Abstract
Efficient fractionation of lignocellulosic biomass is an important step toward the replacement of fossil-based products. However, the utilisation of all of the components in biomass requires various fractionation techniques. One promising process configuration is to apply steam explosion for the recovery of hemicelluloses and a subsequent hydrotropic extraction step for the delignification of the remaining solids. In this work, the influence of residence time, temperature and biomass loading on lignin recovery from birch using sodium xylene sulphonate as a hydrotrope was investigated. Our results show that residence time, temperature and biomass loading correlate positively with lignin extraction, but the effects of these parameters were limited. Furthermore, when steam explosion was implemented as the initial step, hydrotropic extraction could be performed even at room temperature, yielding a lignin extraction of 50%. Also, hydrothermal degradation of the material was necessary for efficient delignification with sodium xylene sulphonate, regardless of whether it occurs during steam explosion pretreatment or is achieved at high temperatures during the hydrotropic extraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The production of chemicals and biofuels from lignocellulosic biomass is important for reaching the Sustainable Development Goals, which have been set by the United Nations [1]. Lignocellulosic biomass comprises 3 main components: cellulose, hemicelluloses and lignin. Each of these components has the potential to replace fossil resources as a raw material for the production of various products. For example, cellulose can be used as a feedstock for the sugar platform, serving as a precursor for various fuels and chemicals [2]; it can also be used in the production of pulp and other materials, such as nanocellulose [3]. Hemicellulose can also be used as a feedstock in the sugar platform [4] and has several specialty applications, such as hydrogels, thermoplastics and the production of films and coatings [5,6,7]. Commercial applications for lignin include such products as vanillin, dispersants and concrete additives [8,9,10]. Ongoing research is examining the production of fuels and chemicals from lignin [10,11,12].
Due to differences in the structure and composition of cellulose, hemicellulose and lignin, these components require different types of chemical conversion routes for valorisation. Additionally, these components are tightly bound together in the complex structure of the lignocellulosic matrix. Thus, many commercial biomass fractionation processes target 1 or perhaps 2 products, neglecting the full potential of much of the biomass. This problem could be addressed by finding efficient fractionation strategies to separate each of the components into distinct streams.
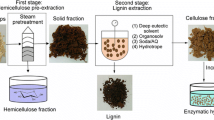
The successive combination of a steam pretreatment step and hydrotropic extraction (HEX) has been proposed to isolate cellulose, hemicelluloses and lignin into separate streams [13, 14]. Steam pretreatment, commonly referred to as steam explosion (STEX), opens up the rigid structure of biomass and solubilises hemicelluloses [15, 16]. Because most of the cellulose and lignin remain in the solid phase, steam pretreatment enables the recovery of hemicellulose through filtration. HEX, a method for extracting lignin from biomass [17], has recently garnered interest due to its low environmental impact [18, 19]. A hydrotrope is an amphiphilic molecule that increases the solubility of otherwise insoluble compounds in water [20, 21], allowing it to solubilise lignin, which has a hydrophobic structure. The exact mechanism is not yet completely understood. Abranches et al. [22] suggested that the hydrotropic effect is driven by hydrophobic interactions between the hydrotrope and the lignin while Srinivas et al. [20] proposed that the hydrotrope forms layered structures creating microenvironments in which the lignin can be solubilised. After extraction, the dilution of the hydrotropic solution with water causes lignin to precipitate. Several hydrotropes have been evaluated with regard to the extraction of lignin, such as sodium xylene sulphonate (SXS) [19, 23], alkyl benzene sulphonate [24, 25] sodium cumene sulphonate [26, 27] and p-toluene sulphonic acid [28]. Out of these compounds, SXS has emerged as a top candidate due to its extraction efficiency and low cost [18].
To optimise the performance of HEX, the effects of the conditions that are applied must be understood. In the case of HEX with SXS as the hydrotrope, previous studies have examined the following ranges of conditions: temperature 60–190 °C [24, 25, 29, 30], residence time 0.5–12 h [23, 24, 30] and liquid-to-wood ratio 4–10 with regard to biomass loading (BL) [23, 29, 31]. Most studies have evaluated 1 or 2 of these parameters, but none has assessed all 3 and their possible interactions. Furthermore, to the best of our knowledge, only Olsson et al. have reported a parameter study on HEX of steam-pretreated biomass [13], determining the effects of temperature and residence time. However, within the span of conditions that were examined, no substantial differences in lignin extraction were observed, indicating that a broader span of conditions must be analysed to identify the limits of the process. Understanding how the process operates at the limits of these factors could create opportunities for further process optimisation and provide a deeper understanding of its underlying mechanisms.
The main aim of this study was to perform an extensive investigation of the influence of temperature, residence time and BL on HEX of lignin from birch chips that have been pretreated by STEX. Secondary goals included determining the effects of temperature on the structure of the extracted lignin and the influence of pretreatment by STEX before HEX.
2 Materials and methods
2.1 Raw material
Debarked birch chips (Betula pendula) were provided by a Swedish pulp mill (Södra Cell, Mörrum, Sweden). The wood chips were cut into smaller pieces using a knife mill (Retsch GmbH, Haan, Germany) that was fitted with a 20-mm screen and then sieved to recover the 2–10-mm fraction. The composition of the raw material was determined using a National Renewable Energy Laboratory (NREL) standard procedure [32] and is shown in Table 1.
2.2 Experimental procedure
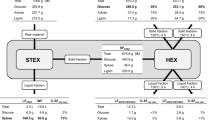
The lignocellulosic biomass was fractionated in 2 sequential steps: autocatalysed STEX to remove hemicelluloses, followed by HEX to extract lignin. The lignin was then precipitated by the addition of water to the hydrotropic solution. The process is illustrated schematically in Fig. 1.
2.2.1 Steam explosion
The chopped wood chips were impregnated with water to a dry matter (DM) content of ~50% and left in a cold storage room at 6 °C overnight. The impregnated chips were treated in batches of 750 g DM at 210 °C for 5 min per previous studies [13, 33] using a 10-L preheated pretreatment reactor, which has been described elsewhere [34]. After pretreatment, the liquid fraction was separated from the solid fraction using a hydraulic filter press (HP5M, Fischer Maschinenfabrik, Germany) at a pressure of 6 bar. To remove solubilised hemicelluloses from the fibres, the solid fraction was washed with warm deionised (DI) water, agitated for 1 h and then filtered at 6 bar. The composition of the solids after STEX is listed in Table 1.
2.2.2 Hydrotropic extraction
HEX was performed in a 2-L stirred batch reactor (Polyclave, Büchi AG, Switzerland) that was connected to a thermostat (Unistat T305, Kältenmaschinenbau AG, Germany), under constant agitation at 350 rpm. The hydrotropic agent was prepared by mixing DI water with 93% pure SXS powder (Stepanate SXS-93, Alsiano, Denmark). The concentration of SXS during extraction was 30% in all experiments. The pretreated material was added to the reactor on a DM basis. The heating and cooling times were 20 min each, regardless of the target temperature during the extraction. After HEX, the liquid fraction was separated from the solid fraction using a Büchner funnel that was connected to a vacuum, equipped with filter paper (Whatman qualitative filter paper, Grade 3). The liquid was stored in a cold storage room at 6 °C until further analysis. The solids were washed sequentially with 0.1 M NaOH, 0.05 M NaOH and DI water and stored at 6 °C. The DM of the solid was measured in triplicate at 105 °C on a scale (Mettler PM100, Mettler Toledo, Ohio, USA) that was equipped with an infrared dryer. The composition of the samples was determined using NREL standard methods.
2.2.3 Precipitation of lignin
To recover the lignin solubilised in the SXS solution after the HEX, the 30% SXS solution was diluted 10-fold with water in accordance with previous studies [23]. The diluted solution was centrifuged in a Sorvall LYNX 4000 Superspeed Centrifuge (ThermoFisher Scientific Inc., Massachusetts, USA) at 15,000 G for 10 min. The supernatant was decanted, and the remaining solids were re-suspended in DI water and centrifuged again. The resulting supernatant was decanted, and the remaining solids dried in an oven at 45 °C.
2.3 Factor-screening experiments
An experimental series was designed to determine the effects of 3 factors on lignin extraction during HEX. A screening design is an efficient way of investigating the effects of factors using a limited number of experiments. In this study, the experimental series was designed according to the 311B screening design (a hybrid design of experiment methodology developed from the central composite design methodology) that was developed by Roquemore [35]. The factors were residence time, temperature and BL. The conditions in the experiment are given in Table 2.
The lignin extraction after HEX was calculated per Eq. (1):
where Exlig is the lignin extraction (%), mlig, SFHEX is the mass of lignin in the solid fraction after HEX (g) and mlig, SFSTEX is the mass of lignin in the solid fraction after STEX (g). The data that were generated in the factor-screening experiments were fitted to a response surface model by multiple linear regression. During the selection of the factors in the model main factors, first-order interactions, second-order interactions and quadratic terms were considered.
2.4 Temperature experiments
After the screening experiments, the influence of temperature on the extraction was examined further at 25 °C, 60 °C, 95 °C, 120 °C, 145 °C, 170 °C, 200 °C, 210 °C, 225 °C and 250 °C. The lowest temperature of 25 °C was chosen, because it was close to ambient conditions and because using lower temperatures would have required active cooling. The highest temperature of 250 °C was selected as a practical upper limit. All extractions were performed at a residence time of 60 min and BL of 5%.
2.5 Pretreatment experiments
A set of experiments were performed to separate the effects of temperature on the hydrotropic extraction from those of the preceding STEX, designed as a 2-factor full-factorial experiment with 4 samples. The factors were the temperature during hydrotropic extraction and the use of STEX pretreatment as a categorical factor. The temperatures for the hydrotropic extraction were 25 °C and 250 °C. The STEX pretreatment was represented by the use of milled birch wood chips (not pretreated) or pretreated birch wood chips, prepared as described in the previous section.
2.6 Analytical procedures
2.6.1 Compositional analysis
The DM content was measured as oven-dry weight at 105 °C. To determine the lignin and carbohydrate content in the birch chips, the solid fractions after STEX (SFSTEX, Fig. 1) and after HEX (SFHEX, Fig. 1) were subjected to acid hydrolysis and then analysed per the NREL [32]. The content of acid-insoluble lignin (often referred to as Klason lignin) was determined using a filter crucible with a maximum pore size of 16 μm to collect the solid residue after the acid hydrolysis. The solid residue was dried at 105 °C overnight and then weighed. Carbohydrates in the liquid hydrolysate were measured by isocratic high-performance anion-exchange chromatography with pulsed amperometric detection on an ICS-3000 chromatography system (Dionex, USA) that was equipped with a Carbo Pac PA1 analytical column (Dionex, USA). Measurements were performed at 30 °C using DI water as the eluent at a flow rate of 1 mL min−1. The liquid fractions of selected samples were analysed per NREL standard procedures for liquids [36]. Carbohydrate content was determined by high-performance liquid chromatography (HPLC) using a refractive index detector (RID-10A, Shimadzu, Kyoto, Japan), with an Aminex HPX-87H column for separation.
2.6.2 Size exclusion chromatography of lignin
Size exclusion chromatography (SEC) was performed on an Alliance 2695 HPLC system (Waters, Milford, MA, USA) that was equipped with a 2487-UV detector (Waters, Milford, MA, USA) at 280 nm and a 2414 RI detector (Waters, Milford, MA, USA). Separation was conducted using 2 columns in series: a Superdex 200 Increase (300 × 10 mm, 9 μm) and a Superdex 30 (300 × 10 mm, 9 μm). The mobile phase was 0.1 M NaOH. Calibration was performed using poly-ethylene glycol standards with molecular weights of 200, 400, 1000, 4000, 10,000 and 35,000 Da. The method was based on previous research by Baumberger et al. [37]. The lignin samples were dissolved in 0.5% (wt/wt) NaOH to a concentration of approximately 1 g/L before analysis.
2.6.3 Combustional elemental analysis of lignin
Total carbon, hydrogen, nitrogen and sulphur in the samples were determined on an elemental analyser (Vario MICRO cube, Elementar Analysensysteme GmbH, Langeselbold, Germany). Oxygen was assumed to have constituted the remainder of the material.
3 Results and discussion
3.1 Hydrotropic extraction factor screening
Several models were fitted to the experimental data on lignin extraction in the factor-screening experiment. Based on the goodness of fit and residual of the regression analysis, the model in Eq. (2) was chosen:
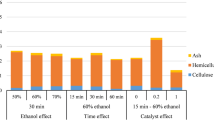
where Exlig is the lignin extraction (%), t is the residence time (min), T is the temperature (°C), BL is the biomass loading and α1–α5 are the experimentally determined constants. The model in Eq. (2) was used to generate response surfaces, as shown in Fig. 2. The estimated coefficients and their corresponding T-statistics and P-values are given in Table 3. The values of the coefficients are based on linear regression using coded variables, from the Roquemore 311B design. This was done to represent the relative effect of each factor on the extraction of lignin within the range of factor values investigated in this study.
Based on the results, lignin extraction correlated positively with temperature, consistent with previous findings that higher temperature increases lignin extraction during HEX of sugarcane bagasse and eucalyptus [24, 30]. The residence time had linear and quadratic effects on lignin extraction, as shown by Eq. (2)—i.e. whereas there was a greater effect initially, increasing the residence time beyond a certain point decreased the lignin extraction, with a local optimum at approximately 45 min, as shown in Fig. 2. This pattern indicates that the maximum achievable lignin extraction, with respect to residence time, was reached within the span investigated (0–60 min). This was compatible with an earlier study that reported similar nonlinear behaviour for sugar cane bagasse [25]; however, the residence time that was required to reach peak lignin extraction by Ansari and Gaikar [25] was considerably higher—approximately 180 min. One explanation for this difference could be the fact that the material in the present study was subjected to STEX before HEX. However, other factors might have accounted for this disparity, such as the use of a different raw material.
Lignin extraction also correlated positively with BL. This result was unexpected, because Ansari and Gaikar reported that the viscosity of a biomass slurry, above a BL of 7.5%, causes problems during HEX [25], indicating that the extraction efficiency decreases at higher BLs due to limitations in mass transfer. However, because this was not observed, another explanation is needed. Findings by McKee [17] might explain this behaviour, reporting that the effectiveness of a hydrotropic solution improves when the lignin content in the solution increases. Higher BLs signify that more lignin is available in the reactor, which can result in greater extraction of lignin.
3.2 Hydrotropic extraction temperature dependence
The effects of temperature on lignin extraction were determined over a range of 25–250 °C. The first notable observation in these experiments was that between 25 and 95 °C, lignin extraction above 45% was consistently achieved, as shown in Fig. 3, demonstrating clearly that temperature has little to no effect on lignin extraction below a certain point. Furthermore, this result indicates that STEX renders 45 to 50% of the lignin in the birch chips available for extraction by SXS.
Between 95 and 200 °C, lignin extraction increased steadily by approximately 20%, as shown in Fig. 3, consistent with the results from the factor-screening experiments. This finding also indicates that a temperature-dependent mechanism makes 20% of the lignin in the pretreated birch available for extraction during HEX, in addition to the 45 to 50% that is already accessible after STEX. One explanation for this pattern could be that mild hydrothermal breakdown of the structure occurs, which breaks bonds and releases bound lignin or reduces the particle size of lignocellulose, further increasing the interface between SXS and lignin. However, the exact mechanisms of this behaviour remain unexplained.
No gains in lignin extraction were observed above 200 °C. However, the selectivity for lignin during HEX decreased significantly, seen in Fig. 3 as an increase in glucan and xylan extraction. This finding is most likely explained by the more extensive degradation of the entire lignocellulosic structure at these temperatures, demonstrating that HEX at high temperatures serves as an extended hydrothermal pretreatment. Furthermore, as the temperature is increased above 200 °C, the overall mass loss during HEX increases from 20% or below to approximately 50% at 250 °C. This means that more of the solid material (in this case mainly cellulose) is found in the liquid fraction after HEX. Therefore, when choosing process conditions, it is important to keep in mind that there is a trade-off between the purity of both the cellulose- and lignin-rich fractions and the potential mass loss that comes with a higher temperature during HEX.
Notably, at 250 °C, the lignin extraction was 45% but reached approximately 68% at 225 °C, for which there are 2 potential explanations. On the face of it, these data indicate that HEX above 250 °C decreases the extractability of the lignin in the pretreated birch. However, previous studies have shown that the Klason lignin content in biomass samples can be overestimated due to the formation of pseudo-lignin [38]. As the calculation of lignin extraction was based on the sum of the Klason lignin and acid-soluble lignin in the solid fraction after HEX (SFHEX, Fig. 1), an overestimation of either of these lignin fractions could be interpreted as a decrease in extracted lignin. The formation of pseudo-lignin correlates with high pretreatment severities and acidic conditions [39]. Considering that higher temperatures are linked to greater severities [40] and that the pH in the liquid fraction after HEX was lower at elevated temperatures (see Table 4), the conditions were suitable for the formation of pseudo-lignin.
Pseudo-lignin is generated from carbohydrates in the material, particularly xylan [41]. In this study, analysis of the liquid samples after HEX showed that the xylan content decreased as the temperature rose above 210 °C, as shown in Table 4. Because the xylan content in the solid fraction declined simultaneously, as shown in Fig. 3, a substantial amount of carbon was unaccounted for in the mass balance. Had the xylan been transformed into pseudo-lignin, it would partly explain the observed decrease in lignin extraction. It could also indicate that the lignin extraction at temperatures above 210 °C is actually higher than what has been shown in this study.
Two notable observations were made with respect to the structure of the lignin precipitated from the liquid fraction after HEX. First, the weighted average molecular weight (Mw) at 200 °C and above decreased significantly, as shown in Table 5, evidenced as a shift toward smaller Mws at higher temperatures (Fig. 4), demonstrating that the lignin that was subjected to HEX at the highest temperatures (200 °C and 250 °C) was reduced in size. Second, in the elemental analysis of the precipitated lignin samples, the oxygen content was lower at 200 °C and 250 °C, as shown in Table 5. This finding could be explained by previous studies that have reported that cleavage of ether bonds under acidic conditions accelerates at temperatures above 160 °C [42]. Because ether bond cleavage is usually a hydrolytic reaction, resulting in the formation of water, the levels of structural oxygen in the lignin would decrease.
These results suggest that HEX at high temperatures is advantageous, because a reduction in the size of lignin particles—i.e. depolymerisation—is often desired for further processing of lignin [43]. Yet, the decrease in structural oxygen could indicate a loss of reactivity, if it was caused by the breaking of aryl ether bonds in the lignin structure because such bonds are often considered to be the most reactive bonds in lignin [44]. Because the usefulness of lignin as a precursor for chemical synthesis is often judged on its reactivity, a loss of reactive bonds is generally undesired.
3.3 Influence of STEX pretreatment
To separate the effects of the preceding STEX from those of temperature during HEX, 2 experiments at each extreme temperature (25 °C and 250 °C) were conducted, in which 2 main observations were made. First, the extraction was 0% for all components when performing HEX without a preceding STEX at 25 °C, as shown in Fig. 5. In contrast, the lignin extraction was 50% when adding a preceding STEX at the same HEX temperature. This result confirms the finding that STEX—i.e. hydrothermal pretreatment—has significant function in making the lignin available for extraction by SXS. The second observation is that at 250 °C, the differences in lignin extraction were small between configurations, as shown in Fig. 5, further supporting that HEX at elevated temperatures represents an extended hydrothermal pretreatment, in addition to its function as an extraction process. These findings underscore the importance of hydrothermal degradation as a mechanism for making lignin available for HEX.
4 Conclusions
In the factor-screening experiments, residence time, temperature and BL correlated positively with lignin extraction during HEX of steam-pretreated birch chips. However, the size of the effects was limited within the range of conditions that were tested, with lignin extraction falling between 45 and 69%. Running HEX at temperatures below 100 °C showed that 45 to 50% of the lignin could always be extracted, regardless of the temperature during HEX. Lignin extraction peaked at 200 °C. Increasing the temperature to above 200 °C did not improve the extraction of lignin; at 250 °C, the lignin extraction was 45%. Furthermore, under these conditions, the selectivity of lignin extraction fell due to extensive hydrothermal degradation of the lignocellulosic structure.
Nevertheless, our results demonstrate that hydrothermal degradation is essential for rendering lignin available for extraction by SXS, regardless of the inclusion of a preceding STEX step. Collectively, our data show that factors other than the HEX conditions, such as the inclusion of a preceding STEX, have a significant influence on the extractability of lignin in birch. Furthermore, performing sequential fractionation of lignocellulosic biomass using the method that has been presented in this paper allows for lignin extraction of up to 50% under mild conditions, which has implications on the suitability of the extracted lignin for further processing. The results presented in this paper show that there is potential for the process to be integrated within a lignocellulosic biorefinery. Efficient fractionation of the 3 main components of the biomass creates new opportunities for efficient utilisation of each fraction.
References
GA U (2015) Transforming our world: the 2030 Agenda for Sustainable Development. Division for Sustainable Development Goals, New York
Brethauer S, Studer MH (2015) Biochemical conversion processes of lignocellulosic biomass to fuels and chemicals–a review. CHIMIA Intl J Chem 69(10):572–581
Osong SH, Norgren S, Engstrand P (2016) Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: a review. Cellulose 23(1):93–123
Wyman CE, Dale BE (2015) Producing biofuels via the sugar platform. Chem Eng Prog 111(3):45–57
Gabrielii I, Gatenholm P, Glasser WG, Jain RK, Kenne L (2000) Separation, characterization and hydrogel-formation of hemicellulose from aspen wood. Carbohydr Polym 43(4):367–374
Jain RK, Sjöstedt M, Glasser WG (2000) Thermoplastic xylan derivatives with propylene oxide. Cellulose 7(4):319–336
Hansen NML, Plackett D (2008) Sustainable films and coatings from hemicelluloses: a review. Biomacromolecules 9(6):1493–1505
Fache M, Boutevin B, Caillol S (2016) Vanillin production from lignin and its use as a renewable chemical. ACS Sustain Chem Eng 4(1):35–46
Chen J, Kazzaz AE, AlipoorMazandarani N, Feizi ZH, Fatehi P (2018) Production of flocculants, adsorbents, and dispersants from lignin. Molecules:23(4)
Bajwa DS, Pourhashem G, Ullah AH, Bajwa SG (2019) A concise review of current lignin production, applications, products and their environment impact. Ind Crop Prod 139:111526
Bhat AH, Dasan YK, Khan I (2015) Extraction of lignin from biomass for biodiesel production. In: Hakeem KR, Jawaid MY, Alothman O (eds) Agricultural Biomass Based Potential Materials. Springer International Publishing, Cham, pp 155–179
Chheda JN, Huber GW, Dumesic JA (2007) Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew Chem Int Ed 46(38):7164–7183
Olsson J, Novy V, Nielsen F, Wallberg O, Galbe M (2019) Sequential fractionation of the lignocellulosic components in hardwood based on steam explosion and hydrotropic extraction. Biotechnol Biofuels:12(1)
Tian D, Chandra RP, Lee J-S, Lu C, Saddler JN (2017) A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol Biofuels 10(1):157
Schultz TP, Biermann CJ, McGinnis GD (1983) Steam explosion of mixed hardwood chips as a biomass pretreatment. Ind Eng Chem Prod Res Dev 22(2):344–348
Galbe M, Zacchi G (2012) Pretreatment: the key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 46:70–78
McKee RH (1943) Recovery of cellulose and lignin from wood. In: Google Patents
Mou H-Y, Fardim P, Wu S (2016) A novel green biomass fractionation technology: hydrotropic pretreatment. In: Mussatto SI (ed) Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery. Elsevier, pp 281–313
Gabov K, Fardim P, da Silva Júnior FG (2013) Hydrotropic fractionation of birch wood into cellulose and lignin: a new step towards green biorefinery. BioResources 8(3):3518–3531
Srinivas V, Rodley G, Ravikumar K, Robinson WT, Turnbull MM (1997) Molecular organization in hydrotrope assemblies. Langmuir 13(12):3235–3239
Gaikar VG, Sharma MM (1993) Separations with hydrotropes. Sep Technol 3(1):2–11
Abranches DO, Benfica J, Soares BP, Leal-Duaso A, Sintra TE, Pires E, Pinho SP, Shimizu S, Coutinho JAP (2020) Unveiling the mechanism of hydrotropy: evidence for water-mediated aggregation of hydrotropes around the solute. Chem Commun 56(52):7143–7146
Korpinen R, Fardim P (2009) Lignin extraction from wood biomass by a hydrotropic solution. O Papel 70(5):69–82
Tan SSY, MacFarlane DR, Upfal J, Edye LA, Doherty WOS, Patti AF, Pringle JM, Scott JL (2009) Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem 11(3):339
Ansari KB, Gaikar VG (2014) Green hydrotropic extraction technology for delignification of sugarcane bagasse by using alkybenzene sulfonates as hydrotropes. Chem Eng Sci 115:157–166
Devendra LP, Pandey A (2016) Hydrotropic pretreatment on rice straw for bioethanol production. Renew Energy 98(Supplement C):2–8
Karthyani S, Pandey A, Devendra LP (2017) Delignification of cotton stalks using sodium cumene sulfonate for bioethanol production. Biofuels:1–10
Chen L, Dou J, Ma Q, Li N, Wu R, Bian H, Yelle DJ, Vuorinen T, Fu S, Pan X (2017) Rapid and near-complete dissolution of wood lignin at≤ 80 C by a recyclable acid hydrotrope. Sci Adv 3(9):e1701735
Gabov K, Gosselink RJ, Smeds AI, Fardim P (2014) Characterization of lignin extracted from birch wood by a modified hydrotropic process. J Agric Food Chem 62(44):10759–10767
Vivian MA, da Silva Júnior FG, Fardim P, Segura TES (2017) Evaluation of yield and lignin extraction from Eucalyptus grandis × Eucalyptus urophylla wood chips with the hydrotropic compound sodium xylenesulphonate (SXS). BioResources 12(3):6723–6735
Gabov K, Hemming J, Fardim P (2017) Sugarcane bagasse valorization by fractionation using a water-based hydrotropic process. Ind Crop Prod 108(Supplement C):495–504
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomas. National Renewable Energy Laboratory, Golden
Novy V, Nielsen F, Olsson J, Aïssa K, Saddler JN, Wallberg O, Galbe M (2020) Elucidation of changes in cellulose ultrastructure and accessibility in hardwood fractionation processes with carbohydrate binding modules. ACS Sustain Chem Eng 8(17):6767–6776
Palmqvist E, Hahn-Hägerdal B, Galbe M, Larsson M, Stenberg K, Szengyel Z, Tengborg C, Zacchi G (1996) Design and operation of a bench-scale process development unit for the production of ethanol from lignocellulosics. Bioresour Technol 58(2):171–179
Roquemore KG (1976) Hybrid designs for quadratic response surfaces. Technometrics 18(4):419–423
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of sugars, byproducts, and degradation products in liquid fraction process samples. In: Laboratory analytical procedure. National Renewable Energy Laboratory, Golden
Baumberger S, Abaecherli A, Fasching M, Gellerstedt G, Gosselink R, Hortling B, Li J, Saake B, de Jong E (2007) Molar mass determination of lignins by size-exclusion chromatography: towards standardisation of the method. Holzforschung 61(4):459–468
Hu F, Jung S, Ragauskas A (2012) Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour Technol 117:7–12
Shinde SD, Meng X, Kumar R, Ragauskas AJ (2018) Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem 20(10):2192–2205
Overend RP, Chornet E (1987) Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos Trans R Soc Lond A Math Phys Sci 321(1561):523–536
Kumar R, Hu F, Sannigrahi P, Jung S, Ragauskas AJ, Wyman CE (2013) Carbohydrate derived-pseudo-lignin can retard cellulose biological conversion. Biotechnol Bioeng 110(3):737–753
Jasiukaitytė-Grojzdek E, Huš M, Grilc M, Likozar B (2020) Acid-catalyzed α-O-4 aryl-ether cleavage mechanisms in (Aqueous) γ-valerolactone: catalytic depolymerization reactions of lignin model compound during organosolv pretreatment. ACS Sustain Chem Eng 8:17475–17486
Chio C, Sain M, Qin W (2019) Lignin utilization: a review of lignin depolymerization from various aspects. Renew Sust Energ Rev 107:232–249
Evstigneyev EI, Shevchenko SM (2019) Structure, chemical reactivity and solubility of lignin: a fresh look. Wood Sci Technol 53(1):7–47
Availability of data and material
Not applicable.
Code availability
Not applicable.
Funding
Open access funding provided by Lund University. This work was conducted with financial support from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), grant number 232-2014-199.
Author information
Authors and Affiliations
Contributions
Johanna Olsson and Michael Persson designed the study. Johanna Olsson performed the data collection and analysis. Johanna Olsson and Michael Persson analysed the data and wrote the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no completing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olsson, J., Persson, M., Galbe, M. et al. An extensive parameter study of hydrotropic extraction of steam-pretreated birch. Biomass Conv. Bioref. 13, 4001–4009 (2023). https://doi.org/10.1007/s13399-021-01425-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01425-w