Abstract
Pure components exhibit different phase behavior and critical properties shift when confined, primarily due to increased molecules-pore wall interactions. While extensive research has focused on modeling this behavior for pure components, there is a need to extend these models to hydrocarbon gas mixtures in tight and shale reservoirs to understand the deviation of gas properties from bulk behavior. The study utilizes the Peng–Robinson equation of state to calculate gas properties, considering the shift in critical properties of pure components due to confinement in EOS parameter calculations. Trend analysis investigates the effect of pore size reduction on gas properties, introducing the concepts of the confinement impact factor and specific pore radius. Correlation analysis explores the relationships between variables. Nonlinear regression analysis leads to the development of a new correlation that accounts for confinement effects on gas properties. The findings reveal that the deviation from bulk properties depends on the pore radius, pressure, temperature, and gas composition. The magnitude of deviation ranges from negligible to exceeding 15% under specific conditions of high pressure, low temperature, small pore radius, and rich gas composition. Gas viscosity experiences the most significant alteration, followed by density, while the gas compressibility factor also displays a noticeable impact. The isothermal gas compressibility coefficient demonstrates minimal to no response to confinement. Decreasing pore radius increases the gas compressibility factor, while gas viscosity and density decrease. The obtained results are crucial for shale and tight reservoir engineering calculations, particularly in adjusting gas properties in reservoir simulation and production modeling software.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Shale and tight reservoirs pose significant challenges to achieving commercial production due to their exceedingly low permeability and porosity resulting from very tight pore structures. These characteristics severely restrict the flow of hydrocarbons, making their extraction economically challenging. The properties of reservoir fluids, including viscosity, density, and compressibility, are highly influenced by the pore size and geometry of the rock formations [1]. Furthermore, the confinement effects of tight pores can induce significant alterations in thermal and molecular diffusion, as well as the phase behavior and critical properties of gas condensate reservoirs, leading to modifications in strategies for wax precipitation, deposition, and prevention [2]. Moreover, the intricate microflow mechanisms and distribution of remaining oil during polymer flooding in tight reservoirs are profoundly influenced by various factors, such as pore structure, fluid rheology, wettability, capillary pressure, and heterogeneity [3, 4]. The physical properties of fluids are determined by a complex interplay between the intermolecular forces between fluid molecules, temperature, and pressure, which can be further influenced by the confinement of the fluid within the pore spaces of shale and tight rocks [5]. In porous media, fluid molecules can interact with each other or with the molecules of the pore walls, and when the pore size is comparable to the size of fluid molecules, the confinement effect becomes significant, affecting the fluid properties due to the restricted free path available to the molecules [6, 7]. Recent experimental studies have revealed that with decreasing pore size, the critical properties of many components change [8, 9]. Several researchers have studied the critical properties shift of gases in nanopores, and various equations have been proposed to quantify this phenomenon for different pore sizes, such as those developed by Zarragoicoechea and Kuz for larger pores and Ma et al. and Jin et al. for smaller pores [9,10,11].
Kamari, Li, and Sheng investigated the impact of rock pore sizes on the pressure–volume-temperature (PVT) properties of oil and gas condensate in shale and tight reservoirs. They modified conventional flash calculations to account for the effects of capillary pressure and critical properties shift and found that pore size had a significant impact on the bubble point and dew point pressures, as well as on interfacial tension, viscosities, and densities of Eagle Ford shale oil and gas condensates [1]. Similarly, Li and Sheng studied the phase behavior of Wolfcamp shale oil using a modified flash calculation coupled with the Peng–Robinson equation of state (PR-EOS) and the Young–Laplace equation and observed significant deviations from the predicted bulk behavior due to the effect of nanopore radius [11].
To date, all research has focused on examining the effect of capillary pressure and shift in critical properties on shale oil and liquid-rich shales. This paper aims to expand on this research by investigating the effect of confinement on the physical properties of hydrocarbon natural gas mixtures in shale gas reservoirs. The research will utilize the Peng–Robinson equation of state to calculate the physical properties of both lean and rich gases across a range of pressures and temperatures. By considering varying pore radii, the study will explore the impact of confinement on gas properties. Understanding these effects is crucial for accurately predicting fluid behavior in shale and tight reservoirs. One important aspect of the research is to analyze the confined gas properties using two concepts: the confinement impact factor and the specific pore radius. These concepts will provide insights into the relationship between pore size reduction and gas properties. Additionally, trend analysis will be conducted to examine the confined gas properties at different reduced pressures and temperatures, allowing for a comprehensive understanding of the confinement effect. The study does not consider interfacial tension and capillary pressure effects, as it deals with a single-phase fluid within the reservoir where no liquid dropout occurs during reservoir depletion. The practical implications of this research are significant. The knowledge gained from studying the behavior of natural gas under confinement can be used to improve production and recovery strategies in shale and tight reservoirs. By accurately modeling the physical properties of confined gas mixtures, engineers can optimize well performance, design efficient stimulation techniques, and enhance hydrocarbon recovery from these challenging formations.
2 Calculation Methodology
This study involved several steps to calculate gas properties without accounting for critical properties shift. Subsequently, the critical properties were modified and the gas properties were recalculated to compare them with the previously calculated values.
Step 1
The initial step in this study involves determining the properties of the pure components required for thermodynamic modeling from published literature [12]. Tables 1 and 2 provide the compositional data for a lean gas and a rich gas, respectively, which will be utilized in this investigation.
Step 2
This step involves the calculation of gas compressibility factor Z using the Peng–Robinson equation of state (PR-EOS). The generalized form of the cubic equation of state is applied in this study as follows [1, 10, 11, 13, 14].
where P is the pressure, psia; T is the temperature, °R; R is the universal gas constant and equals 10.731 psi ft3⋅l bmol−1 °R−1, u = 2, w = 1, a, and b are calculated according to Peng–Robinson as follows [14]
where Tc is the critical Temperature, °R; Pc is the critical pressure, psia; Tr is the reduced temperature; ω is the acentric factor.
For gas mixtures mixing rules must be applied as follows use the equation of state for a hydrocarbon mixture [1, 15,16,17].
where Kij is the binary interaction coefficient. Table 3 shows the set of binary interaction coefficient, Kij, traditionally used when predicting the volumetric behavior of hydrocarbon mixture with the Peng–Robinson equation of state (PR-EOS) [17, 18].
Cubic equations of state could be written as follows [1, 19].
where Z is the compressibility factor, A and B are calculated as follows [1, 17, 18].
It should be noted that Eq. (7) has three roots. However, since this study deals with a single gas phase, there will be two imaginary roots and one positive real root that is considered as the compressibility factor [16, 17].
Gas density, gas formation volume factor, and isothermal gas compressibility coefficient are calculated using the following equations [12, 19, 20].
where ρg is the gas density, Ibm/ft3; Ma is the apparent molecular weight of the gas mixture, Ibm/Ibm mole; βg is the gas formation volume factor, ft3/scf.
Gas viscosities are typically not measured and are instead predicted using correlations [12]. In this study, the Lee-Gonzalez correlation will be employed to calculate gas viscosity. Their proposed correlation is given as follows [20, 21].
Step 3
In this step, the critical properties of the pure components are modified using the correlations developed by Ma et al. and Jin et al. to consider the effect of confinement on hydrocarbon critical properties. These correlations are shown as follows [6, 10].
where ∆Tc, ∆Pc are the critical temperature and pressure shift due to confinement, respectively. Tc and Pc are critical temperature (°R) and critical pressure (psia) for bulk state, respectively. Tcz and Pcz are critical temperature (°R) and critical pressure (psia) under confinement, respectively. D is the pore diameter (nm) and σeff is the effective molecular diameter (nm).
Step 4
In this step, gas properties are calculated for different pore radii. A pore size distribution test conducted on Wolfcamp shale revealed that 93.7% of the pores are smaller than 10 nm, while 5% of the pores are within the range of 20–40 nm [11]. Therefore, the effects of confinement are evaluated at pore radii of 5 nm, 10 nm, and 50 nm. Once the modified critical properties are determined, PR-EOS parameters can be calculated, and all previously calculated gas properties can be recalculated for the specified pore radii. Figure 1 depicts the proposed flow chart for determining the confined gas properties.
3 Results and Discussion
The study utilizes compositional data from Tables 1 and 2 to investigate the effects of nanopores on PVT properties of both lean and rich natural gases. Specifically, the gas compressibility factor, gas density, gas viscosity, and isothermal gas compressibility coefficient are examined to observe how they are affected by different reservoir pressures, temperatures, and pore sizes.
Figures 2 and 3 display the shift in critical temperature and critical pressure, respectively, at three different pore radii for various pure components. The findings highlight that hydrocarbon gases confined in small pores exhibit a more pronounced shift in their critical properties, particularly for heavier hydrocarbons with larger molecular sizes. This observed behavior can be attributed to the confinement of molecules within the limited space of the pores, leading to increased collisions between gas molecules and the pore walls. This observation is visually demonstrated in Fig. 4. Consequently, gas mixtures characterized by a higher proportion of intermediate components, referred to as rich compositions, are anticipated to display more significant deviations in their properties when confined, compared to lean compositions.
Figures 5 and 6 illustrate the calculated bulk and confined gas properties at pore radii of 5 nm and 10 nm for the lean and rich natural gases, respectively, at a reservoir temperature of 640°R. It is observed that the effect of critical properties shift due to confinement on gas properties is insignificant for low pressures below 2000 psia. Notably, the confinement-induced critical properties shift has a negligible effect on gas properties at low pressures below 2000 psia. This lack of pronounced confinement effects can be attributed to the fact that pressure primarily reflects the number of gas molecule collisions with the pore walls. Below 2000 psia, these collisions are comparatively insignificant in relation to molecule–molecule collisions or interactions. However, at high pressures, confined gas properties demonstrate some deviation compared to bulk properties at the same temperature of 640°R. These deviations can be attributed to the increased collisions between gas molecules and the pore walls, leading to altered gas properties. Specifically, as the pore size decreases, the compressibility factor of the gas increases, while gas density and viscosity decrease compared to the unconfined state at the same pressure and temperature. This behavior can be attributed to the influence of pore walls-gas molecules interactions, which partially weaken the molecule–molecule attractions of the gas. Consequently, the compressibility factor increases as the gas molecules experience reduced intermolecular forces. The decreased density indicates that the gas occupies a larger volume due to the partial weakening of molecule–molecule attractions caused by the presence of the pore walls. Additionally, the lower viscosity of the gas is a result of reduced friction between gas molecules resulting from the weakened molecule–molecule attractions.
The lack of significant response of the isothermal gas compressibility coefficient to changing pore radius is an interesting observation. This observation can be explained by referring to Eq. 12, which provides the mathematical definition of this property, and by considering the response of the compressibility factor to changing pore radius at different pressures, as shown in Fig. 5. Equation 12 states that the isothermal gas compressibility coefficient at a specified pressure and temperature is the reciprocal of the pressure minus the derivative of the compressibility factor with respect to pressure at the same pressure and temperature, divided by the compressibility factor itself at those conditions. Analyzing Fig. 5, it becomes evident that confinement has no significant effect on the compressibility factor below 2000 psia. Consequently, the compressibility coefficient remains unchanged in this range of pressures. However, as the pressure exceeds 2000 psia, the compressibility factor starts to increase due to confinement. Concurrently, the derivative of the compressibility factor also increases, resulting in a steeper curve as the pore radius decreases. The simultaneous increase in both the derivative and the compressibility factor has a compensating effect, leading to the cancellation of their overall impact on the isothermal gas compressibility coefficient. As a result, very little or no deviation is observed in the compressibility coefficient. This lack of response can also be explained from a physical perspective. Confinement, which occurs when gas molecules are confined within small pores, does affect the intermolecular forces between gas molecules. However, the impact of confinement on the isothermal compressibility of the gas is overshadowed by the dominant factor of pressure. Pressure primarily determines the distance between gas molecules, influencing their interactions and ultimately governing the compressibility of the gas. While confinement may introduce some alterations in the intermolecular forces, its overall influence on the compressibility is minor compared to the changes induced by pressure.
To gain a comprehensive understanding of the relationship between the variables, a correlation analysis was performed on both the lean and rich gas datasets. The correlation matrix, as depicted in Fig. 7 for the lean gas and the rich gas, was utilized to confirm the previous observations and reveal any hidden observations in the data.
The correlation matrix shows the correlation coefficients between variables. Each element represents the strength and direction of the relationship, ranging from − 1 to 1. In Fig. 7, the correlation coefficient between gas properties and pore radius is higher for rich gas than for lean gas, indicating more pronounced confinement effects in rich gases. Comparing the correlation coefficients of gas properties with pressure and temperature to those with pore radius, we find that the latter is much lower, suggesting that changes in pressure or temperature have a greater impact on gas properties than variations in pore radius.
Figures 8 and 9 provide graphical representations of the calculated confined gas properties at a pore radius of 5 nm for both lean and rich natural gas. These properties are observed at three different assumed reservoir temperatures. The key finding from these figures is that the confined gas properties exhibit a consistent trend across all the reservoir temperatures considered. This means that the behavior observed in the confined gas properties, as illustrated in Figs. 5 and 6 for a reservoir temperature of 640°R, holds true for other reservoir temperatures as well. This consistency is crucial because it assures us that the conclusions derived from the analysis of Figs. 5 and 6 are not limited to a specific reservoir temperature but can be extended to a wider range of temperatures. In other words, the observed behavior and the derived conclusions about the confined gas properties, such as the effect of critical properties shift or the relationship between pore size and gas properties, are applicable and valid across different reservoir temperatures. This provides additional confidence in the generalizability and reliability of the findings obtained from the analysis, as the trend observed is consistent and robust, irrespective of the specific reservoir temperature being considered.
To further investigate the effect of critical properties shift on gas properties, a trend analysis is performed on the calculated confined gas properties versus pore radius in the range of 2.5–100 nm. The analysis is conducted at constant reduced pressure and three different reduced temperatures, as well as at constant reduced temperature and three different reduced pressures, to determine whether the deviation from bulk properties depends on the system’s pressure and temperature.
Figure 10 shows the trend analysis of gas properties calculated for lean and rich gases at a constant reduced pressure of 9 and reduced temperatures of 1.81, 1.67, and 1.52. Conversely, Fig. 11 presents the trend analysis of gas properties calculated for lean and rich gases at a constant reduced temperature of 1.5 and reduced pressures of 9.0, 8.0, and 7.0. These analyses aim to determine whether the deviation from bulk properties is dependent on the system’s pressure and temperature.
The study of trend analysis for confined gas properties introduces two novel concepts, namely the confinement impact factor (CIF) and the specific pore radius (SPR). CIF is calculated at a fixed reduced pressure and temperature, and can be defined mathematically as follows:
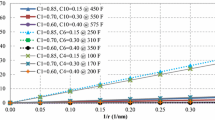
Here, x represents the gas property, such as viscosity, density, or compressibility factor, and the subscripts 100 and 10 represent the values of that property calculated at pore radii of 100 nm and 10 nm, respectively. CIF is defined as the absolute percentage change in a gas property when the pore radius decreases from 100 to 10 nm, at a specified pressure and temperature. The CIF values for all gas properties determine which property is most affected by confinement. By calculating CIF at a constant pressure and a range of temperatures or vice versa, the effect of temperature or pressure on gas properties can be observed. Figure 12 shows CIF versus reduced temperature at a fixed reduced pressure of 9.0 for lean and rich gas, while Fig. 13 shows CIF versus reduced pressure at a fixed reduced temperature of 1.5. It is observed that rich gas has higher CIF values compared to lean gas, and all gas properties exhibit higher deviation from bulk properties at high pressures and low temperatures except for the isothermal gas compressibility coefficient, which only responds to changes in pressure.
The CIF only considers the change in gas properties due to the confinement effect when the pore radius is decreased from 100 to 10 nm, regardless of the actual pore size of the system. Therefore, it provides information about which gas property is most sensitive to confinement at the specified pressure and temperature conditions, but it does not necessarily indicate whether the actual pore size of the system will cause a significant deviation from the bulk properties or not.
The trend analysis of gas properties reveals that as the pore radius decreases, the deviation of gas properties from bulk properties remains insignificant until a certain point is reached. Beyond this point, the increased collisions between gas molecules and the pore walls lead to a significant deviation in gas properties. This point is termed as the specific pore radius (SPR). This specific pore radius (SPR) is an important parameter to consider when studying confinement effects. The SPR can be determined by examining the derivative of gas compressibility factor with respect to pore radius, which helps identify the pore radius at which significant deviations occur.
Figure 14 illustrates the compressibility factor derivative with respect to pore radius at various reduced pressures and temperatures for both lean and rich gases. The SPR is defined as the pore radius at which the gas compressibility factor derivative equals − 1 × 10−4 nm−1. Table 4 displays the calculated SPR values for two cases of lean and rich gases. Interestingly, the SPR is consistently higher for the rich gas compared to the lean gas at the same reduced pressure and temperature. This suggests that the confinement effect is observed for rich gases at relatively larger pore radii. Furthermore, the SPR tends to be higher at low temperatures and high pressures, indicating that confinement effects are observed at relatively larger pore radii under these conditions.
Figure 14 demonstrates that the compressibility factor derivative with respect to pore radii is solely influenced by the reduced pressure, reduced temperature, and pore radius, independent of the gas type. This observation presents a favorable prospect for employing nonlinear regression analysis to uncover the underlying relationship. A comprehensive nonlinear regression analysis is conducted over a broad range of reduced pressures, reduced temperatures, and pore radii. The subsequent expression establishes a correlation between the derivative and reduced pressure, reduced temperature, and pore radii. The validity of this correlation is applicable when Pr < 9, Tr < 2, and pore radii range from 2.5 to 200 nm with average absolute error of 7.8%.
Integrating the Eq. 22 over the range of pore radii from infinity to the specific pore radius of interest leads to a highly valuable equation that can effectively adjust the non-confined compressibility factor to incorporate the effects of confinement. This adjustment can be achieved through the following formulation:
The unconfined compressibility factor, denoted as z∞, represents the compressibility factor at pore radii equal to infinity, disregarding any pore confinement effects. This value can be determined through various means such as utilizing the Standing Katz compressibility chart, employing equations of state, applying correlations, or conducting laboratory experiments. By substituting the obtained z∞ value into Eq. 25, it becomes possible to effectively account for the influence of critical properties shift on the gas compressibility factor. This allows us to calculate the actual compressibility factor, denoted as zr, at the desired pore radius. In doing so, the impact of confinement effects imposed by the pore size and geometry is appropriately considered, leading to more accurate results for gas compressibility at the specific pore radius of interest. Consequently, this adjustment enables the calculation of gas density, viscosity, and the isothermal gas compressibility coefficient.
4 Conclusion
-
1.
Confined hydrocarbon gas mixtures in shale and reservoirs undergo significant property deviations due to increased collisions with pore walls. These deviations can reach up to 15% at high pressures (> 2000 psia), low temperatures, and small pore sizes for rich gases.
-
2.
Intermolecular attraction reduction causes increased compressibility factor and decreased gas density and viscosity. Furthermore, it is important to note that the isothermal gas compressibility coefficient remains primarily governed by pressure. Viscosity is the most affected property, followed by density and compressibility factor, as demonstrated by correlation and trend analysis.
-
3.
The “specific pore radius” marks the point where confinement effects become significant, and this varies with conditions. Specifically, rich gases at high pressure and low temperature exhibit deviations at larger pore sizes, while lean gases at low pressure and high temperature show them at smaller pore sizes.
-
4.
Importantly, we have developed a new correlation that accurately adjusts unconfined gas properties for confinement effects in shale and tight reservoirs. This correlation is applicable for Pr < 9, Tr < 2, and pore radii ranging from 2.5 to 200 nm, with an average absolute error of 7.8%. Furthermore, its implementation in reservoir simulation and production modeling software can greatly enhance their performance. Consequently, our research significantly contributes to the understanding and quantification of confinement effects in unconventional gas reservoirs.
References
Kamari, A.; Li, L.; Sheng, J.J.: Effects of rock pore sizes on the PVT properties of oil and gas-condensates in shale and tight reservoirs. Petroleum 4, 148–157 (2018). https://doi.org/10.1016/j.petlm.2017.06.002
Xu, Y.; Wang, Z.; Hong, J.; Zhou, B.; Pu, H.: An insight into wax precipitation, deposition, and prevention stratagem of gas-condensate flow in Wellbore region. J. Energy Resour. Technol. 145, 1–32 (2023). https://doi.org/10.1115/1.4062084
Fang, Y.; Yang, E.; Guo, S.; Cui, C.; Zhou, C.: Study on micro remaining oil distribution of polymer flooding in class-II B oil layer of Daqing oilfield. Energy 254, 124479 (2022). https://doi.org/10.1016/J.ENERGY.2022.124479
Zhong, H.; He, Y.; Yang, E.; Bi, Y.; Yang, T.: Modeling of microflow during viscoelastic polymer flooding in heterogenous reservoirs of Daqing oilfield. J. Pet. Sci. Eng. 210, 110091 (2022). https://doi.org/10.1016/J.PETROL.2021.110091
Zhang, J.; Zeng, H.: Intermolecular and surface interactions in engineering processes. Engineering 7, 63–83 (2021). https://doi.org/10.1016/J.ENG.2020.08.017
Ahmadi, M.A.; Bahadori, A.: Characterization of Shale Gas. In: Fluid Phase Behavior for Conventional and Unconventional Oil and Gas Reservoirs, pp. 445–481. Elsevier, Netherlands (2017). https://doi.org/10.1016/B978-0-12-803437-8.00009-9
Kuila, U.; Prasad, M.: Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 61, 341–362 (2013). https://doi.org/10.1111/1365-2478.12028
Singh, S.K.; Sinha, A.; Deo, G.; Singh, J.K.: Vapor−liquid phase coexistence, critical properties, and surface tension of confined alkanes. J. Phys. Chem. C 113, 7170–7180 (2009). https://doi.org/10.1021/JP8073915
Zarragoicoechea, G.J.; Kuz, V.A.: van der Waals equation of state for a fluid in a nanopore. Phys Rev E. 65, 021110 (2002). https://doi.org/10.1103/PhysRevE.65.021110
Ma, Y.; Jin, L.; Jamili, A.: Modifying van der Waals equation of state to consider influence of confinement on phase behavior. Proc. SPE Annu. Tech. Conf. Exhib. 6, 4643–4654 (2013). https://doi.org/10.2118/166476-ms
Li, L.; Sheng, J.J.: Nanopore confinement effects on phase behavior and capillary pressure in a wolfcamp shale reservoir. J. Taiwan Inst. Chem. Eng. 78, 317–328 (2017). https://doi.org/10.1016/j.jtice.2017.06.024
Whitson, C.; Brule, M.: SPE Phase Behavior Monograph. (1999)
Gmehling, J.; Kleiber, M.; Kolbe, B.; Rarey, J.: Chemical Thermodynamics for Process Simulation. Wiley, New Jersey (2012)
Prausnitz, J.M.; Lichtenthaler, R.N.; de Azevedo, E.G.: Molecular Thermodynamics of Fluid-Phase Equilibria. Pearson Education, London (1998)
McCain, J.W.D.: Properties of Petroleum Fluids. (1973)
Ahmed, T.: Equations of State and PVT Analysis: Applications for Improved Reservoir Modeling. Second edition. (2016)
Ahmed, T.: Vapor-liquid phase equilibria. In: Reservoir Engineering Handbook, pp. 1109–1225. Elsevier, Netherlands (2019). https://doi.org/10.1016/B978-0-12-813649-2.00015-3
Ahmed, T.: Equations of state and phase equilibria. In: Equations of State and PVT Analysis, pp. 467–597. Elsevier, Netherlands (2016). https://doi.org/10.1016/B978-0-12-801570-4.00005-2
Mahdavi, E.; Suleymani, M.; Rahmanian, N.: Oil and gas properties and correlations. In: Fluid Phase Behavior for Conventional and Unconventional Oil and Gas Reservoirs, pp. 1–64. Elsevier, Netherlands (2017). https://doi.org/10.1016/B978-0-12-803437-8.00001-4
Ahmed, T.: Reservoir-fluid properties. In: Reservoir Engineering Handbook, pp. 29–121. Elsevier, Netherlands (2019). https://doi.org/10.1016/B978-0-12-813649-2.00002-5
Lee, A.L.; Gonzalez, M.H.; Members, J.; Bertram, A.; Eakin, E.: The viscosity of natural gases. J. Petrol. Technol. 18, 997–1000 (1966). https://doi.org/10.2118/1340-PA
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests related to this research.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mamdouh, M., Elsayed, S.K. & El-Rammah, S. Investigation of the Properties of Hydrocarbon Natural Gases Under Confinement in Tight Reservoirs Due to Critical Properties Shift. Arab J Sci Eng 48, 16907–16919 (2023). https://doi.org/10.1007/s13369-023-08210-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08210-z