquid hot water pretreated wheat straw extract (see the “2.1. Materials” section).
-
In case of the standard xylans, 2 g (66.6 mmol) was diluted in 200 mL demineralized water. For standard hydrolysis procedure, 175/350/525 μL (2, 4, 6 wt%) sulfuric acid (H2SO4 72%) were added to the sample (10 mL) in a 10-mL cuvette with screw cap. The cuvette was incubated at 80, 100, 110, 120, and 140 °C in a thermal reactor (TR420 thermal reactor from Merck) for 60, 120, and 180 min. The temperature ranges from 80 °C as to determine the minimum temperature that enables hydrolysis of xylan structures, while at 140 °C humin byproducts are formed rapidly, hence inhibiting the aim of complete monomer release and recovery. Immediately after incubation, the cuvette was cooled in an ice water bath at 0 °C until room temperature was reached. The solution was transferred to a 10-mL centrifuge tube and neutralized to pH 7 by adding CaCO3. The samples were stored at 4 °C for 24 h, then centrifuged (15 min, 4500 min−1), and the supernatants were stored for HPLC measurement. For the unknown sample (LHW-C5 hydrolysate), the same procedure has been executed in the range of 100 to 120 °C found to be the optimal range of hydrolysis parameters for xylans.
-
For standard addition of xylan to hydrolysate, stem solutions were prepared. In case of corn cob xylan, 5.0, 7.5, 10.0, and 12.5 g were diluted in 1000 mL demineralized water. An identical dilution row was prepared with the unknown hydrolysate from the wheat straw (LHW-C5 hydrolysate). Treatment of the samples followed the standard procedure in triplicates, at 110 °C, 2 wt% sulfuric acid and 60 and 90 min reaction time.
2.3 HPLC analysis
The amount of xylose and glucose as well as the degradation products—furfural and acetic acid from xylose, 5-hydroxymethylfurfural and formic acid from glucose—were measured by HPLC (Agilent Infinity II HPLC Series [5.3]). Sugar separation was achieved using a Biorad Aminex HPX-87P column with ionic form H+/CO3− deashing guard column and an Agilent Hi-Plex H+ column. A total of 5 mmol sulfuric acid was used as the mobile phase. Detection has been realized by a refraction index detector. The calibration process followed the adjusted method of NREL [12]. Standard solutions of glucose, xylose, arabinose, formic acid, acetic acid, 5-hydroxymethylfurfural, and furfural were prepared in the range between 0.024 and 24 mg/mL (Table 3).
2.4 Yields and conversions of saccharides and degradation products
The yields of released xylose and arabinose from hemicellulosic xylans as well as the conversion of the aforementioned saccharides towards furfural were determined by the method of Chen et al. [17] and expanded by the addition of arabinose yield. Chen et al.’s pretreatment combines sulfuric acid and moderate temperatures by conventional heating, followed by microwave irradiation heating. The equations for monomer yields and polymer conversion calculations as well as degradation product selectivity, correction factors for saccharides, reaction products, and the combined severity factor are displayed in Table 1. The hemicellulose stands for the total percentage of xylose, arabinose, and furfural converted from hemicellulose; the cellulose conversion designates the total percentage of glucose and HMF converted from cellulose.
2.5 Box-Behnken experimental design
The Box-Behnken design with response surface method was used to optimize the hydrolysis conditions to obtain the maximum amount of converted pentosan and xylose yield, achieving full monomeric recovery. Furthermore, interaction effects of the independent factors on the amount of total converted hemicellulose and xylose yield were studied. The independent variables were hydrolysis temperature (80 to 140 °C), hydrolysis time (60 to 180 min), and acid amount (2 to 6 wt%), which resulted in a three-factor three-level design. Statistical experiment planning was executed by using a commercially available software (Design Expert 9.0 [18]) with a central composite approach. The experimental plan is shown in Table 2 and consists of the abovementioned parameters. A total of at least 17 experimental runs were performed, and the actual predicted values of the response along with the experimental conditions are summarized in Tables 4, 5, 6, 7, 8, and 9.
3 Results and discussion
3.1 Hydrolysis optimization of corn cob xylan
Results
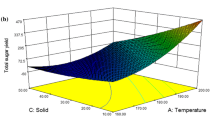
Application of the abovementioned conditions on corn cob xylan illustrated in Fig. 3 shows the released xylose concentrations. Bright (red) colored areas show the highest released xylose concentrations, while decreasing concentrations and eventually the lowest concentration are show in darkened colors, dark (blue) respectively.
The highest amount of converted hemicellulose (97.0%) was detected at 140 °C, 2 wt% sulfuric acid, and 120-min reaction time. The highest amount of xylose (77.4%) was obtained at 100° C, 2 wt% sulfuric acid, and 120-min reaction time. Lowest amounts of xylose were found at 140 °C, 6 wt% sulfuric acid, and 120-min reaction time (18.7%) and 140 °C, 4 wt% sulfuric acid, and 180-min reaction time (20.3%) (Table 4). Correspondingly, the highest furfural concentrations were found (33.3 and 33.4%). Disregarding the <5% impurities of the starting material, the full conversion is achieved and the highest amount of detected xylose increases to 81.4%.
The calculated combined severity factors (CSFs) for corn cob hydrolysis are displayed in Fig. 4. The maximum xylose yields were achieved in the severity range of 1.5 to 2.5 CSF. Increasing severity factors higher than 2.5 CSF show a decline in xylose yields. The decrease of xylose yields at high CSFs indicates the degradation of xylose over time at high temperatures.
Discussion
The results are indicating that higher temperatures and longer reaction times favor the conversion of hemicellulose to water-soluble products up to a maximum temperature and time; hereafter, humin formation is heavily favored [9]. The hemicellulose conversion is directly linked to temperature and time. Increased temperature provides more thermal effects (e.g., molecular collisions) resulting in more frequent cleavage of gylcosidic bonds between xylans. Catalyzing sulfuric acid provided highest conversion yields at 2 wt% addition. Temperatures above 100 °C favor the formation of furfural from xylose and arabinose over time. Hence, the maximum released xylose concentration results in incomplete hemicellulose conversion, while complete hemicellulose conversion lowers the xylose yield due to degradation. The amount of converted furfural can be corrected by the factor of Eq. (13). Compared to the method of Sluiter et al. [12] the optimized parameters (100 °C, 2 wt% acid, 120 min) provide higher released xylose concentrations from corn cob xylan (+14%) by reducing the degradation towards furfural (−22%) at similar hemicellulose conversion rates.
3.2 Hydrolysis optimization of beech wood xylan
Results
Released xylose monomers from beech wood xylan are illustrated in Fig. 5. Following this graphic, the highest amount of converted hemicellulose (74.2%) was detected at 120 °C, 4 wt% sulfuric acid, and 180-min reaction time. The highest amount of xylose (65.1%) was obtained at 100° C, 2 wt% sulfuric acid, and 120-min reaction time. The lowest amounts of released xylose have been measured at 80 °C, 2 wt% sulfuric acid, and 120-min time (6.3%), as well as 80 °C, 4 wt%, and 60 min (8.7%) (Table 5). A yield of ~30% released xylose was detected at 80 °C, 6 wt%, and 120 min and 80 °C, 4 wt%, and 180 min. Thus, the temperature of 80 °C does not provide a complete hydrolysis of xylan structures at the chosen period and acid concentration. The highest amount of furfural (~10%) was found at 120 °C, 6 wt%, 120 min and 120 °C, 4 wt%, 180 min. Disregarding the <5% impurities of the starting material, the hemicellulose conversion the highest amount of detected xylose increases to 74% and 68.5% respectively.
The calculated combined severity factors for beech wood hydrolysis are displayed in Fig. 6. The maximum xylose yields were achieved in the severity range of 2 to 3 CSF. Decreasing severity factors smaller than 2 CSFs show a decline in xylose yields. The lowest CSFs indicate that temperatures below 80°C are insufficient for xylose hydrolysis.
Discussion
In total, beech wood xylan is possibly harder to hydrolyze than corn cob xylan at the chosen conditions. At these conditions, it shows significantly lower xylose concentrations. In addition, beech wood xylan showed poor solubility in water. Potentially unsolved xylan in the stem solution might have contributed to the decreased xylose yields. A solution for this problem could be the inclusion of optimized catalytic ionic acids which show increased conversion rates for oligosaccharides from hard wood [19]. Compared to the method of Sluiter et al. [12], the optimized parameters (120 °C, 2 wt% acid, 120 min) provide a minorly higher released xylose concentrations from beech wood xylan (+2.3%) by reducing the degradation towards furfural (−1.7%) at similar hemicellulose conversion rates.
3.3 Hydrolysis optimization of C5 hydrolysate
Results
The detected monomer and corresponding concentrations of hydrolyzed C5 hydrolysate are displayed in Fig. 7. Hydrolysis of standard xylans showed highest xylose yields at low temperatures and acid concentrations within the chosen parameters. However, the screened conditions for C5 hydrolysis display no particular maximum of the released monomers. Treated C5 hydrolysate consists mainly of xylose (20 g/L) and partly glucose (4 g/L), as well as degradation products HMF (0.3 g/L), furfural (1.3 g/L), and released organic acids (3.8 g/L). The untreated C5 hydrolysate contains an average concentration of 3 g/L monomeric xylose. The highest amounts of released xylose (20.7 g/L) were detected at 110 °C, 2 wt% sulfuric acid, and 180-min reaction time with a furfural concentration of 1.25 g/L (Table 8). NREL conditions (120 °C, 4 wt%, 60 min) resulted in 6.3% fewer released xylose (19.4 g/L) and 21.3% higher concentrations of furfural (1.6 g/L).
The calculated combined severity factors for C5 hydrolysate hydrolysis are displayed in Fig. 8. Xylose concentrations were found in the severity range of 1.5 to 3 CSF. No particular maximum was detected; the reaction parameters have been narrowed for a corridor of optimal hydrolysis conditions.
Standard addition of xylans to C5 hydrolysate was executed to determine the degree of conversion and effects of matrix material. Corn cob xylan was chosen as an additive agent in standard addition, because of its better comparability as stem-like biomass with C5 hydrolysate. The dilution row was prepared for 5.0 to 12.5 g in C5 hydrolysate from wheat straw. Conditions were settled at 110 °C, 2 wt% sulfuric acid, and 60-min and 90-min reaction time.
Discussion
Hydrolysis at 2 wt% acid concentration showed the most promising results with a maximum of released xylose ranging along time and temperature axis in a broad area. This is confirmed by the severity factor which was calculated for C5 hydrolysate and plotted versus the xylose yields for all added sulfuric acid concentrations. Acid concentrations at 2 and 4 wt% result in a similar severity factor inside the optimum corridor, while at 6 wt%, degradation of xylose rapidly increases.
The released xylose concentrations from corn cob xylan standards with a concentration range from 5 to 12.5 g/mol (bottom bar), C5 hydrolysate averaged from 5 samples (top bar), and the combined solutions of both substrates (line) are displayed in Fig. 9 in triplicates including standard deviation. The recovery of monomers indicates a yield of at least 90% for each added amount of xylan to the hydrolysate. The deviation of the sum of added xylan and hydrolysate compared to the mixture is in any case lower than 2% for 60 min hydrolysis and lower than 9% for 90 min hydrolysis. This indicates that no matrix effects from either substrate inhibited the complete hydrolysis. As a result of standard addition, the transfer of the optimal hydrolysis conditions from standard xylans was achieved.
Summed up hydrolysis results from combined corn cob xylan dilution row and C5 hydrolysate displaying the determined concentrations of released monomers; the hydrolysis was executed at 110 °C, 2 wt% sulfuric acid, and 60-min run time. With corn cob xylan solution (bottom bar, dark gray), C5 hydrolysate (top bar, light gray), and combined solution of both substrates (line)
4 Conclusion
The aim was to find the optimal hydrolysis conditions for a maximum recovery of total xylose and the additional inclusion of the degradation products HMF and furfural enabling full monomeric recovery. The hydrolysis of corn cob and beech wood xylan as well as an unknown sample of a C5 hydrolysate has been analyzed by using response surface methodology based on a Box-Behnken design. Quadratic regression was used to define the effects of the variables, temperature, time, and acid concentration as well as their interaction effects on the responses of hemicellulose conversion and xylose yield. Analysis of variance developed a significant model using quadratic regression and predicted both responses with a high level of confidence. The optimized hydrolysis parameters for model xylans and unknown samples enabled the increased recovery of xylose and reduction of furfural formation. For corn cob xylan, the maximum xylose yield was found to be 77.3% (100 °C, 2 wt% acid, 120 min) at 87.5% hemicellulose conversion, and for beech wood xylan, the maximum xylose yield was found to be 65.1% (100 °C, 2 wt% acid, 120 min) at 70.5% hemicellulose conversion. The hydrolysis parameters (110 °C, 60 min, 2 wt% H2SO4) were found optimal for the application on liquid phase hemicellulose xylan structures. Standard addition of xylan from corn cob to C5 hydrolysate showed similar conversion rates (< 2% deviation); no matrix effects were detected. All substrates show increased yields of released xylose and reduced furfural compared to established methods. Recovery of monomers from oligosaccharide hydrolysis was achieved at conversion rates higher than 90% including the application of correction factors for HMF and furfural.
In future works, the hydrolysis condition for hydrolysates from wheat straw and applicability of the presented method will be further investigated focusing on the conversion of wheat straw hydrolysate from steam as promising follow-up substrate.
Data availability
All data will be provided transparently.
References
Dusselier M, Mascal M, Sels BF (2014) Top chemical opportunities from carbohydrate biomass: a chemist’s view of the biorefinery. In: Nicholas KM (ed) Selective catalysis for renewable feedstocks and chemicals. Springer International Publishing, Cham, pp 1–40
Fagerson IS (1969) Thermal degradation of carbohydrates; a review. J Agric Food Chem 17:747–750
Weingarten R, Cho J, Xing R, Conner WC, Huber GW (2012) Kinetics and reaction engineering of levulinic acid production from aqueous glucose solutions. ChemSusChem 5(7):1280–1290. https://doi.org/10.1002/cssc.201100717
Tsai Y-D (2012) Dilute acid hydrolysis of oligomers in hydrothermal pretreatment hydrolyzate into monomers with high yields. Masters Thesis, University of California Riverside
Lamp A, Kaltschmitt M, Lüdtke O (2018) Improved HPLC-method for estimation and correction of amino acid losses during hydrolysis of unknown samples. Anal Biochem 543:140–145. https://doi.org/10.1016/j.ab.2017.12.009
Peter B, Thaler H , Täufel K (1933) Zur Analytik der Pentosane. Mitteilung aus dem Uliversitätsinstitut und der Deutschen Forschungsanstalt für Lebensmittelchemie in München 1933(66):143–57.
Rennie EA, Scheller HV (2014) Xylan biosynthesis. Curr Opin Biotechnol 26:100–107. https://doi.org/10.1016/j.copbio.2013.11.013
Melim Miguel AS, Souza T, EVd CF, Paulo Lobo BW, Maria G (2013) Enzymes in bakery: current and future trends. In: Muzzalupo I (ed) Food Industry. InTech, Princeton
van Zandvoort I, Wang Y, Rasrendra CB, van Eck ERH, Bruijnincx PCA, Heeres HJ et al (2013) Formation, molecular structure, and morphology of humins in biomass conversion: influence of feedstock and processing conditions. ChemSusChem 6(9):1745–1758. https://doi.org/10.1002/cssc.201300332
Lavarack BP, Griffin GJ, Rodman D (2002) The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy 23(5):367–380. https://doi.org/10.1016/S0961-9534(02)00066-1
Tungland BC (2003) Fructooligosaccharides and Other Fructans: Structures and occurrence, production, regulatory aspects, food applications, and nutritional health significance. In: Eggleston G, Cote GL (eds) Oligosaccharides in food and agriculture. American Chemical Society, Washington, DC, pp 135–152
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of sugars, byproducts, and degradation products in liquid fraction process samples: laboratory analytical procedure (LAP); Issue Date: 12/08/2006. Technical Report NREL/TP-510-42623 January 2008
Huynh B-L, Palmer L, Mather DE, Wallwork H, Graham RD, Welch RM, Stangoulis JCR (2008) Genotypic variation in wheat grain fructan content revealed by a simplified HPLC method. J Cereal Sci 48(2):369–378. https://doi.org/10.1016/j.jcs.2007.10.004
Verspreet J, Pollet A, Cuyvers S, Vergauwen R, van den Ende W, Delcour JA, Courtin CM (2012) A simple and accurate method for determining wheat grain fructan content and average degree of polymerization. J Agric Food Chem 60(9):2102–2107. https://doi.org/10.1021/jf204774n
Thomann R (1982) Eine günstige Bestimmungsmethode für lösliche Pentosane. Die Nahrung 26(6):515–518
Reynolds W, Singer H, Schug S, Smirnova I (2015) Hydrothermal flow-through treatment of wheat-straw: detailed characterization of fixed-bed properties and axial dispersion. Chem Eng J 281:696–703. https://doi.org/10.1016/j.cej.2015.06.117
Chen W-H, Ye S-C, Sheen H-K (2012) Hydrolysis characteristics of sugarcane bagasse pretreated by dilute acid solution in a microwave irradiation environment. Appl Energy 93:237–244. https://doi.org/10.1016/j.apenergy.2011.12.014
Anderson M, Whitcomb P (2020) Design Expert 9.0. Minneapolis, Minnesota, USA: Stat-Ease
Matsagar BM, Dhepe PL (2015) Brönsted acidic ionic liquid-catalyzed conversion of hemicellulose into sugars. Catal Sci Technol 5(1):531–539. https://doi.org/10.1039/C4CY01047G
Code availability
None used
Funding
Open Access funding enabled and organized by Projekt DEAL. The ELBE-NH research project was supported via t Project Management Jülich, commissioned by the Federal Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 3156 kb)
Appendix
Appendix
1.1 Acid hydrolysis thermal (oven)
For standard hydrolysis procedure, 355 μL hydrolysis mixture (H2SO4 96%) was added to the sample (10 mL) in a 100-mL glass bottle with screw cap. The bottle was incubated at 121 °C in an oven for 1 h. Immediately after incubations, the bottle was cooled in an ice-water bath at 0 °C to room temperature. The solutions were transferred to 50-mL centrifuge tubes and neutralized by adding CaCO3 until pH 7 was adjusted. The samples were centrifuged (15 min, 4500 rpm), stored at 4 °C for 24 h, again centrifuged and stored for HPLC measurement.
1.2 Optimization of thermal acid hydrolysis
Hydrolysis of oligosaccharides in the thermal oven produced undesired results. Reaction vessels suffered permanent leakage. One hundred-millimeter glass flasks cannot be sealed to fullly extend at a temperature of 140 °C; all contained fluid evaporates within 180 min, thermally decomposing the desired product saccharides. Hence, 130 °C oven temperature is the maximum temperature applicable in the presented method, though still compromised by loss of volume. Hence, thermal oven hydrolysis results are disregarded.
1.3 HPLC Agilent Infinity II HPLC series details
-
Pump: product no. G7162A, Serial no. DEAC90341, made in Germany
-
Auto sampler: product no. G7116A, Serial no. DEAEM05467, made in Germany
-
Thermostat: product no. G7167-60101, Serial no. DEBBP07859, made in Germany
-
RI detector: product no. G7111A, Serial no. DEAEZ01310, made in Germany
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beckendorff, A., Lamp, A. & Kaltschmitt, M. Optimization of hydrolysis conditions for xylans and straw hydrolysates by HPLC analysis. Biomass Conv. Bioref. 13, 3361–3374 (2023). https://doi.org/10.1007/s13399-021-01429-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01429-6