Abstract
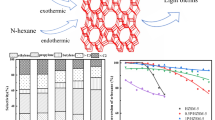
In this study, the effects of different types of acid treatments (H3PO4, H2SO4, and HCl) on the physicochemical properties and catalytic activity of the microporous HZSM-5 catalyst were investigated in methanol to olefins (MTO) reaction. The synthesized catalysts were characterized by XRD, N2 adsorption/desorption, ICP-MS, SEM, TEM, and pyridine-adsorbed DRIFT techniques. The characterization analyses indicated that each acid treatment led to the removal of differently positioned Al atoms in the HZSM-5 framework. Consequently, these acid treatments tuned the physicochemical properties, enhanced mesoporosity, and adjusted the Lewis Acid Site/Brønsted Acid Site (LAS/BAS) ratio of the catalysts by increasing the Si/Al ratio based on the removed Al atoms positioned in the HZSM-5 structure. In the activity tests of the catalysts, acid-treated HZSM-5 catalysts showed more stable and higher average methanol conversion, ethylene, and propylene selectivity compared to the parent catalyst. Among the acid-treated catalysts, the highest total light olefin selectivity (53%) was obtained over phosphoric acid-treated HZSM-5 due to the formation of silicoaluminophosphate-like structures, the generation of mesopores, and the adjusted acidity caused by the altered Si/Al ratio. TOS results showed that the amount of total light olefin selectivity obtained from the parent HZSM-5 was increased by 33% after phosphoric acid treatment. Acid treatments decreased coke deposition over the catalyst from 9.5 to 1.3%. Thus, it is noteworthy to say that the use of phosphoric acid-treated HZSM-5 in the MTO reaction is recommendable.











Similar content being viewed by others
References
Liu, D.; Choi, W.C.; Kang, N.Y.; Lee, Y.J.; Park, H.S.; Shin, C.H.; Park, Y.K.: Inter-conversion of light olefins on ZSM-5 in catalytic naphtha cracking condition. Catal. Today (2014). https://doi.org/10.1016/j.cattod.2013.09.060
Al-Shafei, E.N.; Albahar, M.Z.; Aljishi, M.F.; Aljishi, A.N.; Nasser, G.A.; Sanhoob, M.A.; Alnasir, A.S.; AlAsseel, A.: Effect of zeolite structure and addition of steam on naphtha catalytic cracking to improve olefin production. Fuel (2022). https://doi.org/10.1016/j.fuel.2022.124089
Chen, Z.; Li, Z.; Zhang, Y.; Chevella, D.; Li, G.; Chen, Y.; Guo, X.; Liu, J.; Yu, J.: A green route for the synthesis of nano-sized hierarchical ZSM-5 zeolite with excellent DTO catalytic performance. Chem. Eng. J. (2020). https://doi.org/10.1016/j.cej.2020.124322
Al-Dughaither, A.S.; de Lasa, H.: Neat dimethyl ether conversion to olefins (DTO) over HZSM-5: effect of SiO2/Al2O3 on porosity, surface chemistry, and reactivity. Fuel (2014). https://doi.org/10.1016/j.fuel.2014.07.026
Fatih, Y.; Burgun, U.; Sarioglan, A.; Atakül, H.: Effect of sodium incorporation into Fe-Zn catalyst for Fischer-Tropsch synthesis to light olefins. Mol. Catal. (2023). https://doi.org/10.1016/j.mcat.2022.112866
Wang, D.; Gu, Y.; Chen, Q.; Tang, Z.: Direct conversion of syngas to alpha olefins via Fischer-Tropsch synthesis: process development and comparative techno-economic-environmental analysis. Energy (2023). https://doi.org/10.1016/j.energy.2022.125991
Wang, C.; Zhang, Q.; Zhu, Y.; Zhang, D.; Chen, J.; Chiang, F.K.: p-Xylene selectivity enhancement in methanol toluene alkylation by separation of catalysis function and shape-selective function. Mol. Catal. (2017). https://doi.org/10.1016/j.mcat.2016.12.007
Rostami, R.B.; Lemraski, A.S.; Ghavipour, M.; Behbahani, R.M.; Shahraki, B.H.; Hamule, T.: Kinetic modelling of methanol conversion to light olefins process over silicoaluminophosphate (SAPO-34) catalyst. Chem. Eng. Res. Des. (2016). https://doi.org/10.1016/j.cherd.2015.10.019
Tian, P.; Wei, Y.; Ye, M.; Liu, Z.: Methanol to olefins (MTO): from fundamentals to commercialization. ACS Catal. (2015). https://doi.org/10.1021/acscatal.5b00007
Yang, M.; Fan, D.; Wei, Y.; Tian, P.; Liu, Z.: Recent progress in methanol-to-olefins (MTO) catalysts. Adv. Mater. (2019). https://doi.org/10.1002/adma.201902181
Zhou, Y.; Shen, X.; Li, J.: Crystallization and MTO performance of SAPO-34 zeolite under the influence of hydroxyl radicals. Inorg. Chem. Commun. (2019). https://doi.org/10.1016/j.inoche.2019.107462
Sun, Q.; Xie, Z.; Yu, J.: The state-of-the-art synthetic strategies for SAPO-34 zeolite catalysts in methanol-to-olefin conversion. Natl. Sci. Rev. (2018). https://doi.org/10.1093/nsr/nwx103
Zhang, H.; Ning, Z.; Shang, J.; Liu, H.; Han, S.; Qu, W.; Jiang, Y.; Guo, Y.: A durable and highly selective PbO/HZSM-5 catalyst for methanol to propylene (MTP) conversion. Microporous Mesoporous Mater. (2017). https://doi.org/10.1016/j.micromeso.2017.04.031
He, S.; Wang, S.; Fan, S.; Luo, L.; Yuan, K.; Qin, Z.; Dong, M.; Wang, J.; Fan, W.: Improvement of the catalytic performance of ITQ-13 zeolite in methanol to olefins via Ce modification. Catal. Today (2023). https://doi.org/10.1016/j.cattod.2022.05.049
Zhang, S.; Zhang, B.; Gao, Z.; Han, Y.: Methanol to olefin over Ca-modified HZSM-5 zeolites. Ind. Eng. Chem. Res. (2010). https://doi.org/10.1021/ie901446m
Khezri, H.; Izadbakhsh, A.; Izadpanah, A.A.: Promotion of the performance of La, Ce and Ca impregnated HZSM-5 nanoparticles in the MTO reaction. Fuel Process. Technol. (2020). https://doi.org/10.1016/j.fuproc.2019.106253
Ahmadpour, J.; Taghizadeh, M.: Selective production of propylene from methanol over high-silica mesoporous ZSM-5 zeolites treated with NaOH and NaOH/tetrapropylammonium hydroxide. C. R. Chim. (2015). https://doi.org/10.1016/j.crci.2015.05.002
Wei, Y.; de Jongh, P.E.; Bonati, M.L.; Law, D.J.; Sunley, G.J.; de Jong, K.P.: Enhanced catalytic performance of zeolite ZSM-5 for conversion of methanol to dimethyl ether by combining alkaline treatment and partial activation. Appl. Catal. A (2015). https://doi.org/10.1016/j.apcata.2014.12.027
Ren, S.; Liu, G.; Wu, X.; Chen, X.; Wu, M.; Zeng, G.; Liu, Z.; Sun, Y.: Enhanced MTO performance over acid treated hierarchical SAPO-34. Chin. J. Catal. (2017). https://doi.org/10.1016/S1872-2067(16)62557-3
Louwen, J.N.; Van Eijck, L.; Vogt, C.; Vogt, E.T.: Understanding the activation of ZSM-5 by phosphorus: localizing phosphate groups in the pores of phosphate-stabilized ZSM-5. Chem. Mater. (2020). https://doi.org/10.1021/acs.chemmater.0c03411
Danisi, R.M.; Schmidt, J.E.; Paioni, A.L.; Houben, K.; Poplawsky, J.D.; Baldus, M.; Weckhuysen, B.M.; Vogt, E.T.: Revealing long-and short-range structural modifications within phosphorus-treated HZSM-5 zeolites by atom probe tomography, nuclear magnetic resonance and powder X-ray diffraction. Phys. Chem. Chem. Phys. (2018). https://doi.org/10.1039/C8CP03828G
Meng, X.; Lian, Z.; Wang, X.; Shi, L.; Liu, N.: Effect of dealumination of HZSM-5 by acid treatment on catalytic properties in non-hydrocracking of diesel. Fuel (2020). https://doi.org/10.1016/j.fuel.2020.117426
Rostamizadeh, M.; Yaripour, F.: Dealumination of high silica H-ZSM-5 as long-lived nanocatalyst for methanol to olefin conversion. J. Taiwan Inst. Chem. Eng. (2017). https://doi.org/10.1016/j.jtice.2016.12.003
Lopez-Orozco, S.; Inayat, A.; Schwab, A.; Selvam, T.; Schwieger, W.: Zeolitic materials with hierarchical porous structures. Adv. Mater. (2011). https://doi.org/10.1002/adma.201100462
Valtchev, V.; Majano, G.; Mintova, S.; Perez-Ramirez, J.: Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. (2013). https://doi.org/10.1039/C2CS35196J
Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A.: Hierarchy concepts: classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. (2016). https://doi.org/10.1039/C5CS00599J
Fang, Y.; Yang, F.; He, X.; Zhu, X.: Dealumination and desilication for Al-rich HZSM-5 zeolite via steam-alkaline treatment and its application in methanol aromatization. Front. Chem. Sci. Eng. (2019). https://doi.org/10.1007/s11705-018-1778-8
Tanaka, S.; Fukui, R.; Kosaka, A.; Nishiyama, N.: Development of hierarchical and phosphorous-modified HZSM-5 zeolites by sequential alkaline/acid treatments and their catalytic performances for methanol-to-olefins. Mater. Res. Bull. (2020). https://doi.org/10.1016/j.materresbull.2020.110958
Fan, Y.; Bao, X.; Lin, X.; Shi, G.; Liu, H.: Acidity adjustment of HZSM-5 zeolites by dealumination and realumination with steaming and citric acid treatments. J. Phys. Chem. B (2006). https://doi.org/10.1021/jp0607566
Gorzin, F.; Darian, J.T.; Yaripour, F.; Mousavi, S.M.: Preparation of hierarchical HZSM-5 zeolites with combined desilication with NaAlO 2/tetrapropylammonium hydroxide and acid modification for converting methanol to propylene. RSC Adv. (2018). https://doi.org/10.1039/C8RA08624A
Li, J.; Liu, M.; Li, S.; Guo, X.; Song, C.: Influence of diffusion and acid properties on methane and propane selectivity in methanol-to-olefins reaction. Ind. Eng. Chem. Res. (2019). https://doi.org/10.1021/acs.iecr.8b03969
Balbay, A.; Selvitepe, N.; Saka, C.: Fe doped-CoB catalysts with phosphoric acid-activated montmorillonite as support for efficient hydrogen production via NaBH4 hydrolysis. Int. J. Hydrogen Energy (2021). https://doi.org/10.1016/j.ijhydene.2020.09.181
Yang, L.; Wang, K.; Yang, J.; Zhang, W.: Role of hydrochloric acid treated HZSM-5 zeolite in Sm2Ti2O7/nHZSM-5 composite for photocatalytic degradation of ofloxacin. J. Market. Res. (2020). https://doi.org/10.1016/j.jmrt.2020.07.080
Yu, Z.; Meng, X.; Liu, N.; Shi, L.: A novel disposal approach of deactivated resin catalyst for methyl tert-butyl ether synthesis: Preparation of low-cost activated carbons with remarkable performance on dibenzothiophene adsorption. Fuel (2017). https://doi.org/10.1016/j.fuel.2017.05.053
Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y.: Comparisons of porous, surface chemistry and adsorption properties of carbon derived from Enteromorpha prolifera activated by H4P2O7 and KOH. Chem. Eng. J. (2013). https://doi.org/10.1016/j.cej.2013.08.011
Feng, R.; Yan, X.; Hu, X.; Zhang, Y.; Wu, J.; Yan, Z.: Phosphorus-modified b-axis oriented hierarchical ZSM-5 zeolites for enhancing catalytic performance in a methanol to propylene reaction. Appl. Catal. A (2020). https://doi.org/10.1016/j.apcata.2020.117464
Valecillos, J.; Epelde, E.; Albo, J.; Aguayo, A.T.; Bilbao, J.; Castaño, P.: Slowing down the deactivation of H-ZSM-5 zeolite catalyst in the methanol-to-olefin (MTO) reaction by P or Zn modifications. Catal. Today (2020). https://doi.org/10.1016/j.cattod.2019.07.059
Tian, H.; Liu, S.; Han, Y.; Yang, K.; Xu, W.: Acid treatment to adjust zeolite hydrophobicity for olefin hydration reaction. J. Porous Mater. (2022). https://doi.org/10.1007/s10934-022-01199-0
Akansu, H.; Arbag, H.; Tasdemir, H.M.; Yasyerli, S.; Yasyerli, N.; Dogu, G.: Nickel-based alumina supported catalysts for dry reforming of biogas in the absence and the presence of H2S: effect of manganese incorporation. Catal. Today (2022). https://doi.org/10.1016/j.cattod.2021.12.012
Peng, Q.; Wang, G.; Wang, Z.; Jiang, R.; Wang, D.; Chen, J.; Huang, J.: Tuning hydrocarbon pool intermediates by the acidity of SAPO-34 catalysts for improving methanol-to-olefins reaction. ACS Sustain. Chem. Eng. (2018). https://doi.org/10.1021/acssuschemeng.8b04210
Lian, Z.; Yang, C.; Shi, L.; Meng, X.; Liu, N.; Yang, Y.; Wang, X.: Non-hydrocracking of diesel over hierarchical hzsm-5 zeolite to produce gasoline. Appl. Organomet. Chem. (2018). https://doi.org/10.1002/aoc.4587
Selvitepe, N.; Balbay, A.; Saka, C.: Optimisation of sepiolite clay with phosphoric acid treatment as support material for CoB catalyst and application to produce hydrogen from the NaBH4 hydrolysis. Int. J. Hydrogen Energy (2019). https://doi.org/10.1016/j.ijhydene.2019.04.254
Liu, C.; Li, G.; Hensen, E.J.; Pidko, E.A.: Nature and catalytic role of extraframework aluminum in faujasite zeolite: a theoretical perspective. ACS Catal. (2015). https://doi.org/10.1021/acscatal.5b02268
Silaghi, M.C.; Chizallet, C.; Sauer, J.; Raybaud, P.: Dealumination mechanisms of zeolites and extra-framework aluminum confinement. J. Catal. (2016). https://doi.org/10.1016/j.jcat.2016.04.021
Li, M.; Mo, J.; Gu, X.; Zhou, Y.; Fan, M.; Shen, S.; Chen, Y.: Acid modified carrier on catalytic oxidation of dichloromethane over CeO2/HZSM-5 catalysts. J. Rare Earths (2022). https://doi.org/10.1016/j.jre.2021.08.018
Perez-Uriarte, P.; Gamero, M.; Ateka, A.; Diaz, M.; Aguayo, A.T.; Bilbao, J.: Effect of the acidity of HZSM-5 zeolite and the binder in the DME transformation to olefins. Ind. Eng. Chem. Res. (2016). https://doi.org/10.1021/acs.iecr.5b04477
Shirazi, L.; Jamshidi, E.; Ghasemi, M.R.: The effect of Si/Al ratio of ZSM-5 zeolite on its morphology, acidity and crystal size. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. (2008). https://doi.org/10.1002/crat.200800149
Sanhoob, M.A.; Khan, A.; Ummer, A.C.: ZSM-5 catalysts for MTO: effect and optimization of the tetrapropylammonium hydroxide concentration on synthesis and performance. ACS Omega (2022). https://doi.org/10.1021/acsomega.2c01539
Chen, C.; Zhang, Q.; Meng, Z.; Li, C.; Shan, H.: Effect of magnesium modification over H-ZSM-5 in methanol to propylene reaction. Appl. Petrochem. Res. (2015). https://doi.org/10.1007/s13203-015-0129-7
Gayubo, A.G.; Benito, P.L.; Aguayo, A.T.; Olazar, M.; Bilbao, J.: Relationship between surface acidity and activity of catalysts in the transformation of methanol into hydrocarbons. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. (1996). https://doi.org/10.1002/(SICI)1097-4660(199602)65:2%3c186::AID-JCTB401%3e3.0.CO;2-J
Grau-Crespo, R.; Peralta, A.G.; Ruiz-Salvador, A.R.; Gómez, A.; López-Cordero, R.: A computer simulation study of distribution, structure and acid strength of active sites in H-ZSM-5 catalyst. Phys. Chem. Chem. Phys. (2000). https://doi.org/10.1039/B006490O
Chu, Y.; Yi, X.; Li, C.; Sun, X.; Zheng, A.: Brønsted/Lewis acid sites synergistically promote the initial C–C bond formation in the MTO reaction. Chem. Sci. (2018). https://doi.org/10.1039/C8SC02302F
Losch, P.; Laugel, G.; Martinez-Espin, J.S.; Chavan, S.; Olsbye, U.; Louis, B.: Phosphorous modified ZSM-5 zeolites: impact on methanol conversion into olefins. Top. Catal. (2015). https://doi.org/10.1007/s11244-015-0449-y
Tsunoji, N.; Osuga, R.; Yasumoto, M.; Yokoi, T.: Controlling hydrocarbon oligomerization in phosphorus-modified CHA zeolite for a long-lived methanol-to-olefin catalyst. Appl. Catal. A (2021). https://doi.org/10.1016/j.apcata.2021.118176
Dyballa, M.; Becker, P.; Trefz, D.; Klemm, E.; Fischer, A.; Jakob, H.; Hunger, M.: Parameters influencing the selectivity to propene in the MTO conversion on 10-ring zeolites: directly synthesized zeolites ZSM-5, ZSM-11, and ZSM-22. Appl. Catal. A (2016). https://doi.org/10.1016/j.apcata.2015.11.017
Park, G.; Kang, J.; Park, S.J.; Kim, Y.T.; Kwak, G.; Kim, S.: Effect of acid modification of ZSM-5 catalyst on performance and coke formation for methanol-to-hydrocarbon reaction. Mol. Catal. (2022). https://doi.org/10.1016/j.mcat.2022.112702
Alshafei, F.H.; Park, Y.; Zones, S.I.; Davis, M.E.: Methanol-to-olefins catalysis on ERI-type molecular sieves: towards enhancing ethylene selectivity. J. Catal. (2021). https://doi.org/10.1016/j.jcat.2021.10.025
Rui, P.; Wang, B.; Chen, F.; Xiang, Y.; Yang, J.; Guo, T.; Wu, Z.; Liao, W.; Shu, X.: The hydrothermal synthesis of hierarchical SAPO-34 with improved MTO performance. New J. Chem. (2021). https://doi.org/10.1039/D1NJ01066B
Kaarsholm, M.; Joensen, F.; Nerlov, J.; Cenni, R.; Chaouki, J.; Patience, G.S.: Phosphorous modified ZSM-5: deactivation and product distribution for MTO. Chem. Eng. Sci. (2007). https://doi.org/10.1016/j.ces.2006.12.076
Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A.: Effect of boron incorporation on the structure, products selectivities and lifetime of H-ZSM-5 nanocatalyst designed for application in methanol-to-olefins (MTO) reaction. Microporous Mesoporous Mater. (2015). https://doi.org/10.1016/j.micromeso.2014.10.024
Hambali, H.U.; Jalil, A.A.; Siang, T.J.; Abdulrasheed, A.A.; Fatah, N.A.A.; Hussain, I.; Azami, M.S.: Effect of ZSM-5 acidity in enhancement of methanol-to-olefins process. J. Energy Saf. Technol. (JEST) (2019). https://doi.org/10.11113/jest.v2n1.37
Wu, L.; Magusin, P.C.; Degirmenci, V.; Li, M.; Almutairi, S.M.; Zhu, X.; Mezari, B.; Hensen, E.J.: Acidic properties of nanolayered ZSM-5 zeolites. Microporous Mesoporous Mater. (2014). https://doi.org/10.1016/j.micromeso.2013.08.042
Erdogan, B.; Arbag, H.; Yasyerli, N.: SBA-15 supported mesoporous Ni and Co catalysts with high coke resistance for dry reforming of methane. Int. J. Hydrogen Energy (2018). https://doi.org/10.1016/j.ijhydene.2017.11.127
Liu, J.; Zhang, C.; Shen, Z.; Hua, W.; Tang, Y.; Shen, W.; Yue, Y.; Xu, H.: Methanol to propylene: effect of phosphorus on a high silica HZSM-5 catalyst. Catal. Commun. (2009). https://doi.org/10.1016/j.catcom.2009.04.004
Acknowledgements
The Middle East Technical University Central Laboratory is gratefully acknowledged. Thanks are due for the contributions of Prof. Dr. Nuray Oktar from Gazi University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests regarding the publication of this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Degirmencioglu, P., Arbag, H. Acid Treatment to Improve Total Light Olefins Selectivity of HZSM-5 Catalyst in Methanol to Olefins (MTO) Reaction. Arab J Sci Eng 48, 16123–16136 (2023). https://doi.org/10.1007/s13369-023-08067-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08067-2