Abstract
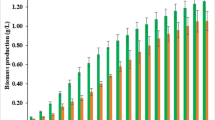
Utilization of harmful algal blooms (HABs) for the cultivation of oleaginous microorganisms can provide dual benefits of mitigating the toxicity from the aquatic reservoirs and generation of copious media for biodiesel production. In the present investigation, microwave-assisted dilute alkali-freeze pretreatment was optimized to develop a low-cost growth medium from HAB dried biomass. The electron micrographs along with the elemental analysis confirmed the efficient breakage of HABs after the microwave-assisted hydrolysis treatment as compared with the acid hydrolysis. Moreover, the sugar analysis revealed ~ 46% higher carbohydrate content in microwave-assisted hydrolysate as compared with acid hydrolysate. The microwave-assisted hydrolysate and conventional dilute acid hydrolysate were then used to cultivate microalga (Chlorella minutissima) and yeast (Trichosporon cutaneum) for biomass and lipid accumulation and compared to artificial media. Microalga showed ~ 1.3- and 2-fold higher dry cell weight (DCW) and lipid content, respectively, while the yeast growth increased by ~ 27% with lipid content of 30%. The fatty acid profiles and biodiesel properties were also amenable to the international biodiesel standards. Hence, the present study provides a proof-of-concept of utilizing HAB hydrolysate for culturing oleaginous microorganisms for potential biodiesel production.





Similar content being viewed by others
References
Kumar V, Kumar S, Chauhan PK, Verma M, Bahuguna V, Joshi HC, Ahmad W, Negi P, Sharma N, Ramola B, Rautela I, Nanda M, Vlaskin MS (2019) Low-temperature catalyst based hydrothermal liquefaction of harmful macroalgal blooms, and aqueous phase nutrient recycling by microalgae. Sci Rep 9:11384. https://doi.org/10.1038/s41598-019-47664-w
Wurtsbaugh WA, Paerl HW, Dodds WK (2019) Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water 6:1373. https://doi.org/10.1002/wat2.1373
Lapointe BE, Burkholder JM, Alstyne KLV (2018) Harmful macroalgal blooms in a changing world: causes, impacts, and management. In: Shumway SE, Burkholder JM, Morton SL (eds) Harmful algal blooms: a compendium desk reference. Wiley, pp. 515–560. https://doi.org/10.1002/9781118994672.ch15
Paerl HW, Gardner WS, Havens KE, Joyner AR, McCarthy MH, Newell SE, Qine B, Scott JT (2016) Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 54:213–222. https://doi.org/10.1016/j.hal.2015.09.009
Sengco MR (2009) Prevention and control of Karenia brevis blooms. Harmful Algae 8:623–6388. https://doi.org/10.1016/j.hal.2008.11.005
Steidinger KA (2009) Historical perspective on Karenia brevis red tide research in the Gulf of Mexico. Harmful Algae 8:549–561. https://doi.org/10.1016/j.hal.2008.11.009
Naruka M, Khadka M, Upadhayay S, Kumar S (2019) Potential applications of microalgae in bioproduct production: a review. Octa J Biosci 7:01–05
Jaiswal KK, Banerjee I, Singh D, Sajwan P, Chhetri V (2020) Ecological stress stimulus to improve microalgae biofuel generation: a review. Octa J Biosci 8:48–54
Arora N, Jaiswal KK, Kumar V, Vlaskin MS, Nanda M, Pruthi V, Chauhan PK Small-scale phyco-mitigation of raw urban wastewater integrated with biodiesel production and its utilization for aquaculture. Bioresour Technol 297:122489. https://doi.org/10.1016/j.biortech.2019.122489
Paudel SR, Banjara SP, Choi OK, Park KY, Kim YM, Lee JW (2017) Pretreatment of agricultural biomass for anaerobic digestion: current state and challenges. Bioresour Technol 245:1194–1205. https://doi.org/10.1016/j.biortech.2017.08.182
An YX, Zong MH, WuH LN (2015) Pretreatment of lignocellulosic biomass with renewable cholinium ionic liquids: biomass fractionation, enzymatic digestion and ionic liquid reuse. Bioresour Technol 192:165–171. https://doi.org/10.1016/j.biortech.2015.05.064
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Baruah J, Nath BK, Sharma R, Kumar S, Deka RC, Baruah DC, Kalita E (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6:141. https://doi.org/10.3389/fenrg.2018.00141
Su TC, Fang Z (2017) One-pot microwave-assisted hydrolysis of cellulose and hemicellulose in selected tropical plant wastes by NaOH-freeze pretreatment. ACS Sustain Chem Eng 5:5166–5174. https://doi.org/10.1021/acssuschemeng.7b00509
Chang JJ, Wu SQ, Dai YR, Liang W, Wu ZB (2013) Nitrogen removal from nitrate laden wastewater by integrated vertical-flow constructed wetland systems. Ecol Eng 58:192–201. https://doi.org/10.1016/j.ecoleng.2013.06.039
Wang XM, Wang LJ, Yu M, Chen H (2013) Freeze-thaw and sulfuric acid pretreatment of wheat straw for fermentable sugar release. Adv Mater Res 724−725:257–260. https://doi.org/10.4028/www.scientific.net/AMR.724-725.257
Tayyab M, Noman A, Islam W, Waheed S, Arafat Y, Ali F, Zaynab M, Lin S, Zhang H, Lin W (2018) Bioethanol production from lignocellulosic biomass by environment-friendly pretreatment methods: a review. Appl Ecol Environ Res 16:225–249. https://doi.org/10.15666/aeer/1601_225249
Huang H, Schwab K, Jacangelo JG (2009) Pretreatment for low pressure membranes in water treatment: a review. Environ Sci Technol 43:3011–3019. https://doi.org/10.1021/es802473r
Dubois M, Gilles KA, Ton JKH, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Kumar V, Kumar R, Rawat D, Nanda M (2018) Synergistic dynamics of light, photoperiod and chemical stimulants influences biomass and lipid productivity in Chlorella singularis (UUIND5) for biodiesel production. Appl Biol Chem 61:7–13. https://doi.org/10.1007/s13765-017-0332-6
Arora N, Patel A, Pruthi PA, Pruthi V (2016) Boosting TAG accumulation with improved biodiesel production from novel oleaginous microalgae Scenedesmus sp. IITRIND2 utilizing waste sugarcane bagasse aqueous extract (SBAE). Appl Biochem Biotechnol 180:109–121. https://doi.org/10.1007/s12010-016-2086-8
Arora N, Gulati K, Patel A, Pruthi PA, Poluri KM, Pruthi V (2017) A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Res 24:29–39. https://doi.org/10.1016/j.algal.2017.03.012
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Wang Q, Wang W, Tan X, Zahoor X, Chen Y, Guo Q, Yu Z, Zhuang X (2019) Low-temperature sodium hydroxide pretreatment for ethanol production from sugarcane bagasse without washing process. Bioresour Technol 291:121844. https://doi.org/10.1016/j.biortech.2019.121844
Arora N, Kumari P, Kumar A, Gangwar R, Gulati K, Pruthi PA, Prasad R, Kumar D, Pruthi V, Poluri KM (2019) Delineating the molecular responses of a halotolerant microalga using integrated omics approach to identify genetic engineering targets for enhanced TAG production. Biotechnol Biofuels 12:2. https://doi.org/10.1186/s13068-018-1343-1
Zheng H, Gao Z, Yin J, TangX JX, Huang H (2012) Harvesting of microalgae by flocculation with poly (γ-glutamic acid). Bioresour Technol 112:212–220. https://doi.org/10.1016/j.biortech.2012.02.086
Chen X, Li Z, Zhang X, Hu F, Ryu DD, Bao J (2009) Screening of oleaginous yeast strains tolerant to lignocelluloses degradation compounds. Appl Biochem Biotechnol 159:591–604. https://doi.org/10.1007/s12010-008-8491-x
Mazumdar S, Lee J, Oh M (2013) Microbial production of 2,3butanediol from seaweed hydrolysate using metabolically engineered Escherichia coli. Bioresour Technol 136:329–336. https://doi.org/10.1016/j.biortech.2013.03.013
Maurya R, Paliwal C, Chokshi K, Pancha I, Ghosh T, Satpati G, Pal R, Ghosh A, Mishra S (2016) Hydrolysate of lipid extracted microalgal biomass residue: an algal growth promoter and enhancer. Bioresour Technol 207:197–204. https://doi.org/10.1016/j.biortech.2016.02.018
Patel A, Arora N, Sartaj K, Pruthi V, Pruthi PA (2016) Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew Sust Energ Rev 62:836–855. https://doi.org/10.1016/j.rser.2016.05.014
Seo YH, Sung M, Han JI (2015) Ferric chloride based downstream process for microalgae based biodiesel production. Fuel 141:222–225. https://doi.org/10.1016/j.biortech.2015.01.004
Chowdhury R, Keen PL, Tao W (2019) Fatty acid profile and energy efficiency of biodiesel production from an alkaliphilic algae grown in the photobioreactor. Bioresource Technol Rep 6:229–236. https://doi.org/10.1016/j.biteb.2019.03.010
Huang C, Luo MT, Chen XF, Qi GX, Xiong L, Lin XQ, Wang C, Li HL, Chen XD (2017) Combined “de novo” and “ex novo” lipid fermentation in a mix-medium of corncob acid hydrolysate and soybean oil by Trichosporon dermatis. Biotechnol Biofuels 10:147. https://doi.org/10.1186/s13068-017-0835-8
Ferreira A, Reis A, Vidovic S, Vladic J, Gkelis S, Melkonyan L, Avetisova G, Congestri R, Acién G, Muñoz R, Collet P, Gouveia L (2019) Combining microalgae-based wastewater treatment with biofuel and bio-based production in the frame of a biorefinery. In: Hallmann A, Rampelotto P (eds) Grand challenges in algae biotechnology. Springer, Cham, pp 319–369. https://doi.org/10.1007/978-3-030-25233-5_9
Trivedi T, Jain D, Mulla NSS, Mamatha SS, Damare SR, Sreepada RA, Kumar S, Gupta V (2019) Improvement in biomass, lipid production and biodiesel properties of a euryhaline Chlorella vulgaris NIOCCV on mixotrophic cultivation in wastewater from a fish processing plant. Renew Energy 139:326–335. https://doi.org/10.1016/j.renene.2019.02.065
Nouri H, Moghimi H, Rad MN, Ostovar M, Sadat S, Mehr F, Ghanaatian F, Talebi AF (2019) Enhanced growth and lipid production in oleaginous fungus, Sarocladium kiliense ADH17: study on fatty acid profiling and prediction of biodiesel properties. Renew Energy 135:10–20. https://doi.org/10.1016/j.renene.2018.11.104
Mondal M, Ghosh A, Tiwari ON, Gayen K, Das P, Mandal MK, Halder G (2017) Influence of carbon sources and light intensity on biomass and lipid production of Chlorella sorokiniana BTA 9031 isolated from coalfield under various nutritional modes. Energy Convers Manag 145:247–254. https://doi.org/10.1016/j.enconman.2017.05.001
Zhang TY, Wang XX, Wu YH, Wang JH, Deantes-Espinosa VM, Zhuang LL, Hu HY, Wu GX (2017) Using straw hydrolysate to cultivate Chlorella pyrenoidosa for high-value biomass production and the nitrogen regulation for biomass composition. Bioresour Technol 244:254–1260. https://doi.org/10.1016/j.biortech.2017.05.095
Li P, Miao X, Li R, Zhong J (2011) In situ biodiesel production from fast-growing and high oil content Chlorella pyrenoidosa in rice straw hydrolysate. J Biomed Biotechnol 141207:1–8. https://doi.org/10.1155/2011/141207
Jain P, Arora N, Mehtani J, Pruthi V, Majumder CB (2017) Pretreated algal bloom as a substantial nutrient source for microalgae cultivation for biodiesel production. Bioresour Technol 242:152–160. https://doi.org/10.1016/j.biortech.2017.03.156
Masri MA, Jurkowski W, Shaigani P, Haack M, Mehlmer N, Brück T (2018) A waste-free, microbial oil centered cyclic bio-refinery approach based on flexible macroalgae biomass. Appl Energy 224:1–12. https://doi.org/10.1016/j.apenergy.2018.04.089
Xu X, Kim JY, Oh YR, Park JM (2014) Production of biodiesel from carbon sources of macroalgae, Laminaria japonica. Bioresour Technol 169:455–461. https://doi.org/10.1016/j.biortech.2014.07.015
Funding
This research work was carried out by the grant provided by DST, Govt. of India under the project (Indo Russian -INT/RUS/RFBR/347). MSV acknowledge the receipt of grant for research work from Russian Foundation for Basic Research (Indo-Russian project No. 18-58-45009).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 156 kb)
Rights and permissions
About this article
Cite this article
Kumar, V., Arora, N., Pandey, S. et al. Microwave-assisted pretreatment of harmful algal blooms for microbial oil-centered biorefinery approach. Biomass Conv. Bioref. 12, 3097–3105 (2022). https://doi.org/10.1007/s13399-020-00941-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00941-5