Abstract
Two different aquatic biomass sources—freshwater hornwort (Ceratophyllum demersum L.) and macroalga (Cladophora glomerata L.)—were used to produce biochars, which were investigated as Cr(III) ion sorbents. Wide range of pyrolysis temperatures from 250 to 800 °C was examined. Resultant biochars were characterized in detail by means of proximate analysis, ultimate analysis, FT-IR, SEM imaging, Boehm titration, and mercury porosimetry. The sorption capacities of the macroalga biochars varied from 104.2 to 163.9 mg g−1, whereas for hornwort biochars from 37.6 to 60.2 mg g−1. Obtained results were compared with literature data, suggesting that pyrolysis temperature and mineral matter content have crucial impact on the sorption capacities of Cr(III) ions. Simple thermal valorization of invasive aquatic macrophytes, i.e., hornwort or macroalga, allows to produce efficient adsorbents for chromium(III) ion removal from water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, a huge environmental concern is the removal of heavy metals and/or toxic metals from wastewaters as they can exhibit harmful impact on human and animal health as well as food purity [1,2,3]. Among heavy metals, much attention is paid to the removal of chromium (Cr) ions. Toxic hexavalent chromium can be easily introduced into water media and migrate through biological membranes (skin) and accumulate in kidneys, lungs, and liver causing their damage. It might also cause allergic reactions, DNA mutations, and as a result cancers [4, 5]. Meanwhile, trivalent chromium is less toxic, but due to the risk of oxidation to hexavalent chromium, the general emissions of chromium must be strictly limited and controlled. The main sources of chromium ions to water, air, and soil are industrial emissions from tannery and energy plants [6]. To reduce the amounts of Cr ions in wastewaters, several techniques are used: (i) precipitation, (ii) ion-exchange, (iii) adsorption [7]. Among these methods, adsorption appears to be the most common. Activated carbons (ACs) are the most popular sorbents, but due to their high price and problems with recovery in industrial scale, cost-effective sorbent materials must be investigated [8]. Biochars are gaining attention as alternative materials for ACs. Biochar is known as a carbon-rich material obtained in thermochemical conversion of biomass (namely pyrolysis process at 300–700 °C in an oxygen-free condition). Due to its properties, i.e., occurrence of abundant functional groups, porous texture, and high specific surface areas, biochar has found applications as heavy metals adsorbent from wastewaters and soils [9,10,11,12]. What is more, adsorption processes with the use of biochar are relatively inexpensive and highly efficient [6]. Additionally, for the production of biochars, a wide range of biomass sources, including waste materials can be used [13, 14]. One of the most important factors, when choosing biomass source, is its availability and simplicity to collect and low amount of preparation stages, which lowers the product price [15]. Aquatic biomass due to the widespread occurrence, diversity and fast growing abilities, might become a valuable source of carbon [16]. Macroalga and hornwort are examples of two different biomass sources, characterized with diverse physical and chemical properties, which might be treated as waste in many water reservoirs. Macroalga and hornwort do not need special growth conditions; they grow fast due to the high concentration of phosphorus and nitrogen in water that causes eutrophication, and thus, such biomasses are easily available [17,18,19]. In agreement with the principles of sustainable development, it is important to use waste as a raw material for other processes [20]. Biochars produced from aquatic biomass are mainly used for the removal of toxic metal ions from wastewater [21, 22] or as a soil amendment [23, 24]. Biochar from freshwater macroalga—Cladophora glomerata was examined as a sorbent of Cr(III) ions [21]; biochar from post-extraction of macroalga residues after oil extraction was used for sorption of Co(II) ions [22]; biochar from water hyacinth was investigated as a sorbent of Zn(II) and Cu(II) ions [25]; biochar from waste marine seaweeds—kelp and hijiki—was applied for sorption of Cd(II), Cu(II), and Zn(II) ions [26]. Hornwort, recognized as an aggressive aquatic weed (pest), easily invades still and flowing waters becoming a dominant species in such basins. Its fast growth may lead to serious ecological problems like weed proliferation. That is why the utilization of aquatic biomass including Ceratophyllum demersum is a forthcoming challenge both from scientific and practical point of view. Nevertheless, little work is devoted to hornwort or hornwort-originated biochars. The biosorption capabilities of hornwort was investigated in Zn(II), Cu(II), and Pb(II) sorption studies [27]. Keskinkan et al. proved that hornwort could be used as a submerged aquatic biomass for the removal of heavy metals. Meanwhile, Liu et al. in their study [28] investigated the leaching toxicity of Cd and Cu in hornwort biochar under different pyrolysis conditions. They proved that thermal treatment significantly improves the stabilization of Cu and Cd in biochars produced from hornwort. All this was an inspiration to investigate pyrolysis as a simple method of hornwort and macroalga conversion into efficient adsorbents of heavy metal ions.
The present work aimed at the utilization of aquatic weeds (freshwater green macroalga and hornwort) as a cheap and available biomass. To achieve this goal, we focused on the temperature optimization of thermal treatment for the production of biochars and to compare their chemical composition and sorption properties. This work is partly a continuation of our recent research [21], where biochars, obtained from freshwater macroalga Cladophora glomerata in pyrolysis at 300, 350, 400, and 450 °C, were successfully applied for Cr(III) ion removal from wastewater—the sorption capacity increased with the increase of pyrolysis temperature and was the highest for 450 °C (87.1 mg g−1). This observation encouraged us to perform further research that included (i) evaluation of the impact of higher pyrolysis temperatures (> 450 °C) on sorption of Cr(III) ions using Cladophora glomerata biochars, (ii) production of biochars derived from another aquatic biomass—hornwort in a wide range of pyrolysis temperatures (250, 300, 400, 500, 600, and 800 °C) and examination their sorption properties towards Cr(III) ions. In the present work, we proposed to use waste aquatic biomass as a new precursor for the production of biochar with good sorption capacities.
2 Materials and methods
2.1 Aquatic biomass collection and preparation
Macroalga (Cladophora glomerata) was collected from a pond in the Tomaszówek village (geographical coordinates: 51° 27′ 21″ N, 20° 07′ 43″ E) in August 2016. Hornwort (Ceratophyllum demersum) was collected from a pond near Zgorzelec (geographical coordinates: 51° 17′ 57″ N, 15° 05′ 37″ E) in July 2017. Both macroalga and hornwort were air-dried, milled, sieved to a particle size < 0.4 mm, and stored in a polypropylene tank for further processing.
2.2 Biomass composition
Cellulose, hemicellulose, and lignin contents were determined according to method described by Adeeyo et al. [29]. First, accurately 2.5 g of raw material was weighed and boiled for 4 h using Soxhlet extraction method with 150 mL of acetone to determine extractives content. After extraction, sample was air-dried overnight and dried again in the oven at 105 °C until constant weight was achieved. Hemicellulose content was determined using 1 g of dry, extractive-free sample. Sample was boiled for 3.5 h in 150 mL, 0.5 mol L−1 of NaOH. After process, solid residue was washed with distilled water to pH 7 and dried at 105 °C to constant weight. Lignin content was determined by the extraction with sulfuric acid. About 0.3 g of dry extractive-free biomass was weighed and mixed with 3 mL of 72% H2SO4 and kept at room temperature for 2 h. Next, 84 mL of distilled water was added. Mixture was autoclaved for 1 h at 121 °C in a Teflon reactor. Cooled sample was washed with distilled water to pH 7 and dried at 105 °C to constant weight. Residue was weighed and ashed to determine lignin content. Cellulose content was calculated by difference, assuming that raw material consists of cellulose, hemicellulose, lignin, ash, and extractives.
2.3 Biochar preparation
Hornwort biochars were obtained at 250, 300, 400, 500, 600, and 800 °C, while macroalga biochars at 500, 600, and 800 °C (lower temperatures were studied in our previous research [21]). Pyrolysis was conducted under nitrogen flow of 20 L h−1. The heating rate was 10 °C min−1 and the residence time 1 h. Biochars were prepared in an electric heated furnace Carbolite CTF 12/65/550 (CARBOLITE GERO, Parsons Lane, Hope Valley, UK). The yield of solid products was determined according to the Eq. (1).
The resultant biochars were designated as follows: shortcut (H) refers to hornwort, while (MA) to macroalga and the number after shortcut to corresponding pyrolysis temperature.
2.4 Biochar characterization
2.4.1 Ultimate analysis
The ultimate analysis of the raw biomass and obtained biochars (C, H, N and S) was conducted using Vario III Elemental Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). The amount of oxygen (O) was calculated by difference. Accurately 5 mg of sample was weighed in a tin boat. The analysis was conducted in two parallel measurements.
2.4.2 Proximate analysis
The proximate analysis of the raw biomass and obtained biochars involved moisture (PN-EN ISO 18134-2:2017-03), ash (PN-ISO 1171), and volatile matter (VM) (PN-EN ISO 18123:2016-01) measurements. For each analysis, accurately 1 g of sample was used. Moisture, ash, and volatile matter were determined by mass difference after measurements. Moisture was established after heating the sample at 105 ± 5 °C for 1.5 h, ash after incineration of sample at 815 ± 10 °C, and volatile matter after heating at 850 ± 10 °C for 7 min. All analyses were conducted in two parallel measurements.
2.4.3 Surface properties
The surface of obtained materials was determined using mercury porosimetry (Micromeritics AutoPore IV 9510, Micromeritics Instrument CORP, Norcross, GA, USA). The operating pressures were in a range of 0.1–400 MPa. The amount of sample used for measurements was about 0.1 g. All analyses were conducted in two parallel measurements.
2.4.4 Fourier transform infrared measurements
To define the occurrence of specific functional groups on the biochar surface, the sample was examined by means of FT-IR (Bruker FT-IR IFS 66/s; Billerica, MA, USA) with KBr beam splitter. The wavelength ranged from 4000 to 400 cm−1.
2.4.5 pHpzc
The value of pHpzc (point of zero charge) of obtained biochars was determined according to the method described by Moreno-Castilla et al. [30]. Prior to the measurement, distilled water was boiled for 3 h to remove the dissolved CO2 and rapidly cooled. Accurately 1 g of biochar was mixed with 20 mL of distilled water for 24 h. The pH value was then measured. All analyses were conducted in two parallel measurements.
2.4.6 SEM images
To investigate the textural properties of hornwort and macroalga biochars obtained at maximum pyrolysis temperature (800 °C), SEM images were performed using Jeol JSM-6610LVnx (JEOL USA Inc., Peabody, MA, USA). The pressure in the chamber at low vacuum mode ranged from 10 to 270 Pa. The accelerating voltage was 9 kV.
2.4.7 Boehm titration
Boehm titration was used to define the oxygen surface functional groups on the biochar surface. Accurately 0.5 g of biochar sample was placed in 100-mL Erlenmayer flask and mixed for 48 h with 0.1 mol L−1 solutions of HCl (to define the basic groups) and NaOH (to define the summarized amounts of carboxylic, lactone, and phenolic groups), respectively. After filtration, accurately 10 mL of solution was placed in a glass flask and titrated using 0.1 mol L−1 HCl or NaOH (for samples mixed with NaOH and HCl, respectively) and methyl red as an indicator. The titrations were made in two parallel measurements [31].
2.5 Sorption kinetics of Cr(III) ions
The solution of Cr(III) ions with initial concentration of 300 mg L−1 was prepared using inorganic salt—Cr(NO3)3 × 9H2O (Honeywell-Fluka, Bucharest, Romania). The pH of the solution was adjusted to 5, using 0.1 mol L−1 solutions of NaOH and/or HCl (Avantor Performance Materials Poland S.A., Gliwice, Poland). Accurately 0.2 g of biochar was mixed with 200 mL of the solution in a 250-mL Erlenmeyer flask. The flask was placed in a laboratory shaker (IKA KS 260 basic, IKA-Werke GmbH & Co., Staufen im Breisgau, Germany) with 200 rpm. The sorption was conducted for 3 h. While conducting the sorption process at distinct time intervals, samples were collected for Cr(III) ions concentration analysis. The measurement of Cr(III) ion concentration was made using UV-Vis spectrophotometer (V-5000, Shanghai Metash Instruments Co., Shanghai, China) at a wavelength of 540 nm. Prior to the measurement, 4 mL of the initial/filtrated solution after sorption was mixed with 0.095 g of EDTA (Avantor Performance Materials Poland S.A., Gliwice, Poland) and placed into a water bath for 10 min at 90 °C, until purple complex of EDTA and Cr(III) ions was formed. The blank sample was made by mixing 0.095 g of EDTA with 4 mL distilled water.
2.6 Equilibrium of sorption of Cr(III) ions
For equilibrium studies, accurately 0.04 g of biochar was mixed with 40 mL Cr(III) ions solution in a 250-mL Erlenmeyer flask. The Cr(III) ions initial concentrations were as follows: 25, 50, 75, 100, 125, 150, 200, 250, and 300 mg L−1. The pH of the solutions was adjusted to 5 as described in point 2.5. The flasks were placed in laboratory shaker (IKA KS 260 basic, IKA-Werke GmbH & Co., Staufen im Breisgau, Germany) with 100 rpm. After 2 h of shaking (determined in the kinetic studies), the equilibrium concentration of Cr(III) ions was measured as described in point 2.5.
2.7 Interpretation of Cr(III) ions sorption studies
The crucial parameter, which gives a direct information about the ability of sorbent towards binding pollutant is sorption capacity qt (mg g−1). It can be calculated from the Eq. (2):
where C0 (mg L−1) and Ct (mg L−1) are Cr(III) ion concentrations of initial solution and after defined time t (min), respectively; W (g) is the mass of biochar used for sorption and V (L) is the volume of the solution.
To describe the sorption process, the mathematical models were applied, i.e., Lagergren (pseudo-first-order model), pseudo-second-order model, Elovich model, and Bangham model (Table 1).
Using parameters, determined from Langmuir equation, a dimensionless parameter RL can be calculated as presented in the Eq. (9):
where C0 (mg L−1) is the initial Cr(III) ion concentration and KL (L mg−1) is the Langmuir equation constant.
3 Results and discussion
3.1 Chemical composition of biochars
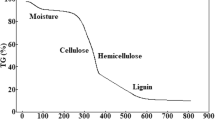
The biomass of macroalga and hornwort were first characterized by means of chemical composition, assuming that the aquatic biomass consists of cellulose, hemicellulose, lignin, extractives (moisture, lipids and chlorophyll), and mineral matter. The obtained results are given in Fig. 1. Both macroalga and hornwort consisted of about 18–20 wt.% of ash. Hornwort contained 20 wt.% of extractives, whereas macroalga 13 wt.%. Nearly 3-fold higher amount of lignin was detected in macroalga in comparison with hornwort. The main difference between the materials was expressed in cellulose and hemicellulose content. Macroalga consisted of 41 wt.% of hemicellulose and 10 wt.% of cellulose, while hornwort 18 wt.% and 39 wt.%, respectively. These differences can have a significant impact on the composition of the final product. Higher amounts of cellulose in the biomass lead to formation of volatile products and char, whereas hemicellulose during decomposition is transformed mainly into volatiles [32, 33]. Hemicellulose during thermochemical treatment is decomposed at lower temperatures (225–325 °C) than cellulose (325–375 °C). Lignin tends to decompose in a wide range of temperatures from 150 to 900 °C to form char and various phenols [34].
Biochars were produced in a wide range of temperatures of 250, 300, 400, 500, 600, and 800 °C for hornwort and 500, 600, and 800 °C for macroalga. Yields of solid products are given in Table 2. Macroalga is characterized with lower yield of solid product than hornwort. This might be due to higher content of hemicellulose in macroalga in comparison with hornwort. Yield of solid products obtained at 800 °C was about 38 and 33 wt.% for hornwort and macroalga, respectively. High ash content in the raw biomass resulted in its high content in biochars [35]. Macroalga biochars contained higher amounts of ash, and for MA800, it was 54.6 wt.%, while for H800, it was 45.1 wt.%. Thus, the mineral matter contents within raw materials were different and might be a result of different nature of aquatic biomass and/or various locations of biomass collection. Such high mineral matter contents also impact the pH point of zero charge values. Due to the presence of alkali metal oxides and salts within the raw biomass, the pHpzc of biochars were very high and varied from 7.82 to 11.20 for hornwort and from 9.83 to 13.18 for macroalga. Significant differences can be also seen in the volatile matter, where for MA500, it was 46.5% (dry, ash-free) and only 13.5% (dry, ash-free) for H500. What is more, the amount of volatile matter for hornwort biochars obtained above 400 °C was rather comparable and decreased slowly along with the rise of the pyrolysis temperature, while for macroalga, there was a significant drop in VM content (about 3-fold) at the temperature range from 600 to 800 °C, probably due to the decomposition of lignin and the formation of phenols, as described above. Hornwort biochars contained about 4-fold higher moisture in comparison with macroalga biochars.
Significant differences can be also seen in the ultimate analysis presented in Table 3. Higher amount of carbon (C) (dry, ash-free) for MA800 (73.3%) in comparison with H800 (64.1%), might be a result of the chemical composition of raw materials. What is more, macroalga biochars were characterized with nearly 3 wt.% content of sulfur (S), whereas for hornwort, it was < 0.5 wt.% (dry basis). The amount of oxygen (O) decreased with the increase of pyrolysis temperature more significantly for macroalga biochars than for hornwort biochars and might be related to the release of oxygen-containing compounds within the volatile matter. Amounts of hydrogen (H) and nitrogen (N) remained comparable for both hornwort and macroalga biochars.
3.2 Morphological characteristics and physicochemical properties of biochars
Mercury porosimetry was employed to have a deeper insight into the surface properties of biochars. The summarized results are given in Table 4. Both hornwort and macroalga biochars were characterized with relatively low surface area of about 20 m2 g−1 for macroalga and about 12 m2 g−1 for hornwort. The total intrusion volume varied from 0.573 to 0.852 cm3 g−1 for hornwort biochars, while for macroalga biochars, it varied from 0.854 to 1.188 cm3 g−1. This value increased along with the increase in pyrolysis temperature for macroalga, while for hornwort rather no significant impact of temperature was observed. The median pore diameter for hornwort varied from 3.9 to 41.4 nm, while for macroalga from 7.9 to 13.5 nm. The average pore diameter suggests that both studied materials are rather macroporous.
Fourier transform infrared spectroscopy was employed to investigate the functional groups over the materials surface, and the spectra are presented in Fig. 2. A characteristic peak can be observed for both raw materials at about 3500–3100 cm−1 and can be assigned to O–H vibrations of carbohydrates and moisture remaining in the material. In macroalga biochars a peak at about 1550 cm−1 can be attributed to aromatic carbon [36]. A presence of a peak at 1400 cm−1 can be related to bending C–H vibrations of CH2. The peak at about 1107 cm−1 was assigned to Si–O–Si and was a result of presence of diatoms within the biomass. Signal of Fe–O was observed at 617 cm−1 [37]. A peak at about 470 cm−1 was assigned to Si–O–Si and might be a result of diatoms present within the biomass and was also observed in macroalga biochars (slightly shifted in comparison with peak at 1050 cm−1 observed in hornwort). At 875 cm−1, a vibration of aromatic C–H bonds was observed for all biochars [21]. For hornwort biochars, until 300 °C, a peak of C–H vibrations was observed at about 2930 cm−1. A peak with decreasing intensity along with the pyrolysis temperature rise at about 1630 cm−1 was assigned to C=C stretching vibrations of aromatic ring [36]. An intensive peak at about 1050 cm−1 present in all hornwort biochars is related to Si–O–Si bridges and is a result of occurrence of ordered silica framework within the biomass [38]. The peak at about 775 cm−1 present in all hornwort samples might be assigned to long aliphatic chains [36]. SEM images were made to investigate the texture of biochars. Microscopic images were made for MA800 (Fig. 3a) and H800 (Fig. 3b) biochars. Dominant complex patterns can be observed for both macroalga and hornwort biochar surfaces. These structures were identified as diatoms—microorganisms present in both salt and freshwater. As it was confirmed using FT-IR, the presence of ordered silica framework might be a result of diatoms occurrence in the biomass [39]. Due to the high ash content, biochars agglomerated into bigger structures as was also reported in our recent work [21].
From Boehm titration, the amounts of basic groups and summarized carboxylic, lactone, and phenolic groups were defined. The obtained results are presented in Fig. 4a and b. The basic groups increased along with the pyrolysis temperature rise and varied from 0.7 to 2.7 mmol g−1 for hornwort samples and from 6.20 to 7.90 mmol g−1. It is also possible that certain amounts of HCl solution might interact with the mineral matter, and as a result, this value is not certain. The summarized amounts of carboxylic, lactone, and phenolic groups decreased along with temperature rise from 2.25 to 0.10 mmol g−1 for hornwort biochars and from 0.50 to 0.05 mmol g−1 for macroalga biochars.
3.3 Sorption properties of biochars
Biochars were examined in terms of sorption of trivalent chromium ions in kinetic experiments. As can be seen in Fig. 5, the sorption appeared to be fast and after about 60 min for hornwort and 120 min for macroalga biochars, the equilibrium was achieved. A rapid sorption at the beginning of the process and rather short time to equilibrium was also observed in our recent work (about 80 min) in sorption of Cr(III) ions [21] and by Lucaci et al. (about 60 min) in sorption of Co(II) ions [22]. This phenomenon was also widely observed in numerous researches dealing with metal sorption onto biochar or activated carbons and might be a result of rapid occupation of the external surface sorption sites, easily available over biochar surface. Thus, the initial rapid sorption might be related to physical sorption [40]. To investigate the behavior of sorbents during the process, Lagergren, pseudo-second-order, Bangham, and Elovich models were applied. The summarized results are given in Table 5. The correlation of Lagergren equation was rather low for all systems, as a result of rapid sorption at the beginning of the process and slower time to the equilibrium state [41]. Thus, the coefficient correlations (R2) varied from 0.887 to 0.989. High R2 value for pseudo-second-order model suggests that the rate-controlling step might be related with chemical sorption or chemisorption, which involves electron exchange or sharing between sorbate and sorbent [41, 42]. The obtained values of sorption capacity (qeq) increased along with the pyrolysis temperature rise for both hornwort and macroalga biochars. In the case of hornwort, the sorption capacity for H800 was 60% higher than for H250, and for macroalga this parameter was also 60% higher for MA800 when compared with MA500. This phenomenon was also observed in our previous work [21], where Cr(III) ion sorption capacity increased from 45.9 mg g−1 for macroalga biochar obtained at 300 °C to 87.1 mg g−1 for biochar obtained at 450 °C. For hornwort biochars, qeq values varied from 37.6 to 60.2 mg g−1, while for macroalga from 104.2 to 163.9 mg g−1. Relatively low coefficient with Bangham equation (R2 below 0.99) suggests that sorption of Cr(III) ions is not related with diffusion within the pores and other mechanisms are involved [43]. Elovich model correlated better with macroalga biochars than hornwort and R2 varied from 0.937 to 0.982. Values of a and b constants give information about the initial sorption rate and chemisorption activation energy, respectively [44]. The value of a constant tends to increase along with the temperature rise, whereas b constant appears to decrease, with certain exceptions. This phenomenon is related to the pyrolysis temperature and might be a result of chemical composition of biomass and obtained biochars. It should be stated that the amount of oxygen functional groups over the biochar surface decreased along with the pyrolysis temperature rise. Thus, the mechanism guiding sorption over biochars obtained at temperatures above 400 °C, might be shifted towards interactions of sorbate with aromatic ring or precipitation. Rivera-Utrilla et al. [45] have studied the Cr(III) ions sorption over ozonized activated carbons. The occurrence of Cπ-cation interactions was suggested as possible mechanism, which ruled the sorption process. When compared to our system, there might be an occurrence of Cπ-cation interactions of aromatic ring with metal ions over biochars obtained at pyrolysis temperatures above 500 °C. From Fourier transform infrared spectroscopy patterns, it can be observed that contribution of aromatic rings increased along with the pyrolysis temperature rise. Higher amounts of oxygen functional groups over macroalga biochars in comparison with hornwort biochars (as detected using Boehm titration), might be reflected in sorption capacities higher for macroalga samples. Nevertheless a synergic effect of several mechanism for Cr(III) ions removal must be taken into account.
The equilibrium of sorption was investigated using Freundlich and Langmuir models (Table 6). Langmuir isotherm is given in Fig. 6. Langmuir model fitted with the experimental data with coefficient R2 ranging from 0.879 to 0.975. The assumption of Langmuir model is a monolayer sorption mechanism, with no interactions between the molecules adsorbed over homogenous surface [46]. The qmax values for hornwort biochars increased with pyrolysis temperature rise, with exception for H400 biochar, where value was 50.25 mg g−1.
The qmax values at higher pyrolysis temperatures fitted also to values of qeq determined from pseudo-second-order model. In the case of macroalga biochars, only MA500 and MA600 measurements were made, due to precipitation of Cr(OH)3 during the sorption by biochar produced at higher temperature, in spite of adjusting pH to value of 5 prior to the measurement. As a result of high pHpzc values, the solution pH after sorption increased up to about 8, and the guiding mechanism of Cr(III) ion removal was shifted towards precipitation. Maximum sorption capacity of macroalga biochar towards Cr(III) ions was much higher than for hornwort—for example, qmax of MA500 was almost 2.5 times higher than for H500. This can result from the properties of both biochars—MA has higher total pore area than H. High pHpzc values might result from the presence of Ca and Mg within the biomass, causing local increase of pH in the solution [47]. Concentration of Ca, Mg, K, and Na in the solution after sorption might also increase due to cation exchange, what was also described by Kidgell et al. [48]. Values of RL parameter varied from 0.039 to 0.140 (Table 6), and these values were relatively close to 0. For RL > 1 sorption is unfavorable, for RL = 1 linear, for the range 0 < RL < 1 sorption is favorable and for RL = 0 irreversible [28]. For all examined biochars, RL values were in a range 0 < RL < 1 what indicates favorable sorption. The lower RL value, the more irreversible sorption of adsorbed pollutant over the sorbent surface [49]. Freundlich isotherms are given in Fig. 7. In the case of Freudnlich model, the experimental data fitted with the equation with coefficient R2 ranging from 0.914 to 0.996. The obtained results vary slightly from the Langmuir model, probably due to the precipitation occurring at low concentrations; nevertheless, the sorption capacities are similar in both models. Taking into account 1/n Freundlich constant values (Table 6), for all examined biochars were lower than 1, what resulted in favorable isotherms [50]. A comparison of Cr(III) ions sorption capacities of biochars from freshwater biomass with data available in literature is given in Table 7. As can be seen, there are only few works on Cr(III) ions sorption by biochars. It can be concluded that macroalga and hornwort biochars investigated in present study are characterized with relatively high sorption capacities. In the case of Cladophora glomerata biochar produced at 800 °C, sorption capacity was much higher, 164 mg g−1, than for the raw algal biomass, 78 mg g−1 (Godlewska et al. [56]), determined for the same experimental conditions (300 mg L−1, 1 g L−1, pH 5). There is no data on the sorption of Cr(III) ions by hornwort. In the work of Keskinkan et al. [27], it was shown that the maximum biosorption capacity of hornwort towards Zn(II), Cu(II), and Pb(II) ions was rather low and equal to 14 mg g−1, 6.2 mg g−1, and 45 mg g−1, respectively [28].
It is worth mentioning that the obtained metal-loaded biochar can be combusted. This solution enables metal and energy recovery. The residual ash can represent a valuable secondary raw material for the production of a given metal (metal smelting) [57, 58]. In our recent work, the desorption of Cr(III) ions was investigated by means of 0.1 mol L HCl solution. It was found that the sorption capacity (qt) decreased 4-fold in the second sorption cycle [51]. Ba et al. [59] investigated the desorption of Cr(III) ions using 2 mol L solutions: HCl, CH3COOH, and NaOH. The obtained results proved that it is difficult to regenerate the sorbent after Cr(III) ion sorption and the yield of desorption was 5% for NaOH solution, whereas for acid solutions desorption was not observed.
4 Conclusions
Easily available aquatic biomasses, i.e., hornwort (H) and macroalga (MA), were used as precursors for the pyrolytic production of efficient heavy metal ions adsorbents. Biochars were successfully applied in removal of Cr(III) ions from aqueous solutions. Sorption capacities of the best two sorbents: H800 and MA800 were 60.2 and 163.9 mg g−1, respectively. Thus, increasing pyrolysis temperature appeared to improve sorption capacity of both tested sorbents. It might be a result of increasing mineral matter within the biochars, that play an important role in the removal of Cr(III) ions in precipitation reactions. Functional groups present over the biochar surface, also took part in the sorption process; thus, a synergic effect of ion removal mechanisms must be taken into account. The differences might also be found in chemical composition of raw materials, i.e., hemicellulose, cellulose, and lignin; thus, different functional groups and porosity of obtained materials was observed. Biomass with higher amounts of hemicellulose (macroalga) than cellulose (hornwort), appeared to produce more valuable functional groups for Cr(III) ion sorption with simultaneously more narrow pore size distribution. Both waste freshwater biomasses can be recommended for the production of biochar, having potential use in the treatment of wastewater.
References
Piechalak A, Tomaszewska B, Baralkiewicz D, Malecka A (2002) Accumulation and detoxification of lead ions in legumes. Phytochemistry 60:153–162. https://doi.org/10.1016/S0031-9422(02)00067-5
Kidgell JT, De Nys R, Paul NA, Roberts DA (2014) The sequential application of macroalgal biosorbents for the bioremediation of a complex industrial effluent. PLoS One 9. https://doi.org/10.1371/journal.pone.0101309
Hernández Rodiguez M, Yperman J, Carleer R et al (2018) Adsorption of Ni(II) on spent coffee and coffee husk based activated carbon. J Environ Chem Eng 6:1161–1170. https://doi.org/10.1016/j.jece.2017.12.045
Paine AJ (2001) Mechanisms of chromium toxicity, carcinogenicity and allergenicity: review of the literature from 1985 to 2000. Hum Exp Toxicol 20:439–451. https://doi.org/10.1191/096032701682693062
Mohan D, Pittman CU (2006) Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J Hazard Mater 137:762–811. https://doi.org/10.1016/j.jhazmat.2006.06.060
Chen T, Zhou Z, Xu S, Wang H, Lu W (2015) Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour Technol 190:388–394. https://doi.org/10.1016/j.biortech.2015.04.115
Elangovan R, Philip L, Chandraraj K (2008) Biosorption of hexavalent and trivalent chromium by palm flower (Borassus aethiopum). Chem Eng J 141:99–111. https://doi.org/10.1016/j.cej.2007.10.026
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645. https://doi.org/10.1016/j.jhazmat.2008.06.042
Dawood S, Sen TK, Phan C (2017) Synthesis and characterization of slow pyrolysis pine cone bio-char in the removal of organic and inorganic pollutants from aqueous solution by adsorption: kinetic, equilibrium, mechanism and thermodynamic. Bioresour Technol 246:76–81. https://doi.org/10.1016/j.biortech.2017.07.019
Komnitsas K, Zaharaki D, Pyliotis I, Vamvuka D, Bartzas G (2015) Assessment of pistachio shell biochar quality and its potential for adsorption of heavy metals. Waste Biomass Valoriz 6:805–816. https://doi.org/10.1007/s12649-015-9364-5
Jian PJ, Jiang J, Kou XR (2014) Removal of Cr(VI) from aqueous solutions by Na2SO3/FeSO4 combined with peanut straw biochar. Chemosphere 101:71–76. https://doi.org/10.1016/j.chemosphere.2013.12.026
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20:358–368. https://doi.org/10.1007/s11356-012-0873-5
Zhou Y, Gao B, Zimmerman AR et al (2013) Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem Eng J 231:512–518. https://doi.org/10.1016/j.cej.2013.07.036
Inyang MI, Gao B, Yao Y et al (2016) A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit Rev Environ Sci Technol 46:406–433. https://doi.org/10.1080/10643389.2015.1096880
Gupta VK, Nayak A, Agarwal S (2015) Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environ Eng Res 20:1–18. https://doi.org/10.4491/eer.2015.018
Bird MI, Wurster CM, de Paula Silva PH, Bass AM, de Nys R (2011) Algal biochar - production and properties. Bioresour Technol 102:1886–1891. https://doi.org/10.1016/j.biortech.2010.07.106
Bulgariu L, Bulgariu D (2020) Bioremediation of toxic heavy metals using marine algae biomass. In: Green materials for wastewater treatment. Springer, Cham, pp 69–98. https://doi.org/10.1007/978-3-030-17724-9_4
Torres MD, Kraan S, Domínguez H (2019) Seaweed biorefinery. Rev Environ Sci Biotechnol 18:335–388. https://doi.org/10.1007/s11157-019-09496-y
Keskinkan O, Goksu MZL, Basibuyuk M, Forster CF (2004) Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum). Bioresour Technol 92:197–200. https://doi.org/10.1016/j.biortech.2003.07.011
Sikdar SK (2003) Sustainable development and sustainability metrics. AICHE J 49:1928–1932. https://doi.org/10.1002/aic.690490802
Michalak I, Baśladyńska S, Mokrzycki J, Rutkowski P (2019) Biochar from a freshwater macroalga as a potential biosorbent for wastewater treatment. Water 11:1390. https://doi.org/10.3390/w11071390
Lucaci AR, Bulgariu D, Ahmad I et al (2019) Potential use of biochar from various waste biomass as biosorbent in Co(II) removal processes. Water 11:1565. https://doi.org/10.3390/w11081565
Bird MI, Wurster CM, de Paula Silva PH et al (2012) Algal biochar: effects and applications. GCB Bioenergy 4:61–69. https://doi.org/10.1111/j.1757-1707.2011.01109.x
De Bhowmick G, Sarmah AK, Sen R (2018) Production and characterization of a value added biochar mix using seaweed, rice husk and pine sawdust: a parametric study. J Clean Prod 200:641–656. https://doi.org/10.1016/j.jclepro.2018.08.002
Nyamunda BC, Chivhanga T, Guyo U, Chigondo F (2019, 2019) Removal of Zn (II) and Cu (II) ions from industrial wastewaters using magnetic biochar derived from water hyacinth. J Eng (United States). https://doi.org/10.1155/2019/5656983
Son EB, Poo KM, Chang JS, Chae KJ (2018) Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci Total Environ 615:161–168. https://doi.org/10.1016/j.scitotenv.2017.09.171
Keskinkan O, Goksu MZL, Yuceer A, Basibuyuk M (2007) Comparison of the adsorption capabilities of Myriophylum spicatum and Ceratophyllum demersum for zinc, copper and lead. Eng Life Sci 7:192–196. https://doi.org/10.1002/elsc.200620177
Liu Z, Lu B, He B, Li X, Wang LA (2019) Effect of the pyrolysis duration and the addition of zeolite powder on the leaching toxicity of copper and cadmium in biochar produced from four different aquatic plants. Ecotoxicol Environ Saf 183:109517. https://doi.org/10.1016/j.ecoenv.2019.109517
Adeeyo O, Oresegun O, Oladimeji T (2015) Compositional analysis of lignocellulosic materials: evaluation of an economically viable method suitable for woody and non-woody biomass. Am J Eng Res 4:14–19
Moreno-Castilla C, López-Ramón MV, Carrasco-Marín F (2000) Changes in surface chemistry of activated carbons by wet oxidation. Carbon N Y 38:1995–2001. https://doi.org/10.1016/S0008-6223(00)00048-8
Shaaban A, Se SM, Dimin MF et al (2014) Influence of heating temperature and holding time on biochars derived from rubber wood sawdust via slow pyrolysis. J Anal Appl Pyrolysis 107:31–39. https://doi.org/10.1016/j.jaap.2014.01.021
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Shafizadeh F (1982) Introduction to pyrolysis of biomass. J Anal Appl Pyrolysis 3:283–305. https://doi.org/10.1016/0165-2370(82)80017-X
Collard FX, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sust Energ Rev 38:594–608. https://doi.org/10.1016/j.rser.2014.06.013
Radhakumari M, Prakash DJ, Satyavathi B (2016) Pyrolysis characteristics and kinetics of algal biomass using tga analysis based on ICTAC recommendations. Biomass Convers Biorefinery 6:189–195. https://doi.org/10.1007/s13399-015-0173-7
Chen Y, Mastalerz M, Schimmelmann A (2012) Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int J Coal Geol 104:22–33. https://doi.org/10.1016/j.coal.2012.09.001
Aboulkas A, Hammani H, El Achaby M et al (2017) Valorization of algal waste via pyrolysis in a fixed-bed reactor: production and characterization of bio-oil and bio-char. Bioresour Technol 243:400–408. https://doi.org/10.1016/j.biortech.2017.06.098
Hernández-Morales V, Nava R, Acosta-Silva YJ et al (2012) Adsorption of lead (II) on SBA-15 mesoporous molecular sieve functionalized with -NH2 groups. Microporous Mesoporous Mater 160:133–142. https://doi.org/10.1016/j.micromeso.2012.05.004
Greenwood JL, Clason TA, Lowe RL, Belanger SE (1999) Examination of endopelic and epilithic algal community structure employing scanning electron microscopy. Freshw Biol 41:821–828. https://doi.org/10.1046/j.1365-2427.1999.00420.x
Wang S, Gao B, Li Y, Mosa A, Zimmerman AR, Ma LQ, Harris WG, Migliaccio KW (2015) Manganese oxide-modified biochars: preparation, characterization, and sorption of arsenate and lead. Bioresour Technol 181:13–17. https://doi.org/10.1016/j.biortech.2015.01.044
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Zhang C, Zhang F, Li L, Zhang K (2016) Adsorption rare earth metal ions from aqueous solution by polyamidoamine dendrimer functionalized soy hull. Waste Biomass Valoriz 7:1211–1219. https://doi.org/10.1007/s12649-016-9514-4
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48. https://doi.org/10.1016/j.jtice.2017.01.024
Sun L, Chen D, Wan S, Yu Z (2015) Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour Technol 198:300–308. https://doi.org/10.1016/j.biortech.2015.09.026
Rivera-Utrilla J, Sánchez-Polo M (2003) Adsorption of Cr(III) on ozonised activated carbon. Importance of Cπ - Cation interactions. Water Res 37:3335–3340. https://doi.org/10.1016/S0043-1354(03)00177-5
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742. https://doi.org/10.1016/S0043-1354(99)00232-8
Li H, Dong X, da Silva EB, de Oliveira LM, Chen Y, Ma LQ (2017) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478. https://doi.org/10.1016/j.chemosphere.2017.03.072
Kidgell JT, De Nys R, Hu Y et al (2014) Bioremediation of a complex industrial effluent by biosorbents derived from freshwater macroalgae. PLoS One:9. https://doi.org/10.1371/journal.pone.0094706
A.O D (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn 2+ unto phosphoric acid modified Rice husk. IOSR J Appl Chem 3:38–45. https://doi.org/10.9790/5736-0313845
Agrafioti E, Kalderis D, Diamadopoulos E (2014) Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J Environ Manag 133:309–314. https://doi.org/10.1016/j.jenvman.2013.12.007
Mokrzycki J, Michalak I, Rutkowski P (2019) Tomato green waste biochars as sustainable trivalent chromium sorbents. Environ Sci Pollut Res:1–11. https://doi.org/10.1007/s11356-019-07373-3
Frolova L, Kharytonov M (2019, 2019) Synthesis of magnetic biochar for efficient removal of Cr(III) cations from the aqueous medium. Adv Mater Sci Eng. https://doi.org/10.1155/2019/2187132
Pan J, Jiang J, Xu R (2013) Adsorption of Cr(III) from acidic solutions by crop straw derived biochars. J Environ Sci (China) 25:1957–1965. https://doi.org/10.1016/S1001-0742(12)60305-2
Zhao JJ, Shen XJ, Domene X, Alcañiz JM, Liao X, Palet C (2019) Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci Rep 9:9–12. https://doi.org/10.1038/s41598-019-46234-4
Wnetrzak R, Leahy JJ, Chojnacka KW et al (2014) Influence of pig manure biochar mineral content on Cr(III) sorption capacity. J Chem Technol Biotechnol 89:569–578. https://doi.org/10.1002/jctb.4159
Godlewska K, Marycz K, Michalak I (2018) Freshwater green macroalgae as a biosorbent of Cr(III) ions. Open Chem 16:689–701. https://doi.org/10.1515/chem-2018-0075
Chouchene A, Jeguirim M, Trouvé G (2014) Biosorption performance, combustion behavior, and leaching characteristics of olive solid waste during the removal of copper and nickel from aqueous solutions. Clean Techn Environ Policy 16:979–986. https://doi.org/10.1007/s10098-013-0680-9
Jiang Y, Lei M, Duan L, Longhurst P (2015) Influence of heating temperature and holding time on biochars derived from rubber wood saw dust via slow pyrolysis. Biomass Bioenergy 83:328–339. https://doi.org/10.1016/j.jaap.2014.01.021
Ba S, Alagui A, Hajjaji M (2018) Retention and release of hexavalent and trivalent chromium by chitosan, olive stone activated carbon, and their blend. Environ Sci Pollut Res 25:19585–19604. https://doi.org/10.1007/s11356-018-2196-7
Acknowledgments
The research was financed by the statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wrocław University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mokrzycki, J., Michalak, I. & Rutkowski, P. Biochars obtained from freshwater biomass—green macroalga and hornwort as Cr(III) ions sorbents. Biomass Conv. Bioref. 11, 301–313 (2021). https://doi.org/10.1007/s13399-020-00649-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00649-6