Abstract
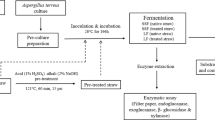
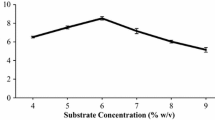
Optimization of saccharification of rice straw plays a crucial role in harnessing maximum fermentable sugars through enzymatic hydrolysis of this complex polymer. The present study was aimed to evaluate the applicability of A. tubingensis NKBP-55 cellulolytic cocktail for generation of fermentable sugars and to carry out parametric optimization of the saccharification process followed by batch ethanolic fermentation of the rice straw hydrolysate. Also, evaluation of the alterations (composition, crystallinity and structural morphology) occurring in the wall polymer structure after sequential treatments was studied. The cellulolytic cocktail, under the statistically optimized conditions (rice straw 4% w/v and pH 5.0) liberated 578 mg fermentable sugars (glucose 463 mg, xylose 114.35 and cellobiose 0.71 mg) per gram of pretreated rice straw showing 2.17-fold enhancement. Candida shehatae NCIM 3501 utilized both glucose and xylose and produced 16.15 g/L bioethanol along with yield (YP/S) 0.50 after 24 h of fermentation. Its bioethanol production was observed to be better than Saccharomyces cerevisiae (13.39 g/L) and their co-culture (15.57 g/L). Sequential changes brought about by the enzymatic treatment of the solid substrate revealed by X-ray diffraction and FTIR analyses showed decrease in the absolute crystallinity (CrI) (54.80 to 48.31%) and total crystallinity index (TCI) (1.002 to 0.991) and confirmed the degradation of the crystalline domain of cellulose by enzyme. The study demonstrated the potential of cellulolytic cocktail produced by A. tubingensis NKBP-55 in rice straw saccharification and its efficient fermentation by conventional and xylose-fermenting yeast in batch ethanolic fermentation.






Similar content being viewed by others
Data availability
Part applicable on reasonable request.
Abbreviations
- 2G:
-
second-generation
- SEM:
-
scanning electron microscopy
- XRD:
-
X-rays diffraction
- FTIR:
-
Fourier-transform infrared
- TGA:
-
thermal gravimetric analysis
- RI:
-
refractive index
- HPLC:
-
high-performance liquid chromatography
- NCIM:
-
National Collection of Industrial Microorganisms
- YP/S :
-
bioethanol yield
- CrI:
-
crystallinity index
- Iam :
-
intensity of amorphous region
- I002 :
-
intensity of crystalline region
- TCI:
-
IR total crystallinity index
- IR:
-
infrared
- CM:
-
copra meal
- APT:
-
alkali pretreated
- AET:
-
alkali enzyme treated
- FPU:
-
filter paperase unit
- CCD:
-
central composite design
- QP :
-
bioethanol productivity or yield or rate
- P:
-
bioethanol production
- S:
-
total fermentable sugar
- QS :
-
sugar consumption rate
- DSE:
-
crystallite size
- U:
-
enzyme unit
- EDX:
-
energy dispersive X-ray
References
Indira D, Jayabalan R (2019) Saccharification of lignocellulosic biomass using seawater and halotolerant cellulasewith potential application in second-generation bioethanol production. Biomass Convers Bioref. https://doi.org/10.1007/s13399-019-00468-4
NguyenTVT UY, Manmai N, Whangchai K, Ramaraj R (2020) Impact and significance of pretreatmenton the fermentable sugar production from low-grade longan fruit wastes for bioethanol production. BiomassConvers Bioref. https://doi.org/10.1007/s13399-020-00977-7
Rezania S, Oryani B, Park J, Hashemi B, Yadav KK, Kwon EE, Hur J, Cho J (2019) Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers Manag 201:112155. https://doi.org/10.1016/j.enconman.2019.112155
Chiranjeevi T, Mattam AJ, Vishwakarma KK, Uma A, Peddy ACR, Gandham S, Velankar HR (2018) Assisted single-step acid pretreatment process for enhanced delignification of rice straw for bioethanol production. ACS Sustain Chem Eng 6(7):8762–8774. https://doi.org/10.1021/acssuschemeng.8b01113
Singh R, Srivastava M, Shukla A (2016) Environmental sustainability of bioethanol production from rice straw in India: a review. Renew Sust Energ Rev 54:202–216. https://doi.org/10.1016/j.rser.2015.10.005
Kshirsagar SD, Waghmare PR, Loni PC, Patil SA, Govindwar SP (2015) Dilute acid pretreatment of rice straw, structural characterization and optimization of enzymatic hydrolysis conditions by response surface methodology. RSC Adv 5(8):46525–46533. https://doi.org/10.1039/C5RA04430H
Khaleghian H, Molaverdi M, Karimi K (2017) Silica removal from rice straw to improve its hydrolysis and ethanol production. Ind Eng Chem Res 56(35):9793–9798. https://doi.org/10.1021/acs.iecr.7b02830
Machinen L (2019) Lignocellulosic biofuel production: review of alternatives. Biomass Convers Bioref. https://doi.org/10.1007/s13399-019-00445-x
Anu KA, Singh D, Kumar V, Singh B (2020) Production of cellulolytic enzymes by Myceliophthora thermophila and their applicability in saccharification of rice straw. Biomass Convers Bioref. https://doi.org/10.1007/s13399-020-00783-1
Alam A, Zhang R, Liu P, Huang J, Wang Y, Hu Z, Madadi M, Sun D, Hu R, Ragauskas AJ, Tu Y, Peng L (2019) A finalized determinant for complete lignocellulose enzymatic saccharification potential to maximize bioethanol production in bioenergy Miscanthus. Biotechnol Biofuels 12:99. https://doi.org/10.1186/s13068-019-1437-4
Deng J, Zhu X, Chen P, He B, Tang S, Zhao W, Li X, Zhang R, Lv Z, Kang H, Yu L, Peng L (2019) Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks. Sustain Energy Fuels 4:607–618. https://doi.org/10.1039/C9SE00906J
Boonsombuti A, Trisinsub O, Luengnaruemitchai A (2020) Comparative study of three chemical pretreatments and their effects on the structural changes of rice straw and butanol production. Waste Biomass Valor 11:2771–2781. https://doi.org/10.1007/s12649-019-00622-z
Guo J-M, Wang Y-T, Cheng J-R, Zhu M-J (2020) Enhancing enzymatic hydrolysis and fermentation efficiency of rice straw by pretreatment of sodium perborate. Biomass Convers Bioref. https://doi.org/10.1007/s13399-020-00668-3
Banoth C, Sunkar B, Tondamanati PR, Bhukya B (2017) Improved physicochemical pretreatment and enzymatic hydrolysis of rice straw for bioethanol production by yeast fermentation. 3 Biotech 7:334. https://doi.org/10.1007/s13205-017-0980-6
Singh A, Bishnoi NR (2012) Optimization of enzymatic hydrolysis of pretreated rice straw and ethanol production. Appl Microbiol Biotechnol 93(4):1785–1793. https://doi.org/10.1007/s00253-012-3870-1
Prajapati BP, Jana UK, Suryawanshi RK, Kango N (2020) Sugarcane bagasse saccharification using Aspergillus tubingensis enzymatic cocktail for 2G bio-ethanol production. Renew Energy 152:653–663. https://doi.org/10.1016/j.renene.2020.01.063
Ghosh M, Prajapati BP, Suryawanshi RK, Dey KK, Kango N (2019) Study of the effect of enzymatic deconstruction on natural cellulose by NMR measurements. Chem Phys Lett 727:105–115. https://doi.org/10.1016/j.cplett.2019.04.063
Liu CG, Xiao Y, Xia XX, Zhao XQ, Peng L, Srinophakun P, Bai FW (2019) Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol Adv 37(3):491–504. https://doi.org/10.1016/j.biotechadv.2019.03.002
Santos ELI, Alanís MR, Saldívar RP, Alvarez AJ (2018) A novel method for bioethanol production using immobilized yeast cells in calcium-alginate films and hybrid composite pervaporation membrane. Bioresour Technol 247:165–173. https://doi.org/10.1016/j.biortech.2017.09.091
Mishra A, Ghosh S (2019) Bioethanol production from various lignocellulosic feedstocks by a novel “fractional hydrolysis” technique with different inorganic acids and co-culture fermentation. Fuel 236:544–553. https://doi.org/10.1016/j.fuel.2018.09.024
Prajapati BP, Suryawanshi RK, Agrawal S, Ghosh M, Kango N (2018) Characterization of cellulase from Aspergillus tubingensis NKBP-55 for generation of fermentable sugars from agricultural residues. Bioresour Technol 250:733–740. https://doi.org/10.1016/j.biortech.2017.11.099
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2011) Determination of structural carbohydrates and lignin in biomass, Laboratory analytical procedure (LAP), Technical Report NREL/TP-510-42618, National Renewable Energy Laboratory Version. 07–0815 p. https://www.nrel.gov/docs/gen/fy13/42618.pdf
Ghose TK (1987) Measurement of cellulase activity. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Chandel AK, Antunes FFA, Anjos V, Bell MJV, Rodrigues LN, Singh OV, Rosa CA, Pagnocca FC, Silva SSD (2013) Ultra-structural mapping of sugarcane bagasse after oxalic acid fiber expansion (OAFEX) and ethanol production by Candida shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels 2013:6,4. https://doi.org/10.1186/1754-6834-6-4
Ahirwar S, Soni H, Rawat HK, Prajapati BP, Kango N (2016) Experimental design of response surface methodology used for utilisation of palm kernel cake as solid substrate for optimised production of fungal mannanase. Mycology 7(3):143–153. https://doi.org/10.1080/21501203.2016.1229697
Antunes FAF, Santos JC, Chandel AK, Carrier DJ, Peres GFD, Milessi TSS, Silva SSD (2019) Repeated batches as a feasible industrial process for hemicellulosic ethanol production from sugarcane bagasse by using immobilized yeast cells. Cellulose 26:3787–3800. https://doi.org/10.1007/s10570-019-02341-z
Germec M, Turhan I (2018) Ethanol production from acid-pretreated and detoxified rice straw as sole renewable resource. Biomass Convers Bioref 8(3):607–619. https://doi.org/10.1007/s13399-018-0310-1
Segal L, Creely JJ, Martin AE Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Res J 29:786–794. https://doi.org/10.1177/004051755902901003
Suryawanshi RK, Jana UK, Prajapati BP, Kango N (2019) Immobilization of Aspergillus quadrilineatus RSNK-1 multi-enzymatic system for fruit juice treatment and mannooligosaccharide generation. Food Chem 289:95–102. https://doi.org/10.1016/j.foodchem.2019.03.035
Kaushal M, Ahlawat S, Makut BB, Goswami G, Das D (2019) Dual substrate fermentation strategy utilizing rice straw hydrolysate and crude glycerol for liquid biofuel production by Clostridium sporogenes NCIM 2919. Biomass Bioenerg 127:105257. https://doi.org/10.1016/j.biombioe.2019.105257
Kim I, Han JI (2012) Optimization of alkaline pretreatment conditions for enhancing glucose yield of rice straw by response surface methodology. Biomass Bioenergy 46:210–217. https://doi.org/10.1016/j.biombioe.2012.08.024
Jeya M, Zhang YW, Kim IW, Lee JK (2009) Enhanced saccharification of alkali-treated rice straw by cellulase from Trametes hirsuta and statistical optimization of hydrolysis conditions by RSM. Bioresour Technol 100(21):5155–5161. https://doi.org/10.1016/j.biortech.2009.05.040
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Bio Rev 62:334–361. https://doi.org/10.1128/MMBR.62.2.334-361.1998
Antunes FAF, Chandel AK, Brumano LPB, Hilares RT, Peres GFD, Ayabe LES, Sorato VS, Santos JR, Santos JC, Silva SSD (2018) A novel process intensification strategy for second-generation ethanol production from sugarcane bagasse in fluidized bed reactor. Renew Energy 124:189–196. https://doi.org/10.1016/j.renene.2017.06.004
Abbi M, Kuhad R, Singh A (1996) Bioconversion of pentose sugars to ethanol by free and immobilized cells of Candida shehatae (NCL-3501): fermentation behavior. Process Biochem 31(6):555–560. https://doi.org/10.1016/S0032-9592(95)00104-2
Zahed O, Jouzani GS, Abbasalizadeh S, Khodaiyan F, Tabatabaei M (2016) Continuous co-production of ethanol and xylitol from rice straw hydrolysate in a membrane bioreactor. Folia Microbiol 61(3):179–189. https://doi.org/10.1007/s12223-015-0420-0
Yuvadetkun P, Reungsang A, Boonmee M (2018) Comparison between free cells and immobilized cells of Candida shehatae in ethanol production from rice straw hydrolysate using repeated batch cultivation. Renew Energy 115:634–640. https://doi.org/10.1016/j.renene.2017.08.033
Meethit P, Ratanaprasit P, Sakdaronnarong C (2016) Candida shehatae and Saccharomyces Cerevisiae work synergistically to improve ethanol fermentation from sugarcane bagasse and rice straw hydrolysate in immobilized cell bioreactor. Eng Life Sci 16(8):706–719. https://doi.org/10.1002/elsc.201500147
Prasad S, Kumar S, Yadav KK, Choudhry J, Kamyab H, Bach QV, Sheetal KR, Kannojiya S, Gupta N (2019) Screening and evaluation of cellulytic fungal strains for saccharification and bioethanol production from rice residue. Energy 190:116422. https://doi.org/10.1016/j.energy.2019.116422
Wu H, Dai X, Zhou SL, Gan YY, Xiong ZY, Qin YH, Ma J, Yang L, Wu ZK, Wang TL, Wang WG, Wang CW (2017) Ultrasound-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Bioresour Technol 241:70–74. https://doi.org/10.1016/j.biortech.2017.05.090
Rahnama N, Mamat S, Md Shah UK, Ling FH, Rahman NAA, Ariff AB (2013) Effect of alkali pretreatment of rice straw on cellulase and xylanase production by local Trichoderma harzianum SNRS3 under solid state fermentation. BioResources 8(2):2881–2896. https://doi.org/10.15376/biores.8.2.2881-2896
Swain MR, Krishnan C (2015) Improved conversion of rice straw to ethanol and xylitol by combination of moderate temperature ammonia pretreatment and sequential fermentation using Candida tropicalis. Ind Crop Prod 77:1039–1046. https://doi.org/10.1016/j.indcrop.2015.10.013
Vu ND, Tran HT, Bui ND, Vu CD, Nguyen HV (2017) Lignin and cellulose extraction from Vietnam’s rice straw using ultrasound-assisted alkaline treatment method. Int J Polym Sci 1063695:1–8. https://doi.org/10.1155/2017/1063695
Phitsuwan P, Permsriburasuk C, Baramee S, Teeravivattanakit T, Ratanakhanokchai K (2017) Structural analysis of alkaline pretreated rice straw for ethanol production. Int J Polym Sci 4876969:1–9. https://doi.org/10.1155/2017/4876969
Mallick D, Poddar MK, Mahanta P, Moholkar VS (2018) Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour Technol 261:294–305. https://doi.org/10.1016/j.biortech.2018.04.011
Tsegaye B, Balomajumder C, Roy P (2019) Alkali delignification and Bacillus sp. BMP01 hydrolysis of rice straw for enhancing biofuel yields. Bull Natl Res Cent 43:136. https://doi.org/10.1186/s42269-019-0175-x
Acknowledgments
Authors thank the sophisticated instrumentation center (SIC) and DST-PURSE (II) scheme Dr. Harisingh Gour V.V., Sagar for SEM, XRD, FTIR, NMR and TGA facilities. Author BPP is grateful to University Grant Commission (UGC) for financial support as NFOBC fellowship.
Author information
Authors and Affiliations
Contributions
Bhanu Pratap Prajapati: conceptualization; data curation; formal analysis; methodology; resources; software; roles/writing—original draft; writing—review and editing. Naveen Kango: funding acquisition; investigation; project administration; supervision; validation; writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 463 kb)
Rights and permissions
About this article
Cite this article
Prajapati, B.P., Kango, N. Rice straw saccharification using cellulolytic cocktail from Aspergillus tubingensis and structure alterations studies of the wall polymer. Biomass Conv. Bioref. 13, 961–975 (2023). https://doi.org/10.1007/s13399-020-01237-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01237-4