Abstract
The photocatalytic performances of three Fe2O3–CeO2 nanocomposites were investigated toward the sulfur removal from a petroleum heavy gas oil (HGO) sample. The three composites were prepared by three different routes namely; auto-combustion, post-precipitation and precipitation. The physio-chemical features and optical properties of the presented composites were determined via proper analytical techniques. Formation of Fe2O3–CeO2 solid solution in all the prepared composites was verified via XRD analysis. These composites were then employed in photo-desulfurization of HGO and their activities were investigated at several operating conditions. The highest photocatalytic desulfurization exploit (91.5%) could be detected for the composite which was prepared via auto-combustion technique, denoted as (Fe20Ce80)ac. This maximum percentage of sulfur removal could be obtained under visible light irradiation at the following optimum operating conditions: 15 g/L (as photocatalyst dose), time of 6 h and 2:1 of H2O2 to oil ratio. The subsequent implementation of a solvent extraction step using N-methyl pyrrolidone was needed to attain the deepest desulfurization of HGO. The efficiencies of the presented composites against the process of sulfur removal were discussed in spot of their textural and optical characteristics as well as the available oxygen vacancies through their lattices structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A significant portion (20–40%) of the distributed high cetane diesel fuel is produced via the fluid catalytic cracking (FCC) of vacuum gas oil (VGO). However, such technique is responsible for 90% of sulfur content of the commercially produced diesel fuels [1]. Therefore, the reduction of their sulfur contents, to about 30 ppm, to meet the Euro-5 specifications is urgently needed. The hydrotreating of FCC-derived diesel fuel can achieve this goal: however, it is a complicated process that may lead to obvious declines in cetane number of the ultimate diesel fuels [2]. Alternatively, the hydrotreatment of original VGO is more reliable to produce preliminary diesel fuel feedstock with sulfur contents of 200–500 ppm [3]. Meanwhile, the conventional hydrotreatment process is suffering from some restrictions in terms of being operated at severe operating conditions of Temperature and pressure as well as utilization of costly catalyst [4]. Thus, it is highly demanded to propose alternative processes to reduce the sulfur content in VGO to generate environmental friendly diesel fuels. As response for such claim, several techniques have been examined to effectively eliminate the refractory sulfur compounds from petroleum diesel oil feedstocks. Bio- desulfurization is one of these efficient techniques: however, it encounters various drawbacks [5]. Particularly, it is a long time consuming process as well as it requires hindered pilot scale capacity. Moreover, such process needs specific microbes have metabolical capability, suitable surrounding growth conditions, and appropriate levels of nutrients and contaminants [6]. Extractive and oxidative-extractive methods, using pure and/or metal oxide doped ionic liquids, were also employed as alternative routed for to removal of dibenzothiophene molecules from diesel fuels [7]. These processes have exhibited sulfur compounds removal of 94–98% at ambient temperature [7, 8]. Despite of these high removal efficiencies, these processes require expensive chemicals, need multi-steps synthesis, cause an environmental hazardous impacts and utilize massive amounts of oxidants [9]. Among other several process, photo-oxidative desulfurization (POD) is considered as a promising candidate due to the use of sunlight as a cheap and abundant energy source. The POD has been widely applied to eliminate the majority of organic pollutants such as azo dyes [10], pharmaceutics [11] aromatics [12] and phenols [13] from wastewater. In line with, the POD process has widely achieved high efficiencies in elimination of Dibenzothiophene and its derivatives from model and real diesel fuels [14], 15. In such process, photocatalytic semiconductors are illuminated with a source of light has energy near or above their band gab to produce electron–hole pairs which are responsible for radicals formation and redox reactions [16]. The attainment of high efficiency by such technique had been commonly dependent to the usage of UV absorptive materials. Among numerous semiconductors, TiO2 was used as photocatalysts for removal of benzothiophene derivatives from fuels. This findings can be attributed to its unique optical, electronic structure and chemical properties [17]. Au and or Ag/TiO2 were also utilized to enhance the TiO2 photocatalytic activity [18]. Meanwhile, utilization of TiO2 usually encounters some restrictions namely; impossible excitation by visible light, low adsorption capacity and difficult post-recovery of the TiO2 particles. Furthermore, modification of titanium oxide via using noble metals, to enhance its visible light absorption, is not favorable in terms of their high prices. Thus, some other semiconductors such as TiO2/biochar [19], CeO2/biochar [20], BiOBr [21] were introduced as photocatalysts.
CeO2 is a good potential for photocatalytic reactions due to its effective Ce4+/Ce3+ redox profile and its narrow band gap [22]. Additionally, CeO2 has strong chemical and photo-corrosion resistance. Although CeO2 can display high photo-activity, its high band gap (3.2 eV) makes it efficient only in UV absorbtion region which represents ~ 5% of photon flux in sunlight. Accordingly, it is obviously beneficial to extend the light absorbance of CeO2 to the visible light range which represents ~ 45% of solar light [23]. Recently, several studies have been devoted to shift the CeO2 light absorbance to the visible range through modifying its structure by doping/loading of some metals oxides. Specifically, doping with Fe3+ ions is highly recommended since it facilitates the formation of hydroxyl radicals which effectively enhance the photocatalytic performances of the obtained composites [24]. Furthermore, Fe2O3 is naturally occurred, has a strong chemical stability and narrow band gap of (2–2.3 eV) with strong light absorbance in range of visible light [25]. Fe2O3–CeO2 nanocompisites were extensively investigated as efficient photocatalyst for degradation of formic and oxalic acids [26]. Doping of 2 Mol%. Fe2O3 with CeO2 to absorb photons in range of visible light could increase the removing efficiency of CeO2 by 3 and 5 times for oxalic and formic acids respectively. The loading of 1.5 Mol.% Fe2O3 has also enhanced the CeO2 ability to eliminate methylene blue dye from wastewater by 12 fold [27]. Fe2O3–CeO2 nanocomposite could also degrade 96% of congo-red dye which is about 50% higher than activity of pure ceria [28].
Despite of the displayed high efficiency of such Fe2O3–CeO2 heterojunction composites, their particles sizes and morphologies are effective feature in their optical properties and photocatalytic performance. The porosity and surface area of photocatalysts are other crucial factors in revealing their high activity [29]. So, it is potentially required to develop a specific synthesis methods controlling such features in photocatalysts to maximize their exploit. Diversity of techniques including precipitation [30] and hydrothermal [31] were examined to synthesize Fe2O3–CeO2 nanocomposites. Meanwhile, hydrothermal process, in some cases, is a time consumer procedures. On the other side, gas phase method was found to be a rapid and superior methodology to produce photocatalysts with controlled morphology and small particles size [32]. Nevertheless, procedures of such technique need high vacuum and/or high temperatures and quite expensive precursors. On the other hand, auto-combustion and self-assembly precipitation procedures are fast and can produce small particles with well controlled porous nature.
The process of photo-desulfurization is a quite effective route for the disposal of sulfur compounds of different diesel fuel fractions. However to elevate the desulfurization activity by such route, the coupling with oxidative and extractive desulfurization methods is strongly recommended [33].
In this research work, the effect of synthesis techniques on Fe2O3–CeO2 nanocomposites activities toward desulfurization of VGO is investigated. The impact of operating conditions, for the proposed technique, on the quality of sulfur removal activity are also studied. Reaction mechanism as well as the reaction rate of the introduced desulfurization process, in this study, are also determined.
2 Experimental
2.1 Materials
Fe(NO3)3·9H2O, Ce(NO3)3·6H2O were utilized as precursors for Fe2O3 and CeO2 respectively. Citric acid was also used as fuel during the process of auto-combustion synthesis. All these chemical reagents were purchased from (sigma-Aldrich) and were utilized as-received with no further treatments. NH4OH (33%) was utilized as precipitating agent and was conducted from El-Naser Company.
2.2 Catalysts Synthesis
In the current research study, three photocatalytic iron oxide/ceria nanocompisites were prepared. The first one was prepared by the co-precipitation method where relative quantities of Fe2O3 and CeO2 precursors (ratio of Fe/Ce equals 20 wt.%) were dissolved in distillate water under vigorous stirring and heated up to 50° C. An amount of 30 mL ammonia solution (NH4OH 33%) were added drop wise until pH of 12 is reached. The formed precipitate was then filtered and dried overnight at 120° C. After drying, the obtained powder was then calcined at 300° C for 3 h to produce the proposed nanocomposite.
Another nanocomposite was prepared by auto-combustion method. Typically, ceria and hematite precursors were separately dissolved in 10 mL distillated water. A solution (0.1 M) of citric acid was prepared and separately added, in adequate portions, to the corresponding amounts of metals precursors in their solutions. The obtained two mixtures were then stirred together vigorously at ambient temperature. The mixture was next heated up to 80° C until sol is observed. The temperature was subsequently raised and was kept at 250 °C until dried powder was obtained. The powder was then calcined at 300 °C for 3 h to ultimately obtain the designated nanocomposite.
The last photocatalyst was produced via precipitation (2 steps) methodology. Specifically, separate solutions (0.1 M) of the two precursors were prepared while considering relative ratio of Fe to Ce = 20%, by weight. The two solutions were mixed together under vigorous stirring. Then, NH4OH solution (5%) was prepared and added to the mixture until the pH of the solution reached the value of 8. The formed collide was further stirred for 15 min and NH4OH solution (33%) was then added until pH of 12 is noticed. The produced precipitate, by end of addition step, was filtered and subsequently washed with distilled water several times. The washed sample was dried overnight and was consequently calcined at 300 °C for 3 h.
The produced nanocomposites were denoted as (Fe20Ce80)cp, (Fe20Ce80)ac and (Fe20Ce80)sp for the synthesized composites by co-precipitation, auto-combustion and two steps precipitation respectively.
2.3 Photocatalysts Characterizations
The samples crystallography was detected by the XRD analysis using X’Pert (Philips, D8-Brucker Model). The apparatus has a Ni filter which is operated at 40 kV, 30 mA current and a scanning speed of 0.02 s−1. The textural and surface properties of the prepared composites were measured by the BET technique via NOVA 3200 apparatus, USA. The samples were first degassed at 250° C for 4 h before being forwarded to analysis. The morphological features of the as-prepared composites were determined via transmission electron microscopy (TEM) using JEM 1230 microscope (JEOL, Japan) at 120 kV. The present functional groups in the studies samples were investigated by FTIR technique via Nicolet spectrophotometer (model Is-10, USA) using KBr method. UV–vis spectrophotometer model V-570, JASCO (Tokyo, Japan) was used to investigate the range of light absorbance by the tested samples, to determine their band gap values. Spectro-fluorophotometer (JASCO FP-6500, Japan) was utilized to determine the samples photoluminescence (PL) at room temperature.
2.4 Photodesulfurization Activity
The activity of the prepared samples was examined toward the photo-oxidative extractive desulfurization (POED) of a petroleum gas oil fraction obtained from fluid catalytic cracking unit. The oil was supplied from the Suze Operating Petroleum Company. The oil has a content of S compounds equals 22,000 ppm and 52% of its composition are aromatic structures.
For typical photo-desulfurization experiments, 10 mL of gas oil was mixed with a 0.1 g catalyst in flask (50 mL) under vigorous stirring. The mixture was kept inside a opaque wooden box for 30 min to attain adsorption equilibrium. The mixture was then illuminated by a tungsten lamp with a wavelength of 550 nm for 5 h. The mixture was then filtered and the produced gas oil after treatment was analyzed. The operational desulfurization conditions namely: catalyst type, catalyst dose (0.05–0.20 g), reaction time (2–6 h), oil volume (10–50 mL) and the catalyst durability, were investigated in order.
3 Results and Discussion
3.1 Catalysts Structural and Textural Properties
Figure 1 shows the diffractograms of the synthesized catalysts. Obviously, all samples possesses peaks at 28.6°, 33.1°, 47.5°, 56.3° and 59.1°. The patterns are attributed to the (111), (200), (220), (311) and (222) miller indices of FCC–CeO2 fluorite structure respectively. In addition, no peaks assigned to the Fe2O3 were recorded. The Fe2O3 patterns absence can be explained via either the formation of tiny Fe2O3 cluster that is not detected by XRD or the fabrication of Fe2O3–CeO2 solid solution [34]. In the second case, the smaller radii Fe3+ (0.046 A°) replaces the large radii Ce4+ (0.078 A°) and causes the ceria lattice shrinkage [35]. The formation of solid solution rout is much convenient as a results of samples low calcination temperature (300 °C) and small Fe percent (20%). Neriet. al. reported the favourable fabrication of Fe2O3–CeO2 solid solution at calcination temperature ≤ 600 °C, while at higher temperature the Fe2O3 segregation is more reliable [36]. So, the solid solution formation is a thermodynamically meta-stable. On the other hand, the application of high calcination temperature results in segregation of Fe2O3 from the ceria lattice is more stable [37]. Additionally, several authors have reported that the formation of such solid solution is commonly limited by addition of ≤ 30% Fe2O3 to ceria lattice [35]38. Reportedly, the Fe–Ce solid solution is commonly characterized by a shift in ceria mean peaks to higher angle with increasing the Fe% [39]. However, in this work the ceria mean characteristic peaks have positioned in their normal place assigned to pure ceria which indicated no lattice distortion. This result is in line with that reported findings in [40]. Also, the (Fe20Ce80)ac sample showed much broad CeO2 peaks with smaller intensities in comparison to the other two samples which revealed the smaller particle sizes of these samples.
The lattice diameter and parameter values were calculated by scherrer equation using the CeO2 main peak at 28.6° and represented in Table 1. As indicated from the table, The (Fe20Ce80)sp lattice value were half of the other two samples. Also, the values of lattice parameter had obviously decreased in all samples than the normal CeO2 lattice of 5.4215 A°. While, the (Fe20Ce80)sp possessed the smallest lattice size and parameter which reveals its strong Fe–Ce interaction. The decline in lattice parameter is due to the lattice shrinkage which confirms the solid solution formation in all samples.
The Raman spectra of the synthesized samples are illustrated in Fig. 1b. The (Fe20Ce80)ac sample showed a high intensity peak at 445 cm−1 which attributed to the F2g active mode caused by the symmetric vibration of oxygen atoms surrounded each Ce4+ cation [41]. The peak was shifted to higher values of 452 and 463 cm−1 in case of (Fe20Ce80)sp and (Fe20Ce80)cp respectively. The peaks red shift is due to the reduction in crystallite size of these structures. The (Fe20Ce80)ac and (Fe20Ce80)cp have possessed a tiny peak at ~ 596 cm−1 which is assigned to the oxygen vacancies in CeO2 lattice [42]. Also, both samples have a peak at ~ 300 cm−1 which is ascribed to the replaced oxygen atoms in CeO2 fluorite structure [43]. The observed peaks imply the CeO2 lattice distortion due to formation of Fe2O3–CeO2 solid solution. The peak at 186 cm−1 could further confirm the CeO2 lattice shrinkage due to the strong CeO2-Fe2O3 interaction.
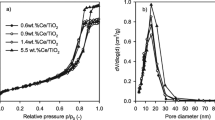
The surface properties and pores distribution were determined via BET analysis and results were displayed in Fig. 1c, d and Table 1. All the samples exhibited a type IV isotherm which is ascribed to the mesoporous materials according to IUPAC classification [44]. The samples had also exhibited a H2 hysteresis loop due to pore blocking/percolation effects in complex or network pores structure. This loop type represents the pores with ink-bottle structure. However, the (Fe20Ce80)cp and (Fe20Ce80)ac exhibited a conventional H2 loop referring to pores with uniform and much smaller neck size than the pore cavity. Meanwhile, the (Fe20Ce80)sp sample possessed an inverse H2 hysteresis which is assigned to the ink bottle with large neck size and small cavity. This can be explained by the pore widening due to the synthesis and/or calcination procedures. The surface area values were 115, 105 and 114.9 m2/g for (Fe20Ce80)cp, (Fe20Ce80)sp and (Fe20Ce80)ac respectively. The calculated average pore diameter was 16.3, 7.9 and 3.35 nm for in the same samples order.
The TEM analysis of the prepared samples possesses a small particle size in nanometre size. The (Fe20Ce80)ac micrograph (Fig. 2a) demonstrated the successive preparation of tiny porous sheets with average diameter of 40 nm. The image shows a uniform structure with a narrow sheet diameter. While, the (Fe20Ce80)sp sample (Fig. 2b) exhibited a small particles size in range of 20 nm. The image revealed, to some extent, the particles agglomeration. Likewise, the (Fe20Ce80)cp showed a much particles agglomeration.
The surface composition and valence state of the involved elements in (Fe20Ce80)ac and (Fe20Ce80)cp nanocomposites were determined by XPS analysis. Figure 3 showed the characteristic peaks of Fe 2p at 713.2 eV and 724.8 eV which assigned to the Fe 2p3/2 and 2p1/2 respectively [45]. The observed peak shift to higher binding energy values, over the commonly known, are due to the new bonds formed during the solid solution formation. This profile can influence the catalyst acidity and organic molecules adsorption onto the nanocomposites [46]. Other two peaks at eV 718.3 and 734.2 eV ascribed to the shake-up Fe satellite were observed. These satellite peaks confirms the presence of Fe3+. Most important that the (Fe20Ce80)ac has a recognisable higher peak intensities and peak shift than the (Fe20Ce80)cp catalyst which indicate its higher catalytic activity. The larger satellite peaks which are detected in (Fe20Ce80)ac indicates higher Fe–Ce lattice conjugation.
The Ce 3d core level spectra showed a multiples due to the 3d5/2 and 3d3/2 spin orbitals. The provided figure could show peaks at 885.7, 899, and 904.3 eV characteristic to Ce3+ species. While, other peaks at 882.2, 901.1, and 916.1 eV were noticed due to the presence of Ce4+ lattice ions [47]. This result revealed the co-existence of Ce3+ and Ce4+ in both samples. Reportedly, the peaks at 904.2 and 885.3 eV are attributed to the 3d10 4f0 ground state of Ce3+. Clearly, those peaks intensity as well as their peak area have recognisable higher values in case of (Fe20Ce80)ac than the (Fe20Ce80)cp. Also, the characteristic peaks of Ce4+ have less intensities than the Ce3+ main peaks in the (Fe20Ce80)ac. All those indicated observations confirm the higher Ce3+ existence in the (Fe20Ce80)ac catalyst than the (Fe20Ce80)cp which prospect the former higher catalytic activity [10].
The O 1 s spectrum of the prepared catalysts have showed a shoulder peaks at ~ 529 eV and 531 eV. Curves fitting possess the existence of 3 peaks at 529.3, 531.8 and 533.2 eV corresponding to the metal–oxygen bonding, oxygen lattice deficiency and adsorbed oxygen respectively. The chemosorbed oxygen is directly related to the oxygen vacancies [48]. Clearly, the (Fe20Ce80)ac had a much higher and broad peaks assigned to oxygen deficiency which revealed its potential catalytic activity than the(Fe20Ce80)cp.
3.2 Optical Properties
The optical properties of the synthesized catalysts were investigated via several techniques. The FTIR analysis (Fig. 4a) revealed that all samples revealed a broad beak at ~ 3450 cm−1 ascribed to the vibrational stretching of the O–H group [49]. This peak has shifted to higher wavenumber of 3500 cm-1 at the (Fe20Ce80)cp spectrum. All samples have peaks at 1050 and 1500 cm−1assigned to the deformation of the –OH vibrations [50]. Also, the peak at 1383 cm−1 attributed to the Ce–O vibration in CeO2 molecules has appeared in all spectrums [51]. However, The (Fe20Ce80)sp, (Fe20Ce80)cp samples exhibited an additional peak at 1620 cm−1. This peak is due to the vibrational stretching and bending of the –OH group [50]. The spectrum of (Fe20Ce80)cp possesses an additional peak at 2300 cm−1which might be due to the C–O stretching of the remained organic molecules during the preparation procedures.
The light absorbance profile of the photocatalysts was measured by the UV–vis technique and results were displayed in Fig. 4b. Clearly, all the photocatalyst have showed an absorption spectra in the visible range at wavelength higher than 400 nm. The catalysts exhibited absorption bands at 405, 410 and 430 nm for (Fe20Ce80)cp, (Fe20Ce80)sp and (Fe20Ce80)ac respectively. The results indicated the ability of Fe2O3 to shift the ceria light absorption from the UV to the visible range. This effect is due to the influenced delay in e−/h+ recombination[27]. Also, the red shift in the (Fe20Ce80)ac spectrum is predicting the decline in its band gab value [52]. Furthermore, The higher absorption edge of the (Fe20Ce80)ac catalyst indicated its higher activity to absorb light in visible range hence, a higher catalytic performance is predicted.
The calculated band gap values of the prepared samples were illustrated as Tauc curves in Fig. 4c. The figure showed the distinguishable lower intensity of (Fe20Ce80)ac while the (Fe20Ce80)sp and (Fe20Ce80)cp samples shows a relative high intensity. This results runs well with the observed red shift assigned to (Fe20Ce80)ac in Fig. 4b. The indicated band gap values were 1.65, 1.2 and 0.9 eV for the (Fe20Ce80)cp, (Fe20Ce80)sp and (Fe20Ce80)ac respectively. The (Fe20Ce80)ac lower band gap can be explained via the originated solid solution whereas, the Fe–Ce strong interaction increased, as the charge transfer is influenced [53]. Also, the strong interaction enhances the quantum confinement effect that created due to the Fe3+ incorporation to the ceria lattice [54]. The declined band gap in (Fe20Ce80)ac can be due to the creation of much oxygen deficiency due the preparation procedures. Such behaviour liberates two electrons that consumed through the Ce4+ to Ce3+ conversion and causes a CeO2 lattice contraction [55].
The optical properties of the synthesized catalysts were further investigated by the Pl technique to determine the e−/h+ recombination rate. The results in Fig. 4d showed the emission peaks at ~ 440 nm for (Fe20Ce80)sp and (Fe20Ce80)ac while (Fe20Ce80)cp had a peak at 470 nm. This results indicated the high light absorbance of the (Fe20Ce80)cp in visible range. Meanwhile, the (Fe20Ce80)sp exhibited a much lower intensity than the other two samples which indicates its higher photocatalytic activity. The lower intensity can be due to the oxygen deficiency and strong Fe–Ce interaction as discussed above [56].
3.3 Catalytic Activity
3.3.1 Activity Due to Preparation Procedures
The results of gas oil Photo-desulfurization under different reaction conditions were illustrated in Fig. 5. The desulfurization efficiencies of the synthesized catalysts were negligible under dark and direct photolysis conditions. Where, the efficiencies of ~ 1.5%, 2% and 4% were recorded to the (Fe20Ce80)cp, (Fe20Ce80)sp and (Fe20Ce80)ac catalysts respectively upon direct photolysis. Furthermore, the H2O2 and solvent to oil ratios were kept constant at 2:1 along the entire work.
The effect of catalyst preparation procedures on its photo-desulfurization activity was studied and results were illustrated in Fig. 5a. In typical experiments, a 0.1 g catalyst was mixed with 10 ml oil and subjected to illumination by 500 W lamp. The figure demonstrated the relatively higher activity of (Fe20Ce80)ac sample over the other samples. The sulphur removal% was 90.5%, 88% and 86% for the (Fe20Ce80)ac, (Fe20Ce80)sp and (Fe20Ce80)cp respectively. The relative catalysts efficiencies can be explained via the catalyst structure through several points. The obtained novel high activity can be attributed to the formed FexCe1-xOx solid solution. Where, the formation of solid solution in all samples can provide a smaller ceria lattice and declined its particle sizes (c.f. Table 1). In addition, the incorporation of Fe3+ ions into the ceria lattice shifts the ceria light absorbance to the visible range as indicated from the UV analysis and calculated band gab. Commonly, the loading of Fe3+ ions to ceria improves its redox activity [57]. Although those proposed causes illustrates the superior catalytic activities, they are insufficient to discuss the distinct (Fe20Ce80)ac desulfurization affinity. Where, all samples have proved, via the XRD, UV and XPS analysis, to have the FexCe1-xOx solid solution. The high activity of the (Fe20Ce80)ac can be due to the observed lower band gap (c.f. Figure 4c). Additionally, the (Fe20Ce80)ac high measured surface area which provides enough active centres for oil absorption and hence increases the reactivity. Most worthy is the higher oxygen deficiency occurred due to the auto-combustion synthesis procedures. As proved from the XPS, the (Fe20Ce80)ac possess a higher intensity and broadening for the oxygen vacancy peak than the (Fe20Ce80)cp catalyst. Moreover, the XPS results showed a higher Ce3+ characteristic peaks than the Ce4+. In addition, the summation of era under the Ce3+ main peaks in (Fe20Ce80)ac is greater than in (Fe20Ce80)cp. The role of oxygen vacancy in photoactivity was recently explained via reducing the band gap and attenuate the electronic structure, thereby accelerating the charge transfer. Furthermore, the present oxygen deficiency declining the energy barrier of O–O bonding and restrict the O–H reverse reaction. Thus, the O2 generation kinetics in defective CeO2 is significantly influenced [58]. Accordingly, the (Fe20Ce80)ac was chosen as the best catalyst.
Reportedly, the oil/catalyst contact time affected directly the photo-desulfurization process[59]. So, the contact time in range of 2–6 h were investigated to desulfurize the gas oil. The experiments were performed by applying 0.15 g (Fe20Ce80)ac photocatalyst to 10 ml oil under constant H2O2/oil and solvent/oil identical ratios of 2:1. The mixture was then illuminated with 500 W lamp in visible range for different contact time. The results in Fig. 5b demonstrated the linear improve in sulfur removal with increasing the contact time. Whereas, the removal percent was 80.5%, 91.5% at contact time of 2 and 6 h respectively. The efficiency did not stabilize or declined along the studied time interval which reveals the unattained equilibrium. On other words, a higher efficiency or even constant value can be obtained if elongated reaction time was allowed. In this study, 6 h as optimum contact time was chosen. The continuous efficiency increase can be explained by the high provided surface oxygen vacancies as active centers for the desulfurization reaction as discussed above.
Addition of suitable photocatalyst amount is crucial to avoid the catalyst overdose and ensure the complete photons absorption from the illuminated light. So, the catalyst amount was optimized for the effective desulfurization along the range of 0.05–0.2 g per 10 ml oil corresponding of 5–20 g/l. Figure 5c demonstrated the improvement in sulfur removal efficiency as the amount of catalyst in g was increased. The efficiency was increased up to 92.5% by utilizing 0.15 g catalyst while, it is sharply decreased to ~ 87% over 0.2 g catalyst. So, the amount of 0.15 g photocatalyst was applied to the next experiments. The increase in removal efficiency up to the utilized 0.15 g catalyst amount can be attributed to the increase inthe available active centers and surface area to the reaction. This increase the number of absorbed photons and hence influences the desulfurization activity. The negative impact of higher catalyst wt.% loading, can be due to the light scattering as a result of hindered light penetration into the oil which decline the catalytic performance.
Investigating further the catalyst efficiency, the oil desulfurization was studied over a 0.15 g (Fe20Ce80)ac photocatalyst with oil content varies from 10–50 ml. in other words, the catalyst efficiency limitation to remove the sulphur content from the oil was indirectly studied. Figure 5d shows a slight decrease in S removal with increasing the oil content to 20 ml. where, a sharp decrease in S% was observed with increasing the oil volume up to 50 ml. The removal efficiency was 91.5%, 90%, 80%, 68% and 61% with at added oil volume of 10, 20, 30, 40 and 50 ml respectively. The declined sulfur removal can be attributed to the lower active centre availability to the ascending sulfur concentration as a result of increasing the oil volume. So, a less sulphur molecules can adsorbed onto the catalyst surface and hence hindered efficiency was obtained. Most worthily, the results demonstrated the high catalyst efficiency even if a double oil/catalyst ratio was utilized. It is also important to mention that, the obtained high sulphur removal efficiency in this work is close to other works which utilize more oxidant concentration of 4–8 to the oil volume [33].
3.3.2 POED Comprehensive Results
The superiority of Fe2O3–CeO2 nanocomposite prepared by auto-combustion in this work over recent literature can be illustrated via Table 2. As clearly indicated, most of previous work concentrated on studying the removal of BT or DBT as sulfur model compounds from model oils. Also the previous work investigate the removal of a low DBT concentration of 500 ppm maximum. Additionally, the literature has not reported a high removal percent of maximum 97% until ionic liquids were utilized. In contrary, authors of this work have so recently reported the novel removal of 94.4% for highly concentrated DBT/paraffin oil (2200 ppm) using the POED process [60].
Meanwhile, the POED Process for real diesel oil is not common. Also, most of literature consider the POED of real diesel with a low sulfur compounds content of 0.1 g/L. However, in this work, the authors have investigated the gas oil obtained from the fluid catalytic unite which contains 22,000 ppm of sulfur compounds. This sulfur content is about 20 times than the usually investigated in literature. As a novel progress, the authors have claimed a sulfur compounds removal of 91.5%. This comparison revealed the synergetic activity of Fe2O3–CeO2 nanocomposites over all the former studied Photo-catalysts in sulfur removal from the real diesel. The obvious high Fe2O3–CeO2 activity can be attributed to the lattice oxygen deficiency, high surface area and particle size as discussed in previous section.
3.3.3 Catalytic Reusability, Kinetics, Systems and Mechanism
The effect of the catalyst reusability on its activity (Fig. 6a) was studied over 0.15 g of (Fe20Ce80)ac for 5 h. Figure 5a showed that the catalyst can repeatedly applied with no significant decline in efficiency for 3 cycles. The sulfur removal efficiency was reduced by 9% which confirms the (Fe20Ce80)ac reasonable reusability.
The desulfurization kinetics of HVGO over the (Fe20Ce80)ac (Fig. 6b) was investigated. Correlation of initial sulfur content and at reaction time t (ln C°/C) to the time gave a liner relationship with R2 value of 0.97.Where, the catalyst exhibited an apparent rate constant of 0.0028 min−1. The high catalyst activity was attributed to the high surface area and oxygen deficiency in Fe2O3–CeO2 lattice.
To clarify the role of each photo-desulfurization reaction component, the reaction under different component systems was performed (Fig. 6c). A ~ 7% and 31.5% desulfurization efficiency was obtained by subjecting the oil to light irradiation and catalyst surface without radiation. The removal can be due to the molecules thermal decomposition and catalyst surface adsorption respectively. Barely introducing the catalyst and H2O2 during the oil irradiation produces a desulfurization efficiency of 1.3% and 40% which reflect the importance of oxidant role in the process. On the other hand, simultaneous loading of H2O2 and catalyst to the oil and irradiating light has influenced the catalyst activity from 1.3 to ~ 28%.Where, in addition to the adsorption over the catalyst surface, the oxidant can provide the.OH−radicals that attacks the sulfur molecules and liberate the sulfur atoms.
The consecutive solvent extraction to the irradiated oil with N-methyl-pyrrolidone has clearly enhanced the desulfurization efficiency from ~ 7 to ~ 65%. Also, the solvent extraction increases the efficiency of catalyst activity in absence of radiation to 85.5% and neat irradiated oil to ~ 65%. Applying a subsequent solvent extraction to the luminance oil that separately contains catalyst and oxidant resulted in achieving 78.6% and 58.4% desulfurization efficiency respectively. Additionally, utilizing the solvent has influenced the efficiency from ~ 28 to 91.5% for the desulfurization system that contain the irradiated oil, catalyst and oxidant. All those results in case of solvent extraction utilization confirmed the role of solvent in removing the oxidized and neat sulfur compounds from the oil.
The desulfurization mechanism (Fig. 6d) can be discussed via the simultaneous visible light absorption by both the Fe2O3 and CeO2. Both oxides can be excited to form electrons and holes. As a result of low energy CB of CeO2, the CeO2 excited electrons are transferred to the CB of Fe2O3 through the lattice interfaces. The lattice oxygen deficiency becomes responsible for the ceria high activity[69]. Where, the excited electrons are trapped through converting the Ce4+ to Ce3+ while the h+ in VB of ceria are transferred to the lower energy VB in Fe2O3[70]. The facile low energy transition from O 2p orbital to VB of Ce3+ results in a CeO2 lattice distortion. The redox profile of the Ce4+/Ce3+ is then effectively decline the e−/h+ recombination[71]. The formed higher oxidation state ions are then reacts with excited electrons to form.O2− radicals and OOH− radicals which then forms the.OH radicals. On the other hand, the holes in VB can interact with the adsorbed H2O molecules or existed in the H2O2 to form.OH. Those radicals attack the sulphur containing molecules to form sulfones.
3.3.4 The properties of produced oil
Table 3 represents the alteration in oil properties due to the visible light exposure over the synthesized catalysts. The table demonstrated an obvious decrease in density and viscosity due to the photocatalytic reaction. The properties decline were enhanced over the catalysts in order of (Fe20Ce80)ac > (Fe20Ce80)sp > (Fe20Ce80)cp. Also, both the pour and flash point were increased in the same order. The increase in such properties could indicate the increase in saturated compounds (n, iso-paraffins and naphthenes) concentrations as results of aromatics degradation. In addition, the flash point was increased due to photo-reaction over all the prepared catalysts. The (Fe20Ce80)a ccatalyst has possessed the highest flash point of 82.5° C which indicates the highest oil thermal stability. Accordingly, the (Fe20Ce80)ac exhibited the highest performance not only to desulfurize the VGO but also influence its physio-chemical properties.
The influence of photo-treatment on the oil feed properties was further confirmed through the colour modification as indicated from Fig. 7. The figure shows an obvious change in samples colour in comparison with the feed. Additionally, the treated oil sample over the (Fe20Ce80)ac catalyst possessed some turbidity due to the concentrated n-paraffin while the other two samples was pretty clear. This observation confirms the obtained results for pour and aniline point in Table 2.
4 Conclusion
In this work, the effect of Fe2O3–CeO2 preparation procedure on the photo-desulfurization of heavy gas oil was investigated. Catalysts were synthesized by auto-combustion, step-precipitation and co-precipitation techniques. The results confirmed the formation of Fe2O3–CeO2 solid solution in all samples. While, (Fe20Ce80)ac possessed the lower lattice parameter, size and surface area. Also, it exhibited the lower band gap value than the other two samples. All those characteristics nominate it to be the most effective catalyst for oil desulfurization with sulphur removal efficiency of 91.5%. The optimum conditions for such high efficiency were to illuminate a 0.15 g catalyst with 500 W lamp in visible light range. Also, application of 2:1 of H2O2:oil and subsequent oil extraction in the same ratio with NMP is required.
References
Brunet, S.; Mey, D.; Pérot, G.; Bouchy, C.; Diehl, F.: On the hydrodesulfurization of FCC gasoline: a review. Appl. Catal. A Gen. 278, 143–172 (2005)
Song, C.: An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today. 86, 211–263 (2003)
Klimov, O.V.; Koryakina, G.I.; Gerasimov, E.Y.; Dik, P.P.; Leonova, K.A.; Budukva, S.V.; Pereyma, V.Y.; Uvarkina, D.D.; Kazakov, M.O.; Noskov, A.S.: A new catalyst for the deep hydrotreatment of vacuum gas oil, a catalytic cracking feedstock. Catal. Ind. 7, 38–46 (2015)
Rodríguez, M.A.; Elizalde, I.; Ancheyta, J.: Comparison of kinetic and reactor models to simulate a trickle-bed bench-scale reactor for hydrodesulfurization of VGO. Fuel 100, 91–99 (2012)
El-Sheshtawy, H.S.; Khalil, N.M.; Ahmed, W.; Abdallah, R.I.: Monitoring of oil pollution at Gemsa Bay and bioremediation capacity of bacterial isolates with biosurfactants and nanoparticles. Mar. Pollut. Bull. 87, 191–200 (2014)
Baselt, R.: Encyclopedia of toxicology (2014).
Ban, L.-L.; Liu, P.; Ma, C.-H.; Dai, B.: Deep extractive desulfurization of diesel fuels by FeCl3/ionic liquids. Chinese Chem. Lett. 24, 755–758 (2013)
Rodríguez-Cabo, B.; Rodríguez, H.; Rodil, E.; Arce, A.; Soto, A.: Extractive and oxidative-extractive desulfurization of fuels with ionic liquids. Fuel 117, 882–889 (2014)
Rang, H.; Kann, J.; Oja, V.: Advances in desulfurization research of liquid fuel. Oil Shale. 23, 164–176 (2006)
Majumder, D.; Chakraborty, I.; Mandal, K.; Roy, S.: Facet-dependent photodegradation of methylene blue using pristine CeO2 nanostructures. ACS Omega 4, 4243–4251 (2019)
Sudhaik, A.; Raizada, P.; Shandilya, P.; Singh, P.: Magnetically recoverable graphitic carbon nitride and NiFe2O4 based magnetic photocatalyst for degradation of oxytetracycline antibiotic in simulated wastewater under solar light. J. Environ. Chem. Eng. 6, 3874–3883 (2018)
Davari, N.; Farhadian, M.; Nazar, A.R.S.; Homayoonfal, M.: Degradation of diphenhydramine by the photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: structural and operational comparison. J. Environ. Chem. Eng. 5, 5707–5720 (2017)
Wang, J.-C.; Ren, J.; Yao, H.-C.; Zhang, L.; Wang, J.-S.; Zang, S.-Q.; Han, L.-F.; Li, Z.-J.: Synergistic photocatalysis of Cr (VI) reduction and 4-Chlorophenol degradation over hydroxylated α-Fe2O3 under visible light irradiation. J. Hazard. Mater. 311, 11–19 (2016)
Zhao, Y.; Zhao, L.; Han, J.; Xu, Y.; Wang, S.: Study on method and mechanism for simultaneous desulfurization and denitrification of flue gas based on the TiO2 photocatalysis. Sci. China Ser. E Technol. Sci. 51, 268–276 (2008)
Na, P.; Zhao, B.; Gu, L.; Liu, J.; Na, J.: Deep desulfurization of model gasoline over photoirradiated titanium-pillared montmorillonite. J. Phys. Chem. Solids. 70, 1465–1470 (2009)
Molinari, R.; Argurio, P.; Lavorato, C.: Review on reduction and partial oxidation of organics in photocatalytic (membrane) reactors. Curr. Org. Chem. 17, 2516–2537 (2013)
Czili, H.; Horváth, A.: Photodegradation of chloroacetic acids over bare and silver-deposited TiO2: Identification of species attacking model compounds, a mechanistic approach. Appl. Catal. B Environ. 89, 342–348 (2009)
Pham, X.N.; Pham, T.D.; Nguyen, B.M.; Tran, H.T.; Pham, D.T.: Synthesis of Al-MCM-41@Ag/TiO2 nanocomposite and its photocatalytic activity for degradation of dibenzothiophene, J. Chem. (2018)
Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H.: A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 222, 391–398 (2019)
Khataee, A.; Gholami, P.; Kalderis, D.; Pachatouridou, E.; Konsolakis, M.: Preparation of novel CeO2-biochar nanocomposite for sonocatalytic degradation of a textile dye. Ultrason. Sonochem. 41, 503–513 (2018)
Li, M.; Huang, H.; Yu, S.; Tian, N.; Dong, F.; Du, X.; Zhang, Y.: Simultaneously promoting charge separation and photoabsorption of BiOX (X= Cl, Br) for efficient visible-light photocatalysis and photosensitization by compositing low-cost biochar. Appl. Surf. Sci. 386, 285–295 (2016)
Hao, Y.; Li, L.; Zhang, J.; Luo, H.; Zhang, X.; Chen, E.: Multilayer and open structure of dendritic crosslinked CeO2-ZrO2 composite: Enhanced photocatalytic degradation and water splitting performance. Int. J. Hydrogen Energy. 42, 5916–5929 (2017)
Ho, C.; Yu, J.C.; Kwong, T.; Mak, A.C.; Lai, S.: Morphology-controllable synthesis of mesoporous CeO2 nano-and microstructures. Chem. Mater. 17, 4514–4522 (2005)
Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Phanichphant, S.; Nakaruk, A.; Koshy, P.; Sorrell, C.C.: Photocatalytic activity under visible light of Fe-doped CeO2 nanoparticles synthesized by flame spray pyrolysis. Ceram. Int. 39, 3129–3134 (2013)
Shao, P.; Ren, Z.; Tian, J.; Gao, S.; Luo, X.; Shi, W.; Yan, B.; Li, J.; Cui, F.: Silica hydrogel-mediated dissolution-recrystallization strategy for synthesis of ultrathin α-Fe2O3 nanosheets with highly exposed (1 1 0) facets: a superior photocatalyst for degradation of bisphenol S. Chem. Eng. J. 323, 64–73 (2017)
Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Phanichphant, S.: Kinetics study of photocatalytic activity of flame-made unloaded and Fe-loaded CeO2 nanoparticles, Int. J. Photoenergy. (2013).
Channei, D.; Inceesungvorn, B.; Wetchakun, N.; Ukritnukun, S.; Nattestad, A.; Chen, J.; Phanichphant, S.: Photocatalytic degradation of methyl orange by CeO2 and Fe-doped CeO2 films under visible light irradiation. Sci. Rep. 4, 1–7 (2014)
Aboutaleb, W.A.; El-Salamony, R.A.: Effect of Fe2O3–CeO2 nanocomposite synthesis method on the Congo red dye photodegradation under visible light irradiation. Mater. Chem. Phys. 236, 121724 (2019)
Xie, S.; Wang, Z.; Cheng, F.; Zhang, P.; Mai, W.; Tong, Y.: Ceria and ceria-based nanostructured materials for photoenergy applications. Nano Energy 34, 313–337 (2017)
Truffault, L.; Yao, Q.W.; Wexler, D.; Nevirkovets, I.P.; Konstantinov, K.; Devers, T.; Nightingale, S.: Synthesis and characterization of Fe doped CeO2 nanoparticles for pigmented ultraviolet filter applications. J. Nanosci. Nanotechnol. 11, 4019–4028 (2011)
Hirano, M.; Inagaki, M.: Preparation of monodispersed cerium (IV) oxide particles by thermal hydrolysis: influence of the presence of urea and Gd doping on their morphology and growth. J. Mater. Chem. 10, 473–477 (2000)
Samerjai, T.; Tamaekong, N.; Wetchakun, K.; Kruefu, V.; Liewhiran, C.; Siriwong, C.; Wisitsoraat, A.; Phanichphat, S.: Flame-spray-made metal-loaded semiconducting metal oxides thick films for flammable gas sensing. Sens. Actuators B Chem. 171, 43–61 (2012)
Zaid, H.F.M.; Chong, F.K.; Mutalib, M.I.A.: Photooxidative–extractive deep desulfurization of diesel using Cu–Fe/TiO2 and eutectic ionic liquid. Fuel 156, 54–62 (2015)
Hernández, W.Y.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A.: In situ characterization of the dynamic gold−support interaction over ceria modified Eu3+. Influence of the oxygen vacancies on the CO oxidation reaction. J. Phys. Chem. C. 114, 10857–10865 (2010)
Bao, H.; Chen, X.; Fang, J.; Jiang, Z.; Huang, W.: Structure-activity relation of Fe2O3–CeO2 composite catalysts in CO oxidation. Catal. Letters. 125, 160–167 (2008)
Neri, G.; Bonavita, A.; Rizzo, G.; Galvagno, S.; Capone, S.; Siciliano, P.: Methanol gas-sensing properties of CeO2–Fe2O3 thin films. Sensors Actuators B Chem. 114, 687–695 (2006)
Pérez-Alonso, F.J.; López Granados, M.; Ojeda, M.; Terreros, P.; Rojas, S.; Herranz, T.; Fierro, J.L.G.; Gracia, M.; Gancedo, J.R.: Chemical structures of coprecipitated Fe−Ce mixed oxides. Chem. Mater. 17, 2329–2339 (2005)
Aboutaleb, W.A.; Gobara, H.M.; Hashim, K.M.; Henein, S.A.; Hassan, S.A.: The catalytic performance of Fe2O3–CeO2 nanocomposite in ethanol conversion. Egypt. J. Chem. 59, 445–463 (2016)
Laguna, O.H.; Sarria, F.R.; Centeno, M.A.; Odriozola, J.A.: Gold supported on metal-doped ceria catalysts (M= Zr, Zn and Fe) for the preferential oxidation of CO (PROX). J. Catal. 276, 360–370 (2010)
Gobara, H.M.; Aboutaleb, W.A.; Hashem, K.M.; Hassan, S.A.; Henein, S.A.: A novel route for synthesis of α-Fe2O3–CeO2 nanocomposites for ethanol conversion. J. Mater. Sci. 52, 550–568 (2017)
Hernández, W.Y.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A.: Structural and catalytic properties of lanthanide (La, Eu, Gd) doped ceria. J. Solid State Chem. 184, 3014–3020 (2011)
Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.-W.; Herman, I.P.: Size-dependent properties of CeO2− y nanoparticles as studied by Raman scattering. Phys. Rev. B. 64, 245407 (2001)
Li, H.; Li, K.; Wang, H.; Zhu, X.; Wei, Y.; Yan, D.; Cheng, X.; Zhai, K.: Soot combustion over Ce1-xFexO2-δ and CeO2/Fe2O3 catalysts: roles of solid solution and interfacial interactions in the mixed oxides. Appl. Surf. Sci. 390, 513–525 (2016)
Sing, K.S.W.: Characterization of porous solids: an introductory survey. In: Studies in Surface Science and Catalysis, pp. 1–9. Elsevier, Amstersdam (1991)
Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A.: X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem. Mater. 2, 186–191 (1990)
Wang, J.; Liu, Z.; Cai, R.: A new role for Fe3+ in TiO2 hydrosol: accelerated photodegradation of dyes under visible light. Environ. Sci. Technol. 42, 5759–5764 (2008)
Majumder, D.; Datta, A.; Mitra, M.K.; Roy, S.: Kinetic analysis of low concentration CO detection by Au-loaded cerium oxide sensors. RSC Adv. 6, 92989–92995 (2016)
Zhao, X.; Lu, M.; Li, H.; Fang, J.; Shi, L.; Zhang, D.: In situ preparation of Ni nanoparticles in cerium-modified silica aerogels for coking-and sintering-resistant dry reforming of methane. New J. Chem. 41, 4869–4878 (2017)
Li, X.; Ni, C.; Yao, C.; Chen, Z.: Development of attapulgite/Ce1−xZrxO2 nanocomposite as catalyst for the degradation of methylene blue. Appl. Catal. B Environ. 117, 118–124 (2012)
Vayssilov, G.N.; Mihaylov, M.; Petkov, P.S.; Hadjiivanov, K.I.; Neyman, K.M.: Reassignment of the vibrational spectra of carbonates, formates, and related surface species on ceria: a combined density functional and infrared spectroscopy investigation. J. Phys. Chem. C. 115, 23435–23454 (2011)
Li, X.; Yu, J.; Jaroniec, M.: Hierarchical photocatalysts. Chem. Soc. Rev. 45, 2603–2636 (2016)
Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y.: Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces. 4, 4024–4030 (2012)
Chae, B.W.; Amna, T.; Hassan, M.S.; Al-Deyab, S.S.; Khil, M.-S.: CeO2-Cu2O composite nanofibers: synthesis, characterization photocatalytic and electrochemical application. Adv. Powder Technol. 28, 230–235 (2017)
Moreno, O.P.; Pérez, R.G.; Merino, R.P.; Portillo, M.C.; Tellez, G.H.; Rosas, E.R.; Tototzintle, M.Z.: CeO2 nanoparticles growth by chemical bath and its thermal annealing treatment in air atmosphere. Optik (Stuttg). 148, 142–150 (2017)
Yu, S.-H.; Cölfen, H.; Fischer, A.: High quality CeO2 nanocrystals stabilized by a double hydrophilic block copolymer. Colloids Surf. A Physicochem. Eng. Asp. 243, 49–52 (2004)
Corma, A.; Atienzar, P.; Garcia, H.; Chane-Ching, J.-Y.: Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat. Mater. 3, 394–397 (2004)
Li, K.; Haneda, M.; Gu, Z.; Wang, H.; Ozawa, M.: Modification of CeO2 on the redox property of Fe2O3. Mater. Lett. 93, 129–132 (2013)
Zhang, Y.-C.; Li, Z.; Zhang, L.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.-J.: Role of oxygen vacancies in photocatalytic water oxidation on ceria oxide: experiment and DFT studies. Appl. Catal. B Environ. 224, 101–108 (2018)
Li, Y.; Zhao, D.; Lin, J.; Yuan, Q.: Preliminary study on oxidative desulfurization of diesel via power ultrasound. Energy Sources Part A 31, 191–198 (2009)
Radwan, M.S.; Aboutaleb, W.A.; El Naggar, A.M.A.; El Sayed, H.A.; Shehata, M.R.; Medany, S.S.: Photo-oxidative extractive desulfurization of dibenzothiofene over Fe2O3–CeO2 nanocomposites at visible light irradiation. J. Photochem. Photobiol. A Chem. 433, 114137 (2022)
Dedual, G.; MacDonald, M.J.; Alshareef, A.; Wu, Z.; Tsang, D.C.W.; Yip, A.C.K.: Requirements for effective photocatalytic oxidative desulfurization of a thiophene-containing solution using TiO2. J. Environ. Chem. Eng. 2, 1947–1955 (2014)
Shafiq, I.; Hussain, M.; Rashid, R.; Shafique, S.; Akhter, P.; Yang, W.; Ahmed, A.; Nawaz, Z.; Park, Y.-K.: Development of hierarchically porous LaVO4 for efficient visible-light-driven photocatalytic desulfurization of diesel. Chem. Eng. J. 420, 130529 (2021)
Mahboob, I.; Shafiq, I.; Shafique, S.; Akhter, P.; Hussain, M.; Park, Y.-K.: Effect of active species scavengers in photocatalytic desulfurization of hydrocracker diesel using mesoporous Ag3VO4. Chem. Eng. J. 441, 136063 (2022)
Ahmad, W.; Ur Rahman, A.; Ahmad, I.; Yaseen, M.; Mohamed Jan, B.; Stylianakis, M.M.; Kenanakis, G.; Ikram, R.: Oxidative desulfurization of petroleum distillate fractions using manganese dioxide supported on magnetic reduced graphene oxide as catalyst. Nanomaterials 11, 203 (2021)
Li, F.; Liu, Y.; Sun, Z.; Zhao, Y.; Liu, R.; Chen, L.; Zhao, D.: Photocatalytic oxidative desulfurization of dibenzothiophene under simulated sunlight irradiation with mixed-phase Fe2O3 prepared by solution combustion, Catal. Sci. Technol. 2, 1455–1462 (2012)
Huo, Q.; Liu, G.; Sun, H.; Fu, Y.; Ning, Y.; Zhang, B.; Zhang, X.; Gao, J.; Miao, J.; Zhang, X.: CeO2-modified MIL-101(Fe) for photocatalysis extraction oxidation desulfurization of model oil under visible light irradiation. Chem. Eng. J. 422, 130036 (2021)
Mousavi-Kamazani, M.; Ashrafi, S.: Single-step sonochemical synthesis of Cu2O-CeO2 nanocomposites with enhanced photocatalytic oxidative desulfurization. Ultrason. Sonochem. 63, 104948 (2020)
Abdullah, W.N.W.; Bakar, W.A.W.A.; Abdullah, N.H.; Mokhtar, W.N.A.W.; Rosid, S.J.M.; Shukri, N.M.: Effect of activated carbon supported Ce/Fe based catalyst for catalytic oxidative desulfurization of Malaysian diesel fuel. In: AIP Conference Proceedings, AIP Publishing LLC, p. 20075 (2019)
Singh, S.P.; Chakradhar, R.P.S.; Rao, J.L.; Karmakar, B.: EPR, FTIR, optical absorption and photoluminescence studies of Fe2O3 and CeO2 doped ZnO–Bi2O3–B2O3 glasses. J. Alloys Compd. 493, 256–262 (2010)
Pei, L.Z.; Liu, H.D.; Lin, N.; Yu, H.Y.: Hydrothermal synthesis of cerium titanate nanorods and its application in visible light photocatalysis. Mater. Res. Bull. 61, 40–46 (2015)
Gharagozlou, M.; Bayati, R.: Photocatalytic characteristics of single phase Fe-doped anatase TiO2 nanoparticles sensitized with vitamin B12. Mater. Res. Bull. 61, 340–347 (2015)
Acknowledgements
The authors are deeply express their gratitude to the Science, Technology and Innovations funding authority (project No. 25 in next scientist generation call).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Radwan, M.S., Aboutaleb, W.A., El Naggar, A.M.A. et al. Synergetic Effect of Fe2O3 Doped-CeO2 Nanocomposites Prepared via Different Techniques on Photocatalytic Desulfurization of Heavy Gas Oil. Arab J Sci Eng 48, 15837–15850 (2023). https://doi.org/10.1007/s13369-023-07633-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-07633-y