Abstract
This investigation is dedicated to producing hybrid nanocomposites based on AA2024, achieved through the utilization of powder metallurgy methods encompassing mechanical milling and hot pressing. The research focused on studying how changes in B4C content influence the mechanical, wear, and corrosion behavior of AA2024/h-BN/B4C hybrid nanocomposites. The findings revealed that as the B4C content increased, the relative density values decreased, and higher porosity values were observed in the hybrid nanocomposite samples. Among the tested samples, the hybrid nanocomposite with 4 wt% B4C exhibited the most favorable properties. It demonstrated significantly higher hardness and ultimate tensile strength compared to AA2024 matrix alloy, with values of 161 HB and 372 MPa, respectively. In contrast, the matrix alloy showed hardness and ultimate tensile strength values of 105 HB and 237 MPa, respectively. Furthermore, the wear resistance of hybrid nanocomposite with 4 wt% B4C displayed approximately 14 times greater wear resistance under 40 N load compared to the unreinforced AA2024 matrix alloy. The corrosion test results have revealed that the corrosion resistance of the hybrid nanocomposite reinforced with 4wt% B4C is nearly 47% higher compared to the corrosion resistance of the unreinforced AA2024 alloy. This nanocomposite exhibits the highest polarization resistance of 5.21 Ω.cm2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal matrix composites (MMCs) are a combination of metal and another material, usually ceramic or nonmetal, aimed to enhance the metal's strength and stiffness while preserving its high electrical and thermal conductivity [1]. These composites find applications in the aerospace sector for reducing the weight of aircraft parts and enhancing their performance under high stress and temperature. In the automotive industry, MMCs improve the durability of engine components by increasing their strength and resistance to wear [2, 3]. Furthermore, MMCs gracefully extend their influence into biomedical engineering, where they serve as sublime implant materials. Their enhanced mechanical properties and inherent biocompatibility make them a choice of elegance, ensuring not only structural integrity but also harmonious integration within the realms of medical innovation. Research indicates that MMCs offer substantial improvements in mechanical properties, including enhanced stiffness, strength, and resistance to wear. Recognized as cutting-edge materials, MMCs deliver significant benefits across various industries due to their advanced physical and mechanical characteristics [4, 5]. Common matrix materials used in MMCs include aluminum, magnesium, copper, titanium, and nickel [6, 7]. Among these, aluminum alloys outshine others with their exceptional strength-to-weight ratio, reliable resistance to fatigue, effective corrosion resistance, and excellent weldability. These superior attributes position aluminum alloys as the predominant choice in MMC manufacturing, highlighting their widespread utilization in comparison with alternative materials. It is commonly used in aerospace, defense, marine, and high-stress structural applications [8].
In its monolithic state, aluminum exhibits a lowered melting temperature, diminished hardness, minimal resistance to wear, and restricted corrosion resistance [9]. These shortcomings in its wear resistance can constrain its applicability, especially in environments characterized by high friction and corrosion. Notably, the corrosion resistance of aluminum faces significant challenges in aggressive chemical settings, particularly in scenarios involving substances like saltwater [10, 11]. The limitations stemming from these weaknesses may constrain the utilization of aluminum alloys in crucial applications, including aerospace engine components, automotive cylinder blocks, industrial pumps, and valves, thereby giving rise to issues like gear system deformations, punctures in chemical plant pipelines, seal wear in industrial pumps, and corrosion in marine equipment [12, 13]. To address these inherent weaknesses, aluminum metal matrix composites (AMCs) are manufactured by incorporating diverse ceramic reinforcements into aluminum-based alloys. This strategic integration is aimed at enhancing the wear and corrosion performance of aluminum, thus mitigating its inherent weaknesses and expanding its utility across a broader spectrum of demanding applications [14, 15].
Commonly used reinforcing materials in AMCs include silicon carbide (SiC), alumina (Al2O3), titanium carbide (TiC), boron carbide (B4C), graphite, etc. [16, 17]. These substances offer enhanced mechanical characteristics, including elevated stiffness, strength, wear resistance, and minimal thermal expansion compared to pure aluminum [18]. The choice of reinforcement material depends on the specific application and the desired properties. For example, SiC is commonly used for applications requiring high strength and stiffness while Al2O3 is often used for applications requiring good wear resistance and low thermal expansion [19, 20]. B4C is one of the ceramic reinforcement materials used in AMCs. It has a high hardness and stiffness, making it an effective reinforcement for AMCs. Incorporating B4C reinforcement into AMCs has the potential to enhance the material's mechanical attributes, encompassing strength, wear resistance, and thermal stability. The dispersion of B4C particles in Al matrix can also influence the thermal expansion coefficient, electrical conductivity and tribological behavior of the composite. However, employing B4C reinforcement may pose challenges, including the difficulty of achieving effective wetting between B4C and Al, as well as potential compatibility issues during both processing and subsequent service [21, 22]
The wear properties of AMCs can be improved by incorporating various types of lubricants into the matrix, which fall into three main categories: solid lubricants, liquid lubricants, and gaseous lubricants [23, 24]. Solid lubricants are substances used to reduce friction and wear within MMCs. Common solid lubricants employed in these composites include graphite[25], molybdenum disulfide (MoS2) [26], tungsten disulfide (WS2) [27], DLC (diamond-like carbon) [28], and hexagonal boron nitride (h-BN) [29]. These materials are known for their low friction coefficient, high wear resistance, and good thermal stability. Solid lubricants are usually added in small amounts to the metal matrix to create a composite with improved tribological properties. h-BN exhibits a planar arrangement featuring a honeycomb lattice structure similar to graphene [30]. It is an insulator with a wide bandgap of ~ 5.97 eV and high thermal conductivity. Comprising alternating boron and nitrogen atoms covalently bonded in a hexagonal arrangement, h-BN's strong covalent bonds contribute to its high thermal and chemical stability [30, 31]. Owing to its large bandgap, h-BN is widely used as an insulator in electronic devices and as a substrate in graphene and other 2D material-based devices to improve their electrical and thermal properties. Additionally, h-BN boasts unique optical properties and serves as a transparent conductor in certain applications. Explored as a solid lubricant in metal matrix composites, h-BN is favored for its low friction coefficient, high wear resistance, and ability to perform effectively under high loads and elevated temperatures [6, 32, 33].
Polat et al. [34] in a study explored the wear behavior of an aluminum-based alloy through the reinforcement of graphene and B4C, revealing a notable 55% increase in wear resistance in the hybrid sample with optimized properties. Harichandran and Selvakumar [35] investigated the enhancement of Al1030 grade aluminum by reinforcing it with varying proportions of B4C and h-BN using a mixing and ultrasound-assisted casting method, indicating substantial improvements in mechanical properties proportional to the B4C ratio increase, with Al-B4C composites containing 6 wt% B4C exhibiting a remarkable 90% increase in hardness and 80% in tensile strength compared to pure aluminum. Notably, the lowest wear rate was observed in Al-6 wt%B4C composites, demonstrating a notable 65% reduction compared to the Al alloy. Celebi et al. [4] reinforced Al2024 alloy with 1 wt% B4C and varying amounts of (1, 2, and 3 wt%) h-BN using the powder metallurgy method, observing a substantial enhancement in wear resistance with increase in h-BN content, resulting in a wear rate decrease of approximately 75% in the composite reinforced with 1 wt% B4C and 3 wt% h-BN compared to Al2024 alloy. In another study, Karabacak et al. [36] utilized the vacuum hot-pressing method to reinforce Al2024 alloy with 0–5 wt% h-BN, leading to increased hardness from 104 to 126 HB and tensile strength from 237 to 299 MPa in the 4 wt% h-BN reinforced sample compared to the Al2024 alloy. However, it was noted that wear resistance decreased with rise in h-BN content, while corrosion resistance increased fivefold. Collectively, these studies in the literature underscore the significant contributions of h-BN and B4C ceramic reinforcements to enhancing the properties of Al alloys.
There are several methods of producing MMCs, including casting [37], powder metallurgy [38, 39], and extrusion [40]. Each approach carries its own set of advantages and disadvantages, and the selection of a method depends on the targeted properties of the MMC and its intended application. AMCs are specifically produced through the powder metallurgy process. In this method, fine metal powders, typical aluminum, are blended with ceramic or metallic reinforcement particles to create a homogeneous mixture [36, 41]. The mixture is then compacted under high pressure and temperature to form green compact. This compact is subsequently sintered to produce solid part. During sintering, the reinforcement particles bond with the Al matrix, forming a composite material with enhanced mechanical properties, such as increased strength and stiffness [23]. Overall, the powder metallurgy method for AMC production offers numerous advantages, including cost-effectiveness, improved mechanical properties, precise dimensional control, high-volume production, and control over the microstructure of the composite [36].
The incorporation of h-BN as solid lubricant in MMCs holds the promise of enhancing their tribological properties, resulting in reduced wear and friction. This improvement has the potential to extend the lifespan and enhance the reliability of components and systems utilizing these composite [42].
The exploration of the microstructural, mechanical, corrosion, and wear properties of AA2024/h-BN/B4C hybrid nanocomposites (HNCs) produced via powder metallurgy method is innovative. His study delves into a novel class of composite materials, amalgamating the distinctive properties of three different materials (AA2024, B4C, and h-BN) to create a material with improved performance compared to each individual component. The powder metallurgy method, employed in this research, offers advantages in processing and cost over traditional manufacturing methods. The findings of this study provide valuable insights into the potential applications and performance of these HNCs across various industries.
2 Experimental Studies
2.1 Materials
The research employed AA2024 alloy powders as the matrix material, sourced from Seydisehir Aluminium in Konya, Turkey. The composition of these powders included 4.850% Cu, 1.310% Mg, 0.667% Mn, 0.254% Fe, 0.110% Si, 0.079% Zn, 0.033% Cr, 0.008%, and the remainder being aluminum. The particle sizes of the AA2024 alloy powders ranged from 90 to 120 µm. Additionally, the B4C and h-BN nanoparticles, acquired from Alfa Aesar Company in Turkey, exhibited an average particle size of 100 nm.
2.2 Fabrication Process
Mechanical milling was utilized to produce hybrid nanocomposite powders (HNPs). The matrix and reinforcing particles underwent milling within a Retsch PM 200 planetary mill, operating at 400 rpm for a duration of 4 h, all conducted under an argon atmosphere. The milling operations were carried out employing 10-mm tungsten carbide balls, maintaining a weight ratio of 10:1 between the balls and the powder. To control the process and prevent cold welding and clumping of the milled powders, stearic acid (0.25 wt%) was introduced as a process control agent. The sample codes are listed in Table 1.
For the production of HNCs, the milled blend was initially placed within a sturdy steel mold, followed by cold compaction at 250 MPa pressure. Before compaction, a layer of zinc stearate was added to the inner surfaces of the mold to prevent powder adhesion and facilitate easy extraction of the sample post-compaction. Subsequently, the green compacts were subjected to hot pressing under vacuum conditions at 560 °C and 500 MPa pressure for a duration of 3 h.
2.3 Characterization
2.3.1 Particle Size and Microhardness
The particle sizes of AA2024 and HNPs were assessed utilizing a particle size analyzer (Malvern, model ‘Mastersizer Hydro 2000e’). The hardness of the powders was evaluated using a Vickers microhardness tester (Innovatest 400™). To ensure accuracy, a minimum of six measurements were performed for each sample, specifically targeting the particles located on the polished surface.
2.3.2 Relative Density Measurement
The relative densities of samples were measured using Archimedes' principle following ASTM B962-08 procedures [43, 44]. A digital balance (Ohaus PA214C) with ± 0.0001 g accuracy was utilized for sample weight measurement.
2.3.3 X-ray Diffraction
X-ray diffraction (XRD) was conducted on the samples using a PANalytical X'Pert3 Pro diffractometer and Cu Kα radiation (1.541874 Å) to analyze the phases of samples. The XRD pattern was captured in a 2θ range of 20–90° with a 0.01°/s scan rate.
2.3.4 Microstructure Characterization
The surface microstructure and distribution of B4C and h-BN nanoparticles in the matrix were analyzed through SEM (Scanning Electron Microscopy) using a Zeiss Evo LS10 instrument, coupled with energy-dispersive spectroscopy (EDS). In addition, an examination of the worn surfaces of the samples was conducted through the utilization of SEM following the wear test.
2.3.5 Hardness and Tensile Test
Brinell hardness values of polished samples were measured using a Innovatest-Nemesis 9000 digital hardness tester according to ASTM E10-18 standard [44, 45]. Hardness test was conducted using a 2.5-mm-diameter ball under a load of 31.25 kg and a 10-s dwell time. The tensile properties of the samples were assessed following the guidelines specified in ASTM E8 standard [44, 46]. Utilizing an MTS Criterion Universal electromechanical testing machine, the tensile test was conducted at a crosshead speed of 1 mm/min under ambient conditions.
2.3.6 Wear Tests
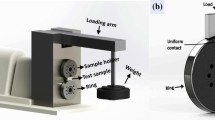
The wear performance of the HNCs was evaluated through a ball-on-disk testing apparatus, conducted in a dry environment with varying loads of 5 N, 10 N, 20 N, and 40 N, all at room temperature, in accordance with the ASTM G99-90 standard [44, 47]. The samples, which took the form of disks with dimensions of 10 mm thickness and 30 mm diameter, were utilized for these tests. A 10-mm-diameter H11 hot-work steel ball was used as the counter material. The sliding velocity was 0.471 m/s (150 rpm rotational speed) and the total sliding distance was 400 m. The change in weight of samples was ascertained by measuring the variance in weight prior to and following the test, with a precision level of 0.0001 mg.
2.3.7 Corrosion Tests
The corrosion characteristics of the samples were analyzed via potentiodynamic polarization experiments conducted on a Gamry Reference 3000 corrosion test machine, adhering to the guidelines specified in the ASTM G59-97 standard [44, 48]. The potentiodynamic polarization experiment was conducted at room temperature using a three-electrode system in a 3.5 wt% NaCl aqueous solution. The counter electrode was a graphite rod, while the reference electrode used was Ag/AgCl (SCE). To ensure stability, a 45-min delay was implemented before each corrosion test to allow the open circuit potential between the electrodes to stabilize. To evaluate the corrosion performance of the samples, the corrosion potential (Ecorr) and corrosion current density (Icorr) were determined using the Tafel extrapolation method from the potentiodynamic polarization curves. The polarization experiment covered a range of ± 500 mV of open circuit potential at a scan rate of 1 mV/s.
3 Results and Discussions
3.1 Powder Morphology
The SEM micrographs depicted in Fig. 1 demonstrate the characteristic features of AA2024 alloy, h-BN, and B4C powder particles in their original state. Analysis of SEM images indicated that the average diameter of aluminum particles (Fig. 1a) was approximately 100 µm. In contrast, both h-BN and B4C particulates had an average diameter of about 100 nm (Fig. 1b and c), a finding also confirmed through SEM observations. Figure 1a also exhibits the SEM image of AA2024 alloy powders, revealing that their initial morphology is predominantly irregular and rod-like, with only a few powders having a spherical shape. The powder morphology of h-BN is spherical (Fig. 1b), whereas B4C exhibits a sharp angular morphology (Fig. 1c), although some of its powders also have a relatively spherical shape.
The morphological and dimensional alterations exhibited by HNPs after 8 h of milling are illustrated in Fig. 2. As depicted, the powders have undergone pronounced changes in both dimension and morphology as a consequence of the milling process. The main cause of this change is the high-energy collisions occurring between powders, balls, and the container during the milling process [49]. The powders, trapped and crushed between the balls, undergo both dimensional changes by breaking and morphological changes by crushing/pounding. The powders, initially displaying a close-to-rod-like morphology, have transformed into angular and polygonal structures after the milling process. Additionally, a significant reduction in powder size can be observed from Figs. 2 and 3. It is clear that increasing the amount of B4C reinforcement has a decreasing effect on powder size. Thus, the mean particle sizes of powders were measured as 100, 48, 39, and 19 µm for A0, C2H2, C4H2, and C6H2 powder samples, respectively. This is because B4C particles are a very hard phase and behave like milling balls against the matrix powders during the milling process. When comparing Fig. 2b and d, it can be seen that there are more agglomerated small powders in Fig. 2d. In other words, smaller particles are formed as a result of breaking the matrix powders during the milling of C6H2 sample. Moreover in Fig. 3, the powder hardness graph of the samples is shown. As can be seen in the graph, a significant increase in powder hardness occurred with the increasing amount of reinforcement, measured as 124, 135, 169, and 217 HV 0.01 for A0, C2H2, C4H2, and C6H2 samples, respectively. The highest increase in powder hardness was observed between C4H2 and C6H2 samples. There are two main reasons for this increase in powder hardness. The first is the milling process, where the powders undergo continuous deformation and plastic deformation, resulting in an expected increase in hardness [50]. This increase becomes more evident, especially with the increase in the amount of B4C, as B4C has a milling ball effect and causes more deformation of the powders. Additionally, the rule of mixtures is a factor that can help explain the increase in powder hardness because the hardness of the h-BN and B4C particles participating in the structure is considerably higher than that of the AA2024 alloy. Anticipated outcomes suggest that the hardness of the alloy powders generated through the milling process will surpass that of the original alloy, and this hardness is projected to escalate with a rise in the quantity of reinforcement.
3.2 Microstructure
SEM microstructure images of sintered pure AA2024 alloy and HNCs in backscattered electron mode are presented in Fig. 4. Additionally, EDS mapping analysis of the C2H2 sample is shown in Fig. 5. In the EDS mapping analysis, the blue, purple, orange, and green colors represent aluminum, copper, carbon, and nitride elements, respectively. These elements illustrate the distribution of aluminum, Al2Cu, B4C, and h-BN in the microstructure. Upon careful examination of Figs. 4 and 5, it is evident that the white areas in the microstructure images are Al2Cu intermetallic phase. The formation of Al2Cu intermetallic phase is inevitable in Al-based alloys with high copper content (approximately 5% by weight). The primary alloying element of the AA2024 alloy approach is Cu, and due to the high proportion of copper, the Al2Cu phase is formed in the Al solid solution during sintering [4, 51]. The presence of this phase is clearly demonstrated in the microstructure images (Fig. 4), EDX analysis (Fig. 5), and XRD analysis (Fig. 6).
The Al2Cu phase plays a crucial role in determining the mechanical properties of the composites. A fine and homogeneously distributed Al2Cu phase leads to an increase in mechanical properties [52]. This is because the fine-sized Al2Cu phase hinders dislocation movements more effectively. Figure 4a shows the microstructure of AA2024, where the Al2Cu phase is predominantly distributed at the particle boundaries and in large sizes. With increase in reinforcement, the distribution of Al2Cu undergoes significant changes. In the microstructure investigations of AMCs, many studies have revealed substantial alterations in the size and distribution of Al2Cu with increase in ceramic reinforcement. Ceramic reinforcements prevent the dissolution of the Al2Cu intermetallics at the particle boundaries, facilitating a more homogeneous dispersion. In addition, since ceramic reinforcements have a lower thermal conductivity coefficient compared to the Al alloy matrix, they reduce the cooling rate of the composite, preventing the aggregation and high content precipitation of Al2Cu. This results in the formation of finer grained and homogeneously distributed Al2Cu deposits. Therefore, based on the SEM pictures in Fig. 4, we can conclude that the Al2Cu phase exhibits a finer size and is more homogeneously distributed with increase in reinforcement.
The dark regions observed along the particle boundaries in Fig. 4 microstructure pictures correspond to clustering regions of B4C and h-BN. Achieving a homogeneous distribution of nano-sized reinforcements in composite material production is challenging. Even when high homogeneity is achieved, it is almost impossible to completely eliminate clustering. Consequently, there will always be clustering regions of nano-sized reinforcements in the microstructure, albeit in small amounts. When examining the microstructures of HNCs, an increase in the number of clustering regions is generally observed with increase in reinforcement. Comparing the microstructures of C2H2 (Fig. 4b) and C4H2 (Fig. 4c) samples, it can be concluded that C4H2 has relatively fewer clustering regions. This can be explained by the fact that the increased B4C supplementation allows more h-BN powders to settle into the Al matrix powder during the milling process, resulting in fewer h-BN particles accumulating at the particle boundaries. However, using B4C reinforcement greater than 4 wt%. (C6H2 sample, Fig. 4d) surpasses the possible critical amount that can settle into the Al matrix during the milling. Consequently, many of the B4C and h-BN nanoparticles cannot enter the matrix and settle at the particle boundaries. This leads to a growth in both the size and quantity of agglomeration regions along the particle boundaries.
The microstructure images reveal small, roughly spherical black regions at the particle boundaries and within the particle, known as voids. These voids appear to increase in number with higher levels of reinforcement. Two factors are thought to contribute to this trend. Firstly, there is an increase in aggregation regions as reinforcement levels rise. Secondly, the presence of reinforcement contributes to an increase in the hardness of the powders, leading to reduced packing during the pressing process and sintering ability.
3.3 XRD Analysis
The X-ray patterns of the AA2024 alloy sample and HNCs are presented in Fig. 6, revealing the presence of Al (JCPDS No: 04-0787), B4C (JCPDS No: 35-0798), and h-BN (JCPDS No: 34-0412) elements. Additionally, intermetallics (Al2Cu (JCPDS No: 25-0012) and Al2CuMg (JCPDS No: 28-0014)) were formed in the structure after mechanical alloying or hot-pressing processes. In Fig. 6, A0 is represented in black, C2H2 in red, C4H2 in green, and C6H2 in blue. In the XRD patterns of sample A0, five main peaks belong to aluminum, detected at 38.4° (111), 44.7° (200), 65.1° (220), 78.2° (311), and 82.4° (222). Therefore, Al2Cu and Al2CuMg peaks were also detected, albeit slightly, in sample A0. The XRD patterns of C2H2, C4H2, and C6H2 samples also exhibit five main Al peaks. However, the peaks belonging to the B4C composition were observed at 20.6° (101), 22.1° (003), 23.5° (012), 31.9° (110), 35° (104), 37.8° (021), and 71.7° (312). These B4C peaks exhibited limited strength, a trend observed as the reinforcement content in the C6H2 sample remained below 5 wt%. In the C6H2 sample, the B4C peaks became stronger, and the number of peaks increased. Moreover, a single prominent h-BN peak is found at 26.6° (002) in the XRD patterns of all HNCs. The XRD patterns of HNCs also show Al2Cu [53] and Al2CuMg [54] intermetallics formed in the Cu and Mg-rich regions in the Al alloy. Al2Cu peaks, as weak peaks, are found at 41.5° (112), 47° (310), and 47.6° (202), while Al2CuMg peaks are found at 34.5° (112) and 40° (041).
Using the Scherrer equation, the crystallite size values of samples were computed, leveraging the broadening observed in the XRD patterns of the peaks. The graph showing the variation of crystallite size of AA2024 alloy and HNCs is given in Fig. 7. The crystallite sizes decrease with the presence of B4C grains in the HNCs, continuously diminishing in direct proportion to the increase in the amount of reinforcement. This reduction is attributed to B4C, which induces a decrease in ionic density, inhibits grain growth, and reduces crystallite size. A dynamic interplay emerges between the amalgamation speed of nanoparticles and the inception pace of aluminum particles, resulting in decrease in crystallite size with the addition of reinforcement. The C6H2 sample exhibits the lowest crystallite size at 36.91 nm, while the crystallite sizes of A0, C2H2, and C4H2 samples are 47.39, 41.52, and 39.61 nm, respectively.
3.4 Relative density, Hardness, and Tensile Strength
The relative density examinations of fabricated samples, according to reinforcement content, are depicted in Fig. 8. As shown, the relative density values of the specimens decline with the increase in the reinforcement ratio. The relative density, approximately 99% in the A0 sample, gradually decreased up to 96% for the C6H2 sample. This decrease can be attributed to the concurrent increase in powder hardness. As illustrated in Fig. 3, the escalating amount of B4C continuously raises the powder hardness. Consequently, the hardness of the powders prepared for the pressing process also increases, reducing the powders' ability to bind and creating resistance to the pressing process. This difficulty in packaging the powders leads to a reduction in relative density measurements, revealing the presence of micro- or nano pores between the powder particles. Another contributing factor to this decline in relative density is the challenges in wetting and bonding at the interface between the matrix and reinforcement phases, as discussed in previous studies [23, 24, 49]. It has been noted that gaps occur at the bonding interfaces between the composite components, particularly between metal and ceramic phases. In this study, it is crucial to emphasize that the expansion of micro- and nanoscale gaps between the matrix and reinforcements, occurring with the escalation in reinforcement quantity, serves as an additional factor influencing the reduction in relative density measurements.
Figure 9 presents the hardness and ultimate tensile strength data for the produced samples. These values exhibit an upward trend as the reinforcement content increases, reaching a peak at the C4H2 composition. Subsequently, a marginal decline is observed in both hardness and tensile strength for the C6H2 sample. Upon examining the A0 and C4H2 samples, a noticeable enhancement in both hardness and tensile strength is evident due to the incorporation of B4C reinforcement. Specifically, in the case of C4H2, the hardness reached 161 HB, and the ultimate tensile strength reached 372 MPa. These values considerably surpass those of the AA2024 matrix alloy, which stands at 105 HB for hardness and 237 MPa for ultimate tensile strength. There is an increase of approximately 55% in both hardness and ultimate tensile strength. This increase can be attributed to the powder properties, specifically the hardness of the B4C particles and Al2Cu phase. First of all, it is expected that h-BN and B4C particles, added to the structure and increasing in amount, will increase the hardness values of the composite according to the mixtures rule. As indicated in the powder properties, the hardness of the milled powders shows continuous growth. Therefore, an increase in the hardness of the samples obtained by pressing these powders is expected. Secondly, the inclusion of nano-sized particles serves as impediments to dislocation mobility within the crystalline lattice of the Al matrix. This effect restricts the movement of dislocations, resulting in a discernible enhancement in both hardness and tensile strength. As demonstrated by Isfahani et al. [55], a hardness augmentation exceeding 100% in AMCs was achieved by incorporating up to 10 wt% nano-sized B4C. The observed improvement was ascribed to the dual mechanisms of grain size refinement and dislocation motion hindrance facilitated by the nano-sized particles. In a separate investigation, Chand et al. [56] noted a substantial advancement in mechanical properties with the escalating quantity of reinforcements in AMC hybrid composites, where Al6061 alloy was reinforced with B4C/BN. This enhancement was attributed to the homogeneous dispersion of reinforcements throughout the structure and the concurrent reduction in grain size. In the C6H2 sample, approximately 50% higher hardness and tensile strength values were obtained compared to the A0 sample, but there is a slight decrease compared to the C4H2 sample. In other words, the increased amount of B4C increased both hardness and tensile strength values up to a point, while decreasing it after a point. While the mechanism that increases the hardness and strength is dispersion hardening, the mechanism that decreases it is the increase in powder hardness and accordingly the decrease in packaging ability. The critical event here is the balance between the hardness feature of B4C and its negative effects on packaging ability and distribution of Al2Cu. The amount of 4 wt% B4C (C4H2 sample) is a critical value for this study. Up to 4 wt% reinforcement, dispersion hardening is the effective mechanism, while at more than 4 wt% reinforcement, the effective mechanism is the formation of porosity and increased dissolution of Al2Cu. Thus, the increased amount of B4C reinforcement causes the fine-grained Al2Cu phase to dissolve at the particle boundaries and a slight decrease in the strength increase resulting from precipitation hardening, as indicated in the microstructure examination. The dissolution of the Al2Cu phase occurring at the particle boundaries undermined the effectiveness of the mechanism responsible for strengthening, as it eliminated the precipitates that hinder dislocation motion [36, 57]. As a result, a marginal reduction was observed in the tensile strength of the C6H2 sample.
3.5 Wear
The tribological performances of both AA2024 alloy and HNCs were evaluated by conducting dry sliding wear tests using a ball-on-disk tribometer. The wear rate values AA2024 and HNCs are analyzed in Fig. 10 under varying applied loads while maintaining a constant sliding distance and speed. Figure 10 visually demonstrates that the wear rate for all test specimens escalates with elevated applied loads. However, the degree of this augmentation is notably contingent on the proportion of reinforcement content. In Fig. 10, it can be observed that the wear rate of HNCs decreases as the amount of B4C increases, reaching a minimum at a 4 wt% reinforcement ratio. For instance, when subjected to wear tests at loads of 5, 10, 20, and 40 N, the wear rate measurements for A0 samples were approximately 0.15, 0.21, 0.28, and 0.37 mm3/m, respectively. In contrast, under the identical test conditions, the wear rate measurements for the C4H2 sample, which exhibited the lowest wear rate, were determined as 0.0018, 0.007, 0.015, and 0.026 mm3/m. Therefore, there was a remarkable reduction of 92–97% in wear rate values for each load. These results highlight that AA2024 alloy exhibited the highest wear loss values, likely due to the lack of resistance in the ductile structure of the matrix material produced without additives against the deformation caused by the abrasive ball during wear. Conversely, the C4H2 sample, comprising 4 wt% B4C and 2 wt% h-BN, exhibited the most minimal wear loss values. This heightened wear resistance is ascribed to the combined impact of h-BN and B4C particles within the matrix, working synergistically. B4C increases the hardness of the composites, thereby enhancing wear resistance, while also contributing to increased wear resistance through the strengthening of the matrix caused by the higher dislocation density [58]. The uniform dispersion of the Al2Cu phase throughout the matrix additionally facilitates the rise in dislocation density as reinforcement content increases [59]. The improved wear resistance of HNCs incorporating nano-sized B4C primarily stems from the load-bearing capacity and the robust interface established between the matrix and the reinforcement. The disparity in thermal expansion coefficients between the nanoparticles and the aluminum matrix results in elevated dislocation density and thermal stresses. These, in turn, function as impediments to dislocation motion, effectively bolstering wear resistance. Consequently, B4C particles enhance wear resistance by increasing composite hardness and forming a protective barrier against surface abrasion [60]. The inclusion of h-BN alongside the robust B4C particles further reduces wear rates. The even dispersion of h-BN solid lubricant particles yields a reduced shear strength within the sliding region. This leads to the formation of a thin lubrication layer rich in h-BN, which effectively curtails direct metal-to-metal contact between surfaces and substantially reduces wear rates [4]. Rajendar and Eswaraiah [42] fabricated AMCs by incorporation of h-BN and B4C, ranging from 0 to 10 wt%, into LM25 Al alloy, and a subsequent examined their wear properties. Similar to our investigation, they documented noteworthy enhancements in wear resistance with increase in h-BN and B4C concentrations compared to the base alloy. They particularly emphasized the exceptional wear resistance exhibited by the sample reinforced with 6 wt% B4C and 2wt% h-BN, demonstrating approximately 19-fold greater resistance compared to the base alloy.
Additionally, it was observed in Fig. 10 that a decrease in wear rates was observed for all loads after a 4 wt% B4C reinforcement. The wear rate values for C4H2 specimens subjected to wear tests at 5, 10, 20, and 40 N loads were approximately 0.0018, 0.007, 0.015, and 0.026 mm3/m, respectively. In contrast, under the identical test conditions, the wear rate values for the C6H2 specimen with 6 wt% B4C reinforcement were approximately 0.012, 0.020, 0.034, and 0.058 mm3/m, suggesting a notable escalation in wear rates. These results demonstrate that the wear rate of HNCs increases when collective weight percentage of h-BN and B4C nanoparticles surpasses 6 wt%. This increase can be attributed to the existence of voids and nanoparticle agglomeration in various regions in the microstructure.
Figure 11 illustrates the average coefficient of friction (AFC) values obtained from wear tests conducted under varying loads, with a sliding speed of 0.471 m/s and a distance of 400 m. It is evident that AFC values increase with higher normal loads for all tested specimens. Notably, the AFC values of HNCs are consistently lower than those of the AA2024 matrix alloy across all loads. Upon closer analysis, it becomes apparent that increasing B4C reinforcement leads to a decrease in both wear rate and AFC values, up to a 4 wt% reinforcement ratio. For instance, under the identical test conditions, at loads of 5, 10, 20, and 40 N, the AFC values for the A0 sample were approximately 0.35, 0.38, 0.39, and 0.43 µ, respectively, while the AFC values for the C4H2 sample were around 0.15, 0.19, 0.21, and 0.24 µ. It is known that the unreinforced AA2024 alloy undergoes softening at elevated temperatures during friction, resulting in reduced load transfer capability and a tendency to adhere to the abrasive ball. For the C4H2 sample, both B4C and h-BN additives exhibit a positive synergistic effect on AFC values. Incorporating h-BN lubricant enhances the capacity of the softer matrix material to withstand abrasion. This improvement is attributed to the lamellar crystal structure of h-BN, which enhances load distribution and transfer [61]. Moreover, the nano-sized h-BN particles facilitate surface lubrication, preventing roughening and maintaining low AFC values [62]. Hard nano-B4C particles, by suppressing heavy plastic deformation induced by the abrasive ball, reduces the effective contact area, thereby contributing to decreased AFC values [63]. However, exceeding a 4 wt% B4C reinforcement ratio adversely affects AFC values by promoting pore formation and agglomeration of high reinforcements. Consequently, the AFC values of HNC specimens increase beyond 4 wt% B4C reinforcement.
Figure 12 exhibits the wear tracks (Fig. 12a–d) and worn surface appearances (Fig. 12a'–d') of AA2024 alloy and HNCs. These observations were made subsequent to wear tests performed under specific conditions: a 20 N load, a sliding speed of 0.471 m/s, and a sliding distance of 400 m. The width of the wear track on the worn surface of A0 sample (unreinforced) was measured as 2763 µm. Notably, due to the soft nature of AA2024 alloy, adhesive regions were prominently observed, as depicted in Fig. 12a. Additionally, clear evidence of grooves formed by severed and adhered Al fragments to the abrasive ball was observed on the sample. The worn surface also displayed cavity regions resulting from plastic deformation induced by the abrasive ball. Furthermore, distinct smearing areas were apparent on the ductile Al matrix due to increased friction force and localized temperature during the wear test. Observable is the inclination of the wear track to deepen, evident through surface deformation and roughness attributed to inadequate resilience against the abrasive ball. The higher magnification SEM image of Fig. 12a (Fig. 12a') revealed a dominant worn surface morphology characterized by delamination zones. Notably, substantial separation of relatively wide layered regions from the surface and surface roughness fluctuations were observed. This indicates a high degree of wear track deformation primarily driven by delamination and deepening. Consequently, the dominant wear mechanism in A0 samples was identified as adhesive wear. Existing literature supports that AA2024 alloys exhibit a lower hardness compared to the abrasive disc, resulting in viscous flow and significant material loss through severe plastic deformation during wear [64]. Figure 12b shows the wear tracks of C2H2. The inclusion of h-BN reinforcement results in a significant decrease in the wear track width (2551 µm) compared to the AA2024 alloy. This reduction is credited to the lubricative influence of h-BN, made possible by its weak van der Waals bonds. The lubricity at the contact points during wear contributes to minimizing material loss. Furthermore, the integration of B4C reinforcement results in heightened hardness, consequently diminishing the formation of grooves. The combined synergistic effect of B4C and h-BN reinforcement demonstrates several advantages over the A0 sample. It is observed that smearing areas are reduced, and the adhesion mechanism is alleviated. The morphology of the extended wear surface of C2H2 sample (Fig. 12b’) reveals that the partially deformed delamination layers tend to cluster at specific points, distinguishing them from the exfoliation layers observed in the A0 sample. Figure 12c shows the wear tracks and worn surface of C4H2 and has the smoothest surface of all samples. In this sample, which has the lowest wear width (1986 µm) compared to other samples, the presence of shallow grooves and abrasive lines indicates a typical abrasive wear.
This phenomenon is attributed to the inclusion of rigid B4C particles within the HNCs, which act to constrain the flow of metal during sliding. The increase in the amount of B4C reinforcement has directly contributed to the enhancement of hardness and wear resistance of the HNCs. The reduction in wear track width suggests a tendency for the worn surfaces to escape the delamination mechanism. Accordingly, an increased amount of B4C reinforcement compared to the C2H2 specimen also reveals a low amount of slight delamination areas. Through the amalgamation of C4H2 with its elevated hardness and the self-lubricating h-BN, which creates a protective tribolayer on the worn surface, this composite displays the most superior tribological attributes. Despite the high content of B4C, the absence of severe abrasive wear mechanism on the worn surface can be credited to the embedding of B4C particles into the AA2024 matrix due to mechanical milling effect, resulting in the creation of a homogeneous wear-resistant surface. Additionally, the h-BN reinforcement within the structure has always maintained the formation of a tribolayer providing lubrication at every additive ratio of the HNCs. The results obtained from Fig. 12d demonstrate the wear track and worn surface of the C6H2 sample. Analysis of the SEM images reveals that the delamination mechanism on the surface is minimized, indicating a reduced occurrence of material detachment. Instead, the predominant wear mechanism observed consists of shallow grooves and abrasive lines. Nonetheless, it is important to highlight that the significant increase in particle hardness due to the higher B4C content has led to the formation of voids within the composite's structure. Consequently, this has resulted in an expansion of the wear track width. Unfortunately, the widening of the wear track negatively impacts the composite's wear resistance and leads to an increased wear rate. Furthermore, Fig. 12d’ illustrates the presence of compressed debris along the wear path, which can contribute to elevated levels of abrasive wear and wear rate. A comparative analysis between the C6H2 and C4H2 specimens indicates a more severe wear mechanism in C6H2. This can be attributed to the presence of shallower grooves, abrasive lines, and additional 3D defects, such as voids and pores, within the C6H2 sample.
3.6 Corrosion Behavior
The corrosion behavior of MMCs has garnered significant attention due to their potential applications in various industries. The presence of physical or chemical heterogeneities within MMCs, such as intermetallic compounds, reinforced particles, and matrix interfaces, can significantly influence their localized corrosion characteristics. These heterogeneities often exhibit different electrochemical potentials compared to the matrix, resulting in galvanic coupling and subsequent corrosion initiation. Additionally, factors such as the distribution and weight percentage of intermetallic compounds, as well as the microstructure of the base alloy, play crucial roles in determining the corrosion performance of the composites [65, 66].
Figure 13 presents the potentiodynamic polarization curves of fabricated samples when immersed in a 3.5% NaCl solution. Overall, HNCs exhibit analogous polarization curves and types of passivity. However, upon closer examination of the polarization curves, it is observed that the corrosion potential (Ecorr) values of the samples range between −643 and −729 mV. This indicates that the inclusion of B4C reinforcement has a minimal impact on the corrosion potential. The corrosion potential, also known as the open circuit potential, plays a pivotal role in determining the corrosion behavior of a material. It serves as an indicator of the thermodynamic driving force for corrosion reactions. A more noble corrosion potential implies that the material's surface is inclined to form a protective oxide layer or passivation film, which acts as a barrier against corrosive substances. This protective film hinders further corrosion by reducing the rates of anodic and cathodic processes. Conversely, a less noble corrosion potential suggests that the material may have difficulties in forming a protective film or that the existing film is less stable [67,68,69]. In the case of the tested samples, the corrosion potential of AA2024 was −685 mV. With increase in B4C reinforcement, the corrosion potential of C2H2 increased to −661 mV and that of C4H2 further increased to −643 mV. Therefore, it can be inferred that an escalation in B4C reinforcement enhances the material's ability to form a passive film on its surface, thereby increasing its resistance to corrosion. In a similar study in the literature, Ramadoss et al. [70] achieved substantial advancements in corrosion resistance by incorporating 3 wt% BN and up to 9 wt% B4C into Al7075 alloy. They ascribed this enhancement to the efficacious contribution of B4C in forming a protective film layer and the barrier function of Al2Cu against undesirable interfacial reactions.
Table 2 provides the polarization parameters extracted from the Tafel curves obtained for these specimens. The polarization parameters include the corrosion potential (Ecorr), anodic slopes (βa), cathodic slopes (βc), and current density (Icorr), all derived from the Tafel curves. By analyzing the Tafel plots, it was possible to quantify the corrosion current and corrosion potential, enabling a comprehensive evaluation of the corrosion resistance exhibited by the nanocomposites at different B4C reinforcement levels. Furthermore, the polarization resistances (Rp) of the samples were computed employing the Stern-Geary equation (Eq. 1) [71]. These parameters collectively offer valuable insights into the corrosion behavior and characteristics of the AA2024 and HNCs under study.
Elevated corrosion resistance, augmented corrosion potential, and diminished corrosion current density collectively signify more effective corrosion protection [72]. The recorded corrosion current densities for the fabricated samples were measured as 4.59 × 10–6 A/cm2 for A0, 2.71 × 10–6 A/cm2 for C2H2, 2.40 × 10–6 A/cm2 for C4H2, and 5.41 × 10–6 A/cm2 for C6H2. Consequently, the C6H2 sample exhibited the highest corrosion current density, indicating that it is the most susceptible to corrosion among the tested samples.
The C6H2 specimen exhibited the lowest polarization resistance among the tested samples, with a value of 3.09 Ω.cm2, while the C4H2 specimen demonstrated the highest polarization resistance at 5.21 Ω.cm2, as clearly indicated in Table 2. However, when examining the data in Table 2, it becomes apparent that the corrosion tendency initially decreased and then increased with the introduction of B4C reinforcement into the AA2024 matrix alloy, within the investigated parameter range. Ceramic nanoparticles like B4C possess remarkable capabilities as galvanic protectors for Al-based matrix. When exposed to corrosive agents, these nanoparticles exhibit preferential corrosion behavior due to their higher anodic nature compared to aluminum. The sacrificial corrosion mechanism exhibited by B4C nanoparticles effectively acts as a shield, protecting the aluminum matrix from direct corrosion. This leads to a notable decrease in the overall corrosion rate. Therefore, utilizing B4C nanoparticles as galvanic protectors presents a meaningful approach to protect aluminum materials by sacrificing themselves in corrosive environments [73,74,75]. However, numerous reports in the literature have highlighted that the incorporation of inert particles into a metal can notably enhance its corrosion resistance by acting as an inert physical barrier [65, 76,77,78]. Notably, there was a substantial decrease in corrosion resistance after incorporating 4 wt% of B4C reinforcement. In fact, it is noteworthy that the corrosion resistance of the C6H2 specimen was observed to be inferior, even when compared to the AA2024 alloy. The reduction in corrosion resistance can be attributed to the notable presence of high porosity. The homogeneous distribution of reinforcing particles within the material's microstructure, combined with low porosity, has a positive effect on its corrosion behavior. Conversely, it has been confirmed by studies in the literature that increased agglomeration resulting from higher reinforcement ratios leads to elevated porosity, thereby promoting microcrack corrosion and reducing the sample's corrosion resistance [65, 79, 80].
4 Conclusion
In this investigation, we utilized mechanical milling as a technique to attain a uniform blend of Al2024 matrix powders along with rigid B4C and lubricating h-BN nanoparticles. Subsequent to this, we effectively generated hybrid nanocomposite materials via the hot-pressing technique, employing the hybrid nanocomposite powders. The outcomes derived from this study bear substantial significance and can be succinctly summarized as follows:
-
It was determined that B4C nanoparticles exhibited a predominantly homogeneous distribution throughout the microstructure as a result of mechanical milling. However, there was a tendency for the B4C nanoparticles to cluster in small amounts. Conversely, h-BN particles were observed to cluster mainly at the particle boundaries.
-
The hardness and tensile strength values increased by approximately 55% with the increasing B4C reinforcement. However, beyond a dual reinforcement ratio of 6%, these values started to decrease.
-
The addition of B4C significantly influenced the wear behavior of the AA2024/h-BN/B4C hybrid nanocomposite specimens. The C4H2 specimen, which exhibited the highest wear resistance, demonstrated a wear rate approximately 14 times lower than that of the unreinforced alloy under identical conditions. However, it was observed that the wear resistance decreased when the dual reinforcement ratio exceeded 6% by weight.
-
The corrosion test results of nanocomposites revealed an interesting trend: as the amount of reinforcement increased, the current density values decreased while the polarization resistance values increased. This suggests that the addition of reinforcement initially improved the corrosion properties. However, it was also observed that there is an optimal ratio of reinforcement beyond which the corrosion properties began to deteriorate.
References
Smagorinski, M.E.; Tsantrizos, P.G.: Development of light composite materials with low coefficients of thermal expansion. Mater. Sci. Technol. 16, 853–861 (2013). https://doi.org/10.1179/026708300101508568
Gudipudi, S.; Nagamuthu, S.; Subbian, K.S.; Chilakalapalli, S.P.R.: Enhanced mechanical properties of AA6061-B4C composites developed by a novel ultra-sonic assisted stir casting. Eng. Sci. Technol. An Int. J. 23, 1233–1243 (2020). https://doi.org/10.1016/J.JESTCH.2020.01.010
Muley, A.V.; Aravindan, S.; Singh, I.P.: Nano and hybrid aluminum based metal matrix composites: an overview. Manuf. Rev. 2, 15 (2015). https://doi.org/10.1051/mfreview/2015018
Çelebi, M.; Çanakçı, A.; Güler, O.; Özkaya, S.; Karabacak, A.H.; Arpacı, K.A.: Investigation of microstructure, hardness and wear properties of hybrid nanocomposites with Al2024 matrix and low contents of B4C and h-BN nanoparticles produced by mechanical milling assisted hot pressing. JOM 74, 4449–4461 (2022). https://doi.org/10.1007/S11837-022-05441-7
Bains, P.S.; Sidhu, S.S.; Payal, H.S.: Fabrication and machining of metal matrix composites: a review. Mater. Manuf. Process. 31, 553–573 (2015). https://doi.org/10.1080/10426914.2015.1025976
Sharma, A.K.; Bhandari, R.; Aherwar, A.; Rimašauskiene, R.: Matrix materials used in composites: a comprehensive study. Mater. Today Proc. 21, 1559–1562 (2020). https://doi.org/10.1016/j.matpr.2019.11.086
Çelebi, M.; Güler, O.; Çanakçı, A.; Çuvalcı, H.: The effect of nanoparticle content on the microstructure and mechanical properties of ZA27-Al2O3-Gr hybrid nanocomposites produced by powder metallurgy. J. Compos. Mater. 55, 3395–3408 (2021). https://doi.org/10.1177/00219983211015719
Karabacak, A.H.; Çanakçı, A.; Erdemir, F.; Özkaya, S.; Çelebi, M.: Effect of different reinforcement on the microstructure and mechanical properties of AA2024-based metal matrix nanocomposites. Int. J. Mater. Res. 111, 416–423 (2020). https://doi.org/10.3139/146.111901
Şenel, M.C.; Kanca, Y.; Gürbüz, M.: Reciprocating sliding wear properties of sintered Al-B4C composites. Int. J. Miner. Metall. Mater. 29, 1261–1269 (2022). https://doi.org/10.1007/S12613-020-2243-5
Maji, P.; Ghosh, S.K.; Nath, R.K.; Karmakar, R.: Microstructural, mechanical and wear characteristics of aluminum matrix composites fabricated by friction stir processing. J. Braz. Soc. Mech. Sci. Eng. 42, 1–24 (2020). https://doi.org/10.1007/S40430-020-02279-5
Keshavarz, H.; Moosavi, S.E.; Eslamian, H.; Kokabi, A.: Corrosion behavior and tribological-microstructural characterization of Al/ZrO2-Gr hybrid composite fabricated via Friction Stir Processing. J. Alloys Comp. 960, 170770 (2023). https://doi.org/10.1016/j.jallcom.2023.170770
Garg, P.; Jamwal, A.; Kumar, D.; Sadasivuni, K.K.; Hussain, C.M.; Gupta, P.: Advance research progresses in aluminium matrix composites: manufacturing & applications. J. Market. Res. 8, 4924–4939 (2019). https://doi.org/10.1016/j.jmrt.2019.06.028
Singh, A.K.; Soni, S.; Rana, R.S.: A critical review on synthesis of aluminum metallic composites through stir casting: challenges and opportunities. Adv. Eng. Mater. 22, 2000322 (2020). https://doi.org/10.1002/adem.202000322
Pelevin, I.A.; Nalivaiko, A.Y.; Ozherelkov, D.Y.; Shinkaryov, A.S.; Chernyshikhin, S.V.; Arnautov, A.N.; Zmanovsky, S.V.; Gromov, A.A.: Selective laser melting of Al-based matrix composites with Al2O3 reinforcement: features and advantages. Materials. 14, 2648 (2021). https://doi.org/10.3390/ma14102648
Sharath, B.N.; Madhu, P.; Verma, A.: Enhancing tribological performance: a review of ceramic reinforced aluminium hybrid composites for high-temperature engineering applications. Hybrid Adv. 4, 100094 (2023). https://doi.org/10.1016/j.hybadv.2023.100094
Nyanor, P.; El-Kady, O.; Yehia, H.M.; Hamada, A.S.; Nakamura, K.; Hassan, M.A.: Effect of carbon nanotube (CNT) content on the hardness, wear resistance and thermal expansion of in-situ reduced graphene oxide (rGO)-reinforced aluminum matrix composites. Met. Mater. Int. 27, 1315–1326 (2021). https://doi.org/10.1007/S12540-019-00445-6
Topcu, I.; Gulsoy, H.O.; Kadioglu, N.; Gulluoglu, A.N.: Processing and mechanical properties of B4C reinforced Al matrix composites. J. Alloys Comp. 482, 516–521 (2009). https://doi.org/10.1016/j.jallcom.2009.04.065
Shuvho, M.B.A.; Chowdhury, M.A.; Kchaou, M.; Rahman, A.; Islam, M.A.: Surface characterization and mechanical behavior of aluminum based metal matrix composite reinforced with nano Al2O3, SiC, TiO2 particles. Chem. Data Collect. 28, 100442 (2020). https://doi.org/10.1016/j.cdc.2020.100442
Azarniya, A.; Azarniya, A.; Sovizi, S.; Hosseini, H.R.M.; Varol, T.; Kawasaki, A.; Ramakrishna, S.: Physicomechanical properties of spark plasma sintered carbon nanotube-reinforced metal matrix nanocomposites. Prog. Mater. Sci. 90, 276–324 (2017). https://doi.org/10.1016/j.pmatsci.2017.07.007
Casati, R.; Vedani, M.: Metal matrix composites reinforced by nano-particles—a review. Metals. 4, 65–83 (2014). https://doi.org/10.3390/met4010065
Manohar, G.; Pandey, K.M.; Maity, S.R.: Effect of microwave sintering on the microstructure and mechanical properties of AA7075/B4C/ZrC hybrid nano composite fabricated by powder metallurgy techniques. Ceram. Int. 47, 32610–32618 (2021). https://doi.org/10.1016/j.ceramint.2021.08.156
Li, J.; Sun, C.; Roostaei, M.; Mahmoudi, M.; Fattahpour, V.; Zeng, H.; Luo, J.L.: Characterization and corrosion behavior of electroless Ni-Mo-P/Ni-P composite coating in CO2/H2S/Cl− brine: effects of Mo addition and heat treatment. Surf. Coat. Technol. 403, 126416 (2020). https://doi.org/10.1016/j.surfcoat.2020.126416
Tosun, G.; Kurt, M.: The porosity, microstructure, and hardness of Al-Mg composites reinforced with micro particle SiC/Al2O3 produced using powder metallurgy. Compos. Part B Eng. 174, 106965 (2019). https://doi.org/10.1016/j.compositesb.2019.106965
Sadhu, K.K.; Mandal, N.; Sahoo, R.R.: SiC/graphene reinforced aluminum metal matrix composites prepared by powder metallurgy: a review. J. Manuf. Process. 91, 10–43 (2023). https://doi.org/10.1016/j.jmapro.2023.02.026
Li, X., Gao, Y., Pan, W., Wang, X., Song, L., International, Z.Z.-C.: Undefined: Fabrication and characterization of B4C-based ceramic composites with different mass fractions of hexagonal boron nitride. Ceram. Int. 41, 27-36 (2015). https://doi.org/10.1016/j.ceramint.2014.07.042
Rouhi, M.; Moazami-Goudarzi, M.; ArdestanI, M.: Comparison of effect of SiC and MoS2 on wear behavior of Al matrix composites. Trans. Nonferrous Metals Soc. China. 29, 1169–1183 (2019). https://doi.org/10.1016/S1003-6326(19)65025-9
Rajeshshyam, R.; Venkatraman, R.; Raghuraman, S.: Tribological investigation and process optimization of dry sliding wear behavior for WS2 solid lubricant layer deposition on Al 6061–T6 surface. Ind. Lubr. Tribol. 74, 178–185 (2022). https://doi.org/10.1108/ILT-07-2021-0272
Guo, C.Q.; Li, H.Q.; Peng, Y.L.; Dai, M.J.; Lin, S.S.; Shi, Q.; Wei, C.B.: Residual stress and tribological behavior of hydrogen-free Al-DLC films prepared by HiPIMS under different bias voltages. Surf. Coat. Technol. 445, 128713 (2022). https://doi.org/10.1016/j.surfcoat.2022.128713
Lakshmanan, M.; Selwin Rajadurai, J.; Chakkravarthy, V.; Rajakarunakaran, S.: Tribological investigations on h-BN/NiTi inoculated Al7075 composite developed via ultrasonic aided squeeze casting. Mater. Lett. 285, 129113 (2021). https://doi.org/10.1016/j.matlet.2020.129113
Wang, J.; Ma, F.; Liang, W.; Sun, M.: Electrical properties and applications of graphene, hexagonal boron nitride (h-BN), and graphene/h-BN heterostructures. Mater. Today Phys. 2, 6–34 (2017). https://doi.org/10.1016/j.mtphys.2017.07.001
Sevik, C.: Assessment on lattice thermal properties of two-dimensional honeycomb structures: graphene, h -BN, h -MoS 2, and h -MoSe 2. Phys. Rev. B: Condens. Matter Mater. Phys. 89, 035422 (2014). https://doi.org/10.1103/physrevb.89.035422
Parveez, B.; Wani, M.; Ali, M.; Reddy Beyanagari, S.; Kandasamy, J.: Tribological performance evaluation of h-BN nanoparticle reinforced AA 7075 and as-cast AA7075 using Taguchi and genetic algorithm. Surf. Topogr. Metrol. Prop. 9, 045009 (2021). https://doi.org/10.1088/2051-672x/ac2795
Agarwal, P.; Kishore, A.; Kumar, V.; Soni, S.K.; Thomas, B.: Fabrication and machinability analysis of squeeze cast Al 7075/h-BN/graphene hybrid nanocomposite. Eng. Res. Express. 1, 015004 (2019). https://doi.org/10.1088/2631-8695/ab26f5
Polat, S.; Sun, Y.; Çevik, E.; Colijn, H.; Turan, M.E.: Investigation of wear and corrosion behavior of graphene nanoplatelet-coated B4C reinforced Al–Si matrix semi-ceramic hybrid composites. J. Compos. Mater. 53, 3549–3565 (2019). https://doi.org/10.1177/0021998319842297
Harichandran, R.; Selvakumar, N.: Microstructure and mechanical characterization of (B4C+ h-BN)/Al hybrid nanocomposites processed by ultrasound assisted casting. Int. J. Mech. Sci. 144, 814–826 (2018). https://doi.org/10.1016/j.ijmecsci.2017.08.039
Karabacak, A.H.; Çanakçı, A.; Çelebi, M.; Güler, O.; Tunç, S.A.; Arpacı, K.A.: Production of Al2024/h-BN nanocomposites with improved corrosion, wear and mechanical properties. Mater. Chem. Phys. 300, 127566 (2023). https://doi.org/10.1016/j.matchemphys.2023.127566
Ramanathan, S.; Karthikeyan, R.; Gupta, M.: Development of processing maps for Al/SiCp composite using fuzzy logic. J. Mater. Process. Technol. 183, 104–110 (2007). https://doi.org/10.1016/j.jmatprotec.2006.09.033
Akgul, B.; Erden, F.; Ozbay, S.: Porous Cu/Al composites for cost-effective thermal management. Powder Technol. 391, 11–19 (2021). https://doi.org/10.1016/j.powtec.2021.06.007
Zhang, X.; Xu, Y.; Wang, M.; Liu, E.; Zhao, N.; Shi, C.; Lin, D.; Zhu, F.; He, C.: A powder-metallurgy-based strategy toward three-dimensional graphene-like network for reinforcing copper matrix composites. Nat. Commun.Commun. 2020(11), 1–13 (2020). https://doi.org/10.1038/s41467-020-16490-4
Reddy, M.P.; Manakari, V.; Parande, G.; Shakoor, R.A.; Mohamed, A.M.A.; Gupta, M.: Structural, mechanical and thermal characteristics of Al-Cu-Li particle reinforced Al-matrix composites synthesized by microwave sintering and hot extrusion. Compos. B Eng. 164, 485–492 (2019). https://doi.org/10.1016/j.compositesb.2019.01.063
Khanna, V.; Kumar, V.; Bansal, S.A.; Prakash, C.; Ubaidullah, M.; Shaikh, S.F.; Pramanik, A.; Basak, A.; Shankar, S.: Fabrication of efficient aluminium/graphene nanosheets (Al-GNP) composite by powder metallurgy for strength applications. J. Market. Res. 22, 3402–3412 (2023). https://doi.org/10.1016/j.jmrt.2022.12.161
Rajendar, K.; Eswaraiah, K.: Experimental investigation and optimization for friction and wear behavior of aluminum LM 25/ h-BN /B4C composites via mixture design and desirability approach. Int. J. Interact. Des. Manuf. (2022). https://doi.org/10.1007/s12008-022-00931-z
B962 Standard Test Methods for Density of Compacted or Sintered Powder Metallurgy (PM) Products Using Archimedes’ Principle, https://www.astm.org/b0962-17.html
Çelebi, M.; Çanakçı, A.; Güler, O.; Karabacak, H.; Akgül, B.; Özkaya, S.: A study on the improvement of wear and corrosion properties of ZA40/Graphene/B4C hybrid nanocomposites. J. Alloys Comp. 966, 171628 (2023). https://doi.org/10.1016/j.jallcom.2023.171628
E10 Standard Test Method for Brinell Hardness of Metallic Materials, https://www.astm.org/e0010-18.html
E8/E8M Standard Test Methods for Tension Testing of Metallic Materials, https://www.astm.org/e0008_e0008m-22.html
G99 Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus, https://www.astm.org/g0099-17.html
G59 Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements, https://www.astm.org/g0059-23.html
Bor, A.; Jargalsaikhan, B.; Uranchimeg, K.; Lee, J.; Choi, H.: Particle morphology control of metal powder with various experimental conditions using ball milling. Powder Technol. 394, 181–190 (2021). https://doi.org/10.1016/j.powtec.2021.08.053
Vajpai, S.K.; Sawangrat, C.; Yamaguchi, O.; Ciuca, O.P.; Ameyama, K.: Effect of bimodal harmonic structure design on the deformation behaviour and mechanical properties of Co-Cr-Mo alloy. Mater. Sci. Eng. C 58, 1008–1015 (2016). https://doi.org/10.1016/j.msec.2015.09.055
Cui, L.; Zhang, Z.; Chen, X.G.: Development of lightweight Al-based entropy alloys for elevated temperature applications. J. Alloys Comp. 938, 168619 (2023). https://doi.org/10.1016/j.jallcom.2022.168619
Liu, G.; Gong, M.; Xie, D.; Wang, J.: Structures and mechanical properties of Al-Al 2 Cu interfaces. JOM 71, 1200–1208 (2019). https://doi.org/10.1007/S11837-019-03333-x
Zhang, Y.; Li, R.; Chen, P.; Li, X.; Liu, Z.: Microstructural evolution of Al2Cu phase and mechanical properties of the large-scale Al alloy components under different consecutive manufacturing processes. J. Alloys Comp. 808, 151634 (2019). https://doi.org/10.1016/j.jallcom.2019.07.346
Staszczyk, A.; Sawicki, J.; Kolodziejczyk, L.; Lipa, S.: Nanoindentation study of intermetallic particles in 2024 aluminium alloy. Coatings 10, 846 (2020). https://doi.org/10.3390/coatings10090846
Nasr Isfahani, M.J.; Payami, F.; Asadabad, M.A.; Shokri, A.A.: Investigation of the effect of boron carbide nanoparticles on the structural, electrical and mechanical properties of Al-B4C nanocomposites. J. Alloys Comp. 797, 1348–1358 (2019). https://doi.org/10.1016/j.jallcom.2019.05.188
Chand, S.; Chandrasekhar, P.; Roy, S.; Singh, S.: Influence of Dispersoid Content on Compressibility, Sinterability and Mechanical Behaviour of B4C/BN Reinforced Al6061 Metal Matrix Hybrid Composites Fabricated via Mechanical Alloying. Met. Mater. Int. 27, 4841–4853 (2021). https://doi.org/10.1007/S12540-020-00739-0
Chen, X.; Ma, X.; Xi, H.; Zhao, G.; Wang, Y.; Xu, X.: Effects of heat treatment on the microstructure and mechanical properties of extruded 2196 Al-Cu-Li alloy. Mater. Des. 192, 108746 (2020). https://doi.org/10.1016/j.matdes.2020.108746
He, Y.; Xu, H.; Jiang, B.; Ji, Z.; Hu, M.: Microstructure, mechanical and tribological properties of (APC+B4C)/Al hybrid composites prepared by hydrothermal carbonized deposition on chips. J. Alloy. Compd. 888, 161578 (2021). https://doi.org/10.1016/j.jallcom.2021.161578
Dong, F.; Yi, Y.; Huang, C.; Huang, S.: Influence of cryogenic deformation on second-phase particles, grain structure, and mechanical properties of Al–Cu–Mn alloy. Journal Alloys and Compounds. 827, 154300 (2020). https://doi.org/10.1016/j.jallcom.2020.154300
Liu, S.; Wang, Y.; Muthuramalingam, T.; Anbuchezhiyan, G.: Effect of B4C and MOS2 reinforcement on micro structure and wear properties of aluminum hybrid composite for automotive applications. Compos. B Eng. 176, 107329 (2019). https://doi.org/10.1016/j.compositesb.2019.107329
Chi, H.; Jiang, L.; Chen, G.; Kang, P.; Lin, X.; Wu, G.: Dry sliding friction and wear behavior of (TiB2 + h-BN)/2024Al composites. Mater. Des. 87, 960–968 (2015). https://doi.org/10.1016/j.matdes.2015.08.088
Paulraj, P.; Harichandran, R.: The tribological behavior of hybrid aluminum alloy nanocomposites at High temperature: role of nanoparticles. J. Market. Res. 9, 11517–11530 (2020). https://doi.org/10.1016/j.jmrt.2020.08.044
Abbas Shafqat, Q., Rafi-Ud-Din, Shahzad, M., Khan, M., Mehmood, S., Syed, W.A., Basit, A., Mehboob, N., Ali, T.: Mechanical, tribological, and electrochemical behavior of hybrid aluminum matrix composite containing boron carbide (B4C) and graphene nanoplatelets. J. Mater. Res. 34, 3116–3129 (2019). https://doi.org/10.1557/jmr.2019.24
Nagaral, M.; Deshapande, R.G.; Auradi, V.; Boppana, S.B.; Dayanand, S.; Anilkumar, M.R.: Mechanical and wear characterization of ceramic boron carbide-reinforced Al2024 alloy metal composites. J. Bio- Tribo-Corros. (2021). https://doi.org/10.1007/S40735-020-00454-8
Güler, O.; Çelebı, M.; Dalmış, R.; Çanakçi, A.; Çuvalci, H.: Novel ZA27/B4C/graphite hybrid nanocomposite-bearing materials with enhanced wear and corrosion resistance. Metall. and Mater. Trans. A. 51, 4632–4646 (2020). https://doi.org/10.1007/S11661-020-05863-5
Karabacak, A.H.; Çanakçı, A.; Erdemir, F.; Özkaya, S.; Çelebi, M.: Corrosion and mechanical properties of novel AA2024 matrix hybrid nanocomposites reinforced with B4C and SiC particles. SILICON (2022). https://doi.org/10.1007/S12633-021-01582-7
Brossia, C.S.; Cragnolino, G.A.: Effect of palladium on the corrosion behavior of titanium. Corros. Sci. 46, 1693–1711 (2004). https://doi.org/10.1016/j.corsci.2003.10.003
Akinwamide, S.O.; Venter, A.; Akinribide, O.J.; Babalola, B.J.; Andrews, A.; Olubambi, P.A.: Residual stress impact on corrosion behaviour of hot and cold worked 2205 duplex stainless steel: a study by X-ray diffraction analysis. Eng. Fail. Anal. 131, 105913 (2022). https://doi.org/10.1016/j.engfailanal.2021.105913
Wang, J.; Du, M.; Li, G.; Shi, P.: Research progress on microbiological inhibition of corrosion: a review. J. Clean. Prod. 373, 133658 (2022). https://doi.org/10.1016/j.jclepro.2022.133658
Ramadoss, N.; Pazhanivel, K.; Anbuchezhiyan, G.: Synthesis of B4C and BN reinforced Al7075 hybrid composites using stir casting method. J. Mater. Res. Technol. 9, 6297–6304 (2020). https://doi.org/10.1016/j.jmrt.2020.03.043
Dinu, M.; Hauffman, T.; Cordioli, C.; Vladescu, A.; Braic, M.; Hubin, A.; Cotrut, C.M.: Protective performance of Zr and Cr based silico-oxynitrides used for dental applications by means of potentiodynamic polarization and odd random phase multisine electrochemical impedance spectroscopy. Corros. Sci. 115, 118–128 (2017). https://doi.org/10.1016/j.corsci.2016.11.018
Wu, Y.; He, Y.; Zhou, T.; Chen, C.; Zhong, F.; Xia, Y.; Xie, P.; Zhang, C.: Synergistic functionalization of h-BN by mechanical exfoliation and PEI chemical modification for enhancing the corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 142, 105541 (2020). https://doi.org/10.1016/j.porgcoat.2020.105541
Chen, C.; Feng, X.; Shen, Y.: Synthesis of Al–B4C composite coating on Ti–6Al–4V alloy substrate by mechanical alloying method. Surf. Coat. Technol. 321, 8–18 (2017). https://doi.org/10.1016/j.surfcoat.2017.04.042
Xie, X.; Hosni, B.; Chen, C.; Wu, H.; Li, Y.; Chen, Z.; Verdy, C.; Kedim, O.E.I.; Zhong, Q.; Addad, A.; Coddet, C.; Ji, G.; Liao, H.: Corrosion behavior of cold sprayed 7075Al composite coating reinforced with TiB2 nanoparticles. Surf. Coat. Technol. 404, 126460 (2020). https://doi.org/10.1016/j.surfcoat.2020.126460
Zhou, Y.T.; Zan, Y.N.; Wang, Q.Z.; Xiao, B.L.; Ma, Z.Y.; Ma, X.L.: Atomic-scale quasi in-situ TEM observation on the redistribution of alloying element Cu in a B4C/Al composite at the initial stage of corrosion. Corros. Sci. 174, 108808 (2020). https://doi.org/10.1016/j.corsci.2020.108808
Hariche, L.; Ballim, Y.; Bouhicha, M.; Kenai, S.: Effects of reinforcement configuration and sustained load on the behaviour of reinforced concrete beams affected by reinforcing steel corrosion. Cement Concr. Compos. 34, 1202–1209 (2012). https://doi.org/10.1016/j.cemconcomp.2012.07.010
Kumar, D.; Singh, S.; Angra, S.: Effect of reinforcements on mechanical and tribological behavior of magnesium-based composites: a review. Mater. Phys. Mech. 50, 439–458 (2022). https://doi.org/10.18149/mpm.5032022_8
Swamy, P.K.; Mylaraiah, S.; Gowdru Chandrashekarappa, M.P.; Lakshmikanthan, A.; Pimenov, D.Y.; Giasin, K.; Krishna, M.: Corrosion behaviour of high-strength Al 7005 alloy and its composites reinforced with industrial waste-based fly ash and glass fibre: comparison of stir cast and extrusion conditions. Materials. 14, 3929 (2021). https://doi.org/10.3390/ma14143929
Rahmani, K.; Majzoobi, G.H.; Bakhtiari, H.; Sadooghi, A.: On the effect of compaction velocity, size, and content of reinforcing particles on corrosion resistance of Mg–B4C composites. Mater. Chem. Phys. 271, 124946 (2021). https://doi.org/10.1016/j.matchemphys.2021.124946
Arab, M.; Azadi, M.; Mirzaee, O.: Effects of manufacturing parameters on the corrosion behavior of Al–B4C nanocomposites. Mater. Chem. Phys. 253, 123259 (2020). https://doi.org/10.1016/j.matchemphys.2020.123259
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Çanakçı, A., Karabacak, A.H., Çelebi, M. et al. A Study on the Optimization of Nano-B4C Content for the Best Wear and Corrosion Properties of the Al-Based Hybrid Nanocomposites. Arab J Sci Eng (2024). https://doi.org/10.1007/s13369-024-08736-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13369-024-08736-w