Abstract
The influence of branched N, N′-bis(p-hydroxybenzoyl) containing propylenediamine (PDA) and triethylenetetramine (TETA) composites for corrosion inhibition of carbon steel in acidic solution (1 M HCl) was investigated using several quantum chemical, electrochemical impedance spectroscopy and potentiodynamic polarization as electrochemical techniques. The investigated molecules were posteriorly characterized by proton nuclear magnetic resonance (1H NMR) and Fourier transform infrared spectroscopy (FTIR) while the surfaces of carbon steel test coupons were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM) techniques. The obtained results revealed that the two amino compounds, N, N′-bis(p-hydroxybenzoyl) propanediamine (N, N′-HBPDA) and N, N′-bis(p-hydroxybenzoyl) triethylenetetramine (N, N′-HBTETA), have significant efficiency toward steel corrosion attack and its inhibition performance was significantly boosted by increasing concentration of di- and tetramine containing inhibitors. The two inhibitors achieved a maximum corrosion inhibition efficiency of 99.1% as indicated from polarization measurements. The isotherm feature of Langmuir adsorption appeared to be proper factor for associating the experimental gains with an applicable mechanism of inhibition process. The free energy ∆Gads was calculated to be − 27.5, 29.1 kJ/mol based on the adsorption isotherm model, indicating physical adsorption on the carbon steel surface. Further, images of the morphological analysis exhibited various features of attack owing to the aggressive medium and the employed concentration of the inhibitor. These synthesized amines supplied many favorable scores in the fabrication of functional mixed-type inhibitors. The computational studies reveal that N, N′-HBPDA and N, N′-HBTETA molecules could absorb via several lone pairs and π clouds, confirming their ability to be good corrosion inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A wide range of studies and several academic scholars have employed a lot of chemical materials and green components that can fabricate significant impediments to conserve the surface of steel from corrosion hazards [1]. For the aim of corrosion inhibition of metallic surfaces based fundamentally on steel materials in processing domains, organic inhibitors are extremely appropriate compounds because of their high effectiveness and obviously promoting ecological attentions [2, 3]. Continual searching for reliable corrosion inhibitors encouraged the efforts for utilizing natural and synthetic macromolecules for corrosion inhibition in saline media [4, 5]. Furthermore, fabricating and preparing of modern inhibitors-based organic compounds show magnificent features for responding demands [6, 7]. Adequate organic composites containing heteroatoms and branched molecules have been reported as new trends of significant corrosion inhibitors such as marine processing by-products [8], ionic liquids [9], non-ionic surfactants [10], ester molecules [11] and Schiff bases [12, 13]. In general, the inhibition performance of these organic macromolecules appears with their adsorption effect on the steel surface throughout their functional groups [14]. Highly aggressive environment like hydrochloric acid medium against steel surface is mainly employed for acidizing, pickling (within the concentration range 5–28%) and cleaning objectives in many manufacturing proceedings [15,16,17,18]. HCl acid gains the priority in the pickling process over the other mineral acids (H2SO4, HNO3, H3PO4, etc.) as it pickles more quickly, with better surface quality and metal chlorides formed in the pickling are more soluble than metal phosphates and sulfates [19]. The petroleum sector often uses the acidizing procedure to stimulate oil wells in order to dissolve drilling mud and mineral rocks for oilfield exploration and development. Concentrated HCl or HF/HCl solutions are frequently injected into an oil well to boost oil output and wellbore penetrability [20]. For such industrial implementations, the attention for developing low cost and effective eco-friendly organic compounds as corrosion inhibitors are increased. Mainly, the employed hydrocarbons for corrosion inhibition of metallic materials contain different atoms like nitrogen, oxygen and sulfur and also consist of numerous types of bonds in their chemical structures in which they are capable to well adsorb on the metal surface [21]. A protective layer can be composed throughout the bond formation between steel and the electron pair of the heteroatoms that inhibiting bad behavior of solution [22]. Several formulations of Schiff bases discussed the inhibition mechanism based on several aggressive media [23,24,25,26]. It is possible that these studies did not take into consideration that the functional imine group contained Schiff base composition, in some cases, is not prolonged steady in acid media and may undergo hydrolysis over time leading to the formation of the corresponding amine [27, 28]. Further, corrosion inhibition efficacy of iron-based metallic surface in definite concentration of molar hydrochloric acid aqueous solutions using ethylenediamine (EDA) and its derivates in absence and presence of substituted aliphatic or aromatic sites have been particularly characterized [29,30,31,32]. The aforementioned researches appeared that diamine inhibitor was clearly reduced the offensive attack of metal and the corrosion inhibition rate was minimized in which the inhibition performance affected by EDA content in the corrosive medium. From the literatures, protection effect of EDA is weak when it employed lonely [14]. In addition, triethylenetetramine (TETA) as an inhibitory organic polyamine compound has been utilized for corrosion control of steel-based materials in different acidic and chloride aqueous solutions [33,34,35]. EDA and TETA are active primary aliphatic amines with four and six reactive hydrogen atoms which capable of interacting well with the active sites of many compounds or molecules [36, 37]. Wherefore, both aliphatic and aromatic polyamines and their derivatives, especially TETA, are mostly employed as hardeners or crosslinkers in several curing techniques as well as in some sensitive medical fields react as chelating agents [38,39,40,41]. Hence, the fabrication of branched macromolecules containing diamine and polyamine constituents modify its behavior against the aggressive attack of steel corrosion. Thus the prime object of the research is the characterization of the prepared corrosion inhibitors, named; N, N`-bis (p-hydroxybenzoyl) propane diamine (N, N`-HBPDA) and N, N`-bis (p-hydroxybenzoyl)triethylenetetramine (N, N`-HBTETA), respectively, as shown in Fig. 1, on the open interface of metal specimens in acidic aqueous solution by standard thermodynamic measurements. The reactive impacts of inhibitor composition and its concentration value on the corrosion control efficiency were also discussed. The carbon steel superficies were examined prior and after the corrosion tests using different spectroscopic techniques such as XRD and SEM, respectively.
2 Experimental
2.1 Synthesis of PDA and TETA-Based Corrosion Inhibitors
All the solvents and reagents, p-hydroxy benzoic acid (PHBA), propanediamine (PDA), triethylenetetramine (TETA), p-toluene sulfonic acid (PTSA), xylene, acetone and hydrochloric acid (HCl), were obtained from Sigma Company and utilized as their purifications. Briefly, the corrosion inhibitors used in this research work were prepared throughout refluxing process of one mole of propanediamine or triethylenetetramine with two moles of p-hydroxy benzoic acid with the addition of p-toluene sulfonic acid as catalyst and xylene as solvent to produced N, N`-bis (p-hydroxybenzoyl) propanediamine (N, N`-HBPDA) and N, N`-bis (p-hydroxybenzoyl) triethylenetetramine (N, N`-HBTETA), respectively. The prepared compound was purified by washing it several times with petroleum ether then separated under vacuum and dried in oven at 60 °C. The synthesis route and chemical composition of the two corrosion inhibitors, N,N'-HBPDA and N,N'-HBTETA (Fig. 1) are compatible with the standard procedures of pure amines reaction [42].
2.2 Specimen’s Preparation
Proper dimensions of Carbon steel coupons were prepared and utilized for the standard proceedings of the corrosion evaluations. 1 M HCl aqueous solution was prepared as artificial attack medium. The implemented measurements occurred according to the common corrosion procedures of steel specimens after recorded period of immersion in environment under the influence of amine inhibitor at definite concentrations [9].
2.3 Electrochemical Measurements
Three electrodes were used in the electrochemical experiments: a saturated calomel reference electrode (SCE), a platinum wire as a counter electrode (CE) and carbon steel as a working electrode (WE). All electrochemical measurements were performed at 303 K using a Volta lab 40 Potentiostat PGZ 301. Steel in an aggressive solution that was given potentiodynamic polarization (PDP) by altering the electrode potential automatically from around −800 to −300 mV vs. RE at open circuit potential (Eocp) with a scan rate of 2 mVs−1. Furthermore, electrochemical impedance spectroscopy (EIS) measurements were taken using an AC signal at open circuit potential with amplitude peaks of 10 mV in the frequency range of 100 kHz to 0.05 Hz. ZSimpWin 3.60 was used to analyze the impedance data to fit the equivalent electrical circuits.
2.4 Spectroscopic Analyses
FTIR was obtained using an ATI Mattson model Genesis Series (USA) infrared spectrophotometer with KBr. 1H NMR analysis was detected with advanced Mercury 300BB spectrometer (NMR300) processing with 5 repetitions at 300 MHz at temperature 303 K for total time 19 min. The obtained amino inhibitors were resolved throughout dimethyl sulfoxide (DMSO) as a solvent. The chemical shift ranges are recorded in parts per million (ppm) which characterize the different functional groups-based structure. Powder X-ray diffraction (XRD) with Cu Kα1 radiation was used to record XRD patterns for the corrosion inhibitors in the range 2θ = 4–80°. A JEOL model JSM-53000 SEM is employed to analyze the surface of steel before and after immersion in 1 M HCl medium with and without definite concentrations of the tested corrosion inhibitors.
2.5 Computational Details
The optimization of the geometric and electronic structures of N, N`-HBPDA and N, N`-HBTETA molecules were processed in the gas and aqueous phases via Dmol3 module included in material studio software [43]. The calculations were done under the following condition: The correlation by local density combined with Perdew–Wang parametrizations (LDA—PWC) [44, 45], The solvent effect was added using COSMO controls and the basis set was double numerical plus d-functions (DND-4.4). The global reactivity descriptors had been calculated on the basis of the values of frontier orbitals (HOMO, LUMO) using the following equations [46, 47]:
Monte Carlo simulations utilizing the adsorption locator module were used to investigate the presence of N, N'-HBPDA and N, N'-HBTETA molecules on steel surfaces [48]. The steel surface simulated by Fe (110) surface as the most stable miller index iron surface [49]. The Fe (110) simulation box has a dimension of 22.341 × 22.341 × 48.422 Å including a 30 Å vacuum layer. The three-dimensional Fe (110) constructed from 10 × 10 × 10 atoms with periodicity. Beside N, N`-HBPDA and N, N`-HBTETA molecules, other molecules like H2O, H3O+ and Cl– were added to the adsorption system to simulate the aqueous and protonated experimental conditions. The simulations were done under the following condition: The charges were force field assigned and group based, atom-based summation methods for the electrostatic and Van Der Waals interactions, respectively, during the optimization using COMPASS.
3 Results and Discussion
3.1 Fourier Transform Infrared Spectroscopy (FTIR)
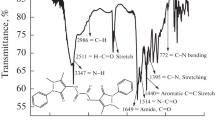
The FTIR spectroscopy is deemed as the common influential technique to investigate the active groups of the various chemical compounds to supply many characteristic features and specifications over classic methods utilized in some types of chemical analyses [50]. The FTIR diagram as presented in Fig. 2, reveals a sharp broad band at 3417.46 cm−1 which is referred to the stretching OH groups of alcohols and phenols. Meanwhile, there are characteristic vibrations at 2950 and 2850 cm−1 owing to the stretching C–H bands. The existence of stretching band of C=O group at 1709.50 cm−1 is due to the association of the polar site with the aldehyde structures. The absorption vibration at 1600 cm−1 is referred to C=O group conjugated with amine. The fundamental stretching C=C group at 1650 cm−1 is shifted to lower values like 1600.23 and 1461.62 cm−1. Anti-symmetric stretching peak appeared at 1361.86 cm−1 related to COO and NH groups and vibration at 1278.32 cm−1 of C–O aromatic. The spectra at 1169.5 cm−1 match the peaks of the bending NH. The vibrations appeared at of 826.79 cm−1 matches to out of plane of substituent phenyl group. The FTIR vibration of the amino inhibitors was a significant method for detecting the aspect of represented oxygenated molecules-based aliphatic and aromatic structures such as alcohols, carboxylic acids, carbonyls which also assured by further investigation [51].
3.2 Proton Nuclear Magnetic Resonance (1H NMR)
In general, NMR spectroscopy technique considers to be the proper analytical way to recognize and examine the different organic structures of individual compounds or molecular fragments-based complex mixtures. By using a combined analytical methods (such as FTIR and NMR) it is prospective to clarify the spectroscopic results and distinguish a set of fundamental composites that consist of considerable data about the product [50]. In the current work, the elucidation of the obtained spectra was particularly investigated by 1H NMR spectroscopy as shown in Fig. 3.
Broadly, the main segments of the 1H NMR analysis manifest as enduring allocations of undissolved spectra, proposing the existence of crosslinked combination of materials and molecules [52]. In this section, the entire spectra regions of the hydrogen atoms can be described according to the main dependent sequence: H–C (0.8–1.5 ppm); H–C–C = (2.3–2.7 ppm); H–C–O (3.5–4.2 ppm); Ar–H (6.7–7.8 ppm) and O–H (4.2–7.8 ppm). A lot of methylene protons CH2 spectra appeared at the range of 0.8–1.5 ppm due to series of aliphatic protons combined with several alkyl linkages. Moreover, the significant sharp spectra at region 2.4–2.6 ppm matches to the chemical convey of aliphatic protons bounded to carbon atoms neighboring amine bonding structure (CH2–NH). The vibration at 2.6 ppm coincide to aromatic alcohol (Ph–OH), identified the existence of OH–Ar–C structure which pointed that it involves hydroxyl group bonded aromatic cycle. The spectra range of 3.5–4.4 ppm corresponded to ethylene group bonded to monoamine neighboring functional carbonyl form (CH2–CH2–NH–CO) in both compounds. The presence of several amine forms (NH) of PDA and TETA appeared by chemical shift at 6.7 ppm identify the massive amine protons exist. [50, 53].
3.3 Electrochemical Impedance Spectroscopy (EIS)
Electrochemical Impedance Spectroscopy (EIS) was employed to characterize the corrosion inhibition performance of the obtained inhibitors, N, N'-HBPDA and N, N'-HBTETA, on the metal surface in acid medium and the data are collected in Table 1. The standard object here is to study as well as estimate the basic kinetic factors of the electrochemical interactions that prevalently occurred among the active specimens and the environment based on Nyquist plots phenomena [54]. As shown in Fig. 4, it represents the Nyquist plots of the carbon steel electrode after inundation in 1 molar hydrochloric acid medium with/without definite contents (25–250 ppm) of the inhibitors.
The first observation is confirmed that Nyquist plots were perfectly fitted to the equivalent circuit which appears in Fig. 5 wherein, chi-square (χ2) values were used to evaluate the accuracy of the fitted data, and the low χ2 values (around 10–4) of all experimental results indicate that the fitted data are correlated well with the experimental data [10, 55]. In the simulated equivalent circuit, Cdl refers to the double layer capacitance, Rp refers to the polarization resistance and Rs refers to the solution resistance.
The capacitive loop was lightly disappointed for the blank experimental environment in absence of inhibitor as obviously exhibited from Nyquist plots. This performance intends that the carbon steel attack in the acidic solution is fundamentally monitored and proceeded according to definite charge transfer parameters named, the polarization resistance (Rp) value is briefly represented by charge transfer resistance (Rct) and diffuse layer resistance (Rd).
Moreover, the loop capacity is raised progressively by raising amount of corrosion inhibitors. With the excess of inhibitor content to 250 ppm, the corrosion inhibitor components capable to well adsorb on the metal. As consequence, a preservative adsorption thin film performed on the surface of steel which make a successful hindrance against the corrosive influence of the acidic medium [56, 57]. On the other hand, with increasing focus of inhibitors, the frequency points start to shift down orderly. This frequent trend is owing to protective characteristics of the inhibitors which have a strong effect on breakdown points of frequency values. The corrosion inhibitor composition reacts directly with the open surface area of carbon steel specimens throughout electrochemical process and substitute several ions and molecules over the functional inhibitor structure [58]. As a comparison study, the obtained characteristic measurements of electrochemical impedance spectroscopy (EIS) based on the Nyquist plots phenomena appeared that triethylenetetramine (TETA) containing inhibitor (Fig. 4a) has a frequency point values more than inhibitor structure consist of propanediamine (PDA) compound (Fig. 4b) in the same acidic medium. This clear manner was also detected with increasing the concentration of inhibitor gradually. The result is attributed to the long chain of adsorbed amine groups-based TETA increased the passivation influence of carbon steel surface against the aggressive attack of acid [57, 59]. Nevertheless, the good adsorption of inhibitor components effect on the charge sedimentation process in the acidic medium and retard the penetration of the aggressive ions to the steel surface. The polarization resistance (Rp) measurements were elevated to 720 Ω cm2 for PDA-based corrosion inhibitor and increased more to 1100 Ω cm2 for inhibitor containing TETA, respectively.
CPE (constant phase element) in the equivalent is used to optimize the effect of the adsorption of corrosion inhibitor molecules on charge transfer to obtain an ideal electrochemical process. The Cdl obtained from CPE as follows [60]:
A decrease in the Cdl values is noticed in the presence of the inhibitors due to decline in the local dielectric constant and the increase in the thickness of the electric double layer. It means that the inhibitor molecules with a lower dielectric constant gradually replace the water molecules adsorbed on the steel surface to inhibit the corrosion of steel [61]. The values of n ranged from 0.8555 to 0.9108, suggesting the inhomogeneity of the steel surface after corrosion in the solution with or without inhibitors, and the corrosion of steel in the solution is primarily controlled by the charge transfer process [62, 63].
The Bode plots (Fig. 6) have only one time constant and the lines at the intermediate frequency range are slanting lines, indicating that the systems have a major capacitive performance. The slopes (S) of these slanting lines decreased by the addition of the inhibitors molecules, indicating increasement of the capacitive behavior (the slope in case of pure capacitor = − 1) [64]. Compared to 1 M HCl aggressive solution, the phase angle (α°) values increased by the addition of several concentrations of the inhibitor’s molecules, implying the formation of an adhesive and protective barrier which supports a major capacitance effect in the presence of PDA and TETA molecules. Those changes in the values of slopes and phase angle confirm the effective adsorption of PDA and TETA molecules onto the steel surface to prevent the corrosion reaction.
3.4 Potentiodynamic Polarization
The present section is represented the potentiodynamic polarization (PDP) technique which employed to characterize the corrosion and the inhibition mechanism. The experimental measurements of potentiodynamic polarization based on definite concentration ranges (25, 50, 100, 150, 200 and 250 ppm) of amino composed inhibitors (N, N’-HBPDA and N, N’-HBTETA) in 1 M HCl medium were studied as shown in Table 2 and Fig. 7. Under the influence of inhibitor, the degradation performance at the anodic side was varied significantly. Based on the initial perception, the actual strength manner not raised purposefully close to the value of corrosion potential (Ecorr) in the definite potential flow, whereas the curve line shifts to more positive sites. The obtained behavior is due to the composition of steady layer on the outer metal surface that makes an effective impediment against the offensive impact of acidic media. With extra electrode potential value “more positive conditions,” intensity rate of desorption approach of the organic inhibitor was changed perfectly. At the high positive values, adsorption of amino inhibitor occurred, while the efficiency of inhibition method was reduced. Further, when the potential “E” value of the polarization arrived to −0.55 V, the intensity of anodic current raised significantly. The existence of aggressive ions like chloride anion-based acidic environment perform the main role for the anodic degeneration of carbon steel. As detected, desorbed corrosion inhibitor components were decreased the adsorption performance of Cl− anions at the metal surface [9, 12]. On the other hand, at the cathodic potential site, the polarization manner shows that the current density values were brought down as a proof for impeding influence of adsorbed components of inhibitor on hydrogen evolution reaction mechanism in acidic solution. Based on this phenomenon, the high close-fitting molecules of amino corrosion inhibitor at the steel surface may restrain the outlet of H3O+ cations to the interface. Otherwise, current values of cathodic field were purposefully decreased close to (ECorr) value. The electrode potential was strolled toward more negative values, means that the metal surface is negatively charged and adsorption efficiency of inhibitor molecules becomes less owing to the different Columbic interactions occurred. Surely, the hindrance of corrosion was observed to be correlated to the inhibitor content, therefore the high impact was exhibited at inhibitor concentration of 250 ppm. The variation of polarization values in case of PDA-based inhibitor (Fig. 7b) not much more than inhibitor containing TETA (Fig. 7a), in which the latter appeared more reducing in the current density values owing to the more covering area. Also, the corrosion current density (icorr) and Tafel slopes (β) as important kinetic parameters were specified from the cathodic site by induction of the current potential plots to the identical corrosion potential value. The inhibitor molecules blocked both anodic and cathodic reaction sites without much alter the cathodic & anodic Tafel slopes and inhibitor molecules worked as mixed type of inhibitor. The cathode Tafel slope (βc) (from − 86 to − 174 mV/dec) and anode Tafel slope (βa) (from 84 to 184 mV/dec) values rarely altered when the inhibitor was introduced, showing that the process of hydrogen evolution stayed intact. That might be due to the inhibitor molecule adhesion on the metallic substrate, lessening the degree of active spots available for the reaction while sustaining the charge transfer process of hydrogen generation.
The icorr values were measured utilizing the amount of metal ions found according to the following equation, taking into account the state of Faraday’s law [65].
where \({M}_{Fe}\) is the equivalent molar weight of iron (g mol−1), A is electrode area (cm2), \({m}_{Fe}\) is the amount of iron (g), and t is the exposure time (s), consecutively. The conversion factor used for calculation of the total mass of decayed carbon steel amount owing to corrosion attack is defined by the constant value of 1.021. The following equation is used to calculate the inhibition efficiencies by using icorr values [66]:
where icorr and i’corr, are used for absence and existence of corrosion inhibitor, consecutively. The anodic PDP diagrams strongly influenced by the alteration of steel/acid interface producing significant adsorption of inhibitor on the positively charged surface of steel. Moreover, well desorbed inhibitor components give substantial hindrance against the charge convey at the metallic surface. Briefly, the inhibitor compounds are concluded that frequently be more efficient on an anodic site to prevent corrosion reaction with the impact on the hydrogen emission mechanism at the cathodic area of the steel surface. Only as the change in Ecorr value was more than 85 mV, a compound could be recognized as an anodic or a cathodic type inhibitor Therefore, the inhibitor might act as a mixed-type inhibitor [9, 12, 67].
3.5 Effect of Temperature
PDP and EIS experiments were conducted at various temperatures (30, 40 and 50 °C) in the absence and presence of the two inhibitors at concentrations of 50, 150 and 250 ppm. The PDP and EIS Nyquist plots at 40 and 50 °C are collected in Figs. S1 and S2. It is clear that for both the blank and the investigated inhibitors, the anodic and cathodic portions grew in more positive directions and the diameter of the semi-circle decreased with an increase in temperature. As a result, as the temperature rises, both the hydrogen evolution reaction and the reaction of iron dissolution are elevated [68]. Since ionic conductivity increases with temperature, Nyquist plots' diameters (Rp) for both blank and inhibitors solutions drop as the temperature rises. This suggests that the amount of surface that the inhibitor molecules cover decreases. The corrosion characteristics from polarization experiments at 40 and 50 °C are listed in Table 3.
As a result of the corrosion rate accelerating at high temperatures, the data demonstrate that the corrosion current densities have increased with temperature. The inhibition effectiveness is reduced at high N, N'-HBTETA inhibitor concentrations (250 ppm) from 99.1% to 85.79 and 82.30% at 40 and 50 °C, respectively. It also appears that N, N'-HBTETA and N, N'-HBPDA are still regarded as corrosion inhibitors for carbon steel across a wide range of temperatures due to the creation of a durable protective coating from the adsorbed inhibitor molecules on the metal surface. Regarding EIS measurements, the data at 40 and 50 °C are collected in Table 4.
For all temperatures, it is clear that the Rp values for carbon steel in 1.0 M HCl containing 50, 150 and 250 ppm of the studied inhibitors were greater than those of the blank solution. The Rp values have dropped in the case of the blank solutions from 80.7 Ω cm2 at 30 °C to 64.84 and 51.26 Ω cm2 at 40 and 50 °C, respectively. Regarding the electrical double layer capacitance, the values of Cdl rise as the temperature rises as a result of the thinned adsorption layer [69].
3.6 Adsorption Isotherm and Thermodynamic Parameters
Prior to discuss, the adsorption isotherm terminology in general represents a considerable guide to describe the expected interface reactions among carbon steel open surface and inhibitor components. Thus, the isotherm evaluation of adsorption process will be highly affected by different parameters such as chemical composition of corrosion inhibitor constituents, structure of steel material as well as activity performance of the metallic surface [65]. The PDP data had been examined against several adsorption isotherms as shown in Fig. S3 and the most suitable one had been chosen according to R2 values closest to unity. The adsorption route of employed organic inhibitor on carbon steel surface is found compatible with the Langmuir isotherm phenomena. The adsorption is realized by the following equation [70].
where “Cinh” is inhibitor concentration and “Kads” is the adsorption equilibrium constant for the adsorption–desorption process. Surface coverage ratio “θ” was measured from efficiency of corrosion inhibition action. From Fig. 8, it is clearly seen that graphs of Cinh/θ as a function of Cinh appeared as straight lines in both inhibitors (N, N’-HBPDA and N, N’-HBTETA).
The gained linear association coefficient (R2) is 0.99. Moreover, the adsorption equilibrium constant (Kads) value was calculated from the plot intercept which was 1.15 × 104 M−1 as shown in Table 5. The obtained Kads value is an evidence for large adsorption ratio of corrosion inhibitor composites on the steel surface [71].
One of the fundamental parameters is the adsorption free energy (ΔGoads) which can be detected by applying the following equation.
The calculated value of \(\Delta {\mathrm{G}}_{\mathrm{ads}}^{\mathrm{o}}\) was found −31 kJ mol−1. As reported in several literatures, it is declared that if the value of the adsorption free energy \(\Delta {\mathrm{G}}_{\mathrm{ads}}^{\mathrm{o}}\) is equal to −20 kJ mol−1 or less than this value, that means the physisorption process only exists and there is a feeble electrostatic attraction throughout the steel surface and inhibitor components. In case of \(\Delta {\mathrm{G}}_{\mathrm{ads}}^{\mathrm{o}}\) value reached −40 kJ mol−1 or more than value, this action indicated to linkage formation of covalent bonding among the steel surface with the adsorbed constituents in which the chemisorption is the prevalent regime. Therewith, most of the employed isotherms which concern with the adsorption process mechanism have several postulates around nature of metal surface such as purity, identity and interface action, meanwhile general investigations for authentic models probably not match all these conditions. As a look, the moderately high value of adsorption free energy \(\Delta {\mathrm{G}}_{\mathrm{ads}}^{\mathrm{o}}\) is indicated that the electrostatic interaction of amino inhibitor components with the steel surface is strong and the current adsorption mode is well stretched along the metal, which may be represented to both physisorption and chemisorption on the open surface of carbon steel [14, 66]. The gained attitude of adsorption free energy is a great confirmation for active electrochemical interactions happened on the prolonged interface of steel metal in the permanence of inhibitor components. The expected trend of chemical adsorption route is referred to a type of coordination linkage of unoccupied electron orbitals of iron atoms with delocalized electrons of inhibitor contained functional groups. Moreover, the obtained negative value of \(\Delta {\mathrm{G}}_{\mathrm{ads}}^{\mathrm{o}}\) indicates that the adsorptive technique of the inhibitor molecules on the metal surface is a spontaneous process [12, 14].
3.7 Scanning Electron Microscopy (SEM) Analysis
Broadly, the structure topography and morphology technique represent essential characteristics of organic compositions [72]. In the present study, the morphological structure of metal was detected using SEM technique under the effect of 250 ppm ratio in 1 M HCl medium after definite exposure time. In case of free hydrochloric acid system, several deteriorated signs were plainly exposed along the steel surface as shown in Fig. 9a. Further, the aggressive attack of acidic medium left strong intelligible marks such as pored and profound bores on the metal surface. One the other hand, SEM graphs under acid system were demonstrated as mild superficies without perforations for TETA contained inhibitor (Fig. 9b) and moderate performance in case of inhibitor consisting of PDA (Fig. 9c). The conservative surface manner under the effect of long-chain TETA system is due to efficient inhibition of corrosion process as a result of good adsorptive capacity of inhibitor components on the carbon steel surface [73]. Therefore, the metal surface has preventive adsorption film and high covering capacity content. The obtained observations are compatible with the employed electrochemical investigations.
3.8 X-ray Diffraction (XRD) Analysis
From the X-ray diffraction (XRD) spectra (Fig. 10), in absence of inhibitor effect, it is distinctly observed that the exposed carbon steel specimens which immersed in the acidic environment were manifested with large crystalline structure as well as large intensity measurements as exhibited in Fig. 10a. After adding inhibitor, this performance was decreased slowly for inhibitor structure containing PDA (Fig. 10c) and substantially in case of TETA-based inhibitor system (Fig. 10b). This anticipated attitude is due to the extended chain length and the high molecular mass of the chemical composition of inhibitor which minimize the attack impact of the offensive medium and conserve the carbon steel interface from the excessive corrosion [73].
3.9 Quantum Chemical Calculations
3.9.1 Global Reactivity Descriptors
Snapp shots for the optimized structures, HOMO and LUMO were taken after Dmol3 calculations for N, N’-HBTETA and N, N’-HBPDA molecules and are collected in Fig. 11. The molecular skeleton of the two molecules is approximately planner with a small deviation for the terminal aromatic moieties. The two molecules possess a similar skeleton but the spacer between the two terminal aromatic moieties increases by two nitrogen atoms and CH2 groups in case of N,N'-HBTETA molecule.
This extra size associated with adsorption sites (two nitrogen atoms) may enhance N, N’-HBTETA molecule to cover more surface area of steel than N, N’-HBPDA molecule and give it priority to protect against corrosion. The reactivity of the two molecules was characterized by frontier molecular orbitals (FMO). HOMO sites are the predicted sites for electron donation while LUMO sites are the predicted sites for accepting electrons [74]. As shown in Fig. 11, HOMO sites are distributed on the middle of the molecular skeleton of the two molecules. So, the nitrogen atom donates electrons for facilitating adsorption. On the other hand, one of the terminal aromatic moieties gained the LUMO character and ready for accepting electrons. The numerical values of the calculated global parameters associate with N, N’-HBTETA and N, N’-HBPDA molecules are tabulated in Table 6. The high energy values of electrons presented in HOMO reflect their ability for donation to steel surface [75].
The EHOMO values in Table 6 showed that neutral N, N’-HBTETA molecule has higher values of EHOMO than the neutral N, N’-HBPDA molecule, either in the gas or aqueous phase. This increase in energy can be attributed to the donating power of the two extra nitrogen atoms. Upon protonation of the four nitrogen atoms in N, N’-HBTETA molecule, EHOMO values are significantly decrease compared to N, N’-HBPDA molecule with only two protonated nitrogen atoms. This confirms the vital role of nitrogen atoms in donating electrons. Protonation enhances the accepting power of N,N'-HBTETA molecule via decreasing the values of ELUMO and strength its binding to metal surface via back donation [76]. The energy gap between the FMO (ΔE = ELUMO—EHOMO) is a crucial factor in determining the reactivity of any chemical species [77]. Usually highly reactive molecules are characterized by low values of energy gap but most stable (low reactivity) molecules are characterized by high values of energy gap [78]. In our case N, N’-HBTETA molecule is more reactive with low values of ΔE than N, N’-HBPDA molecule even at the protonated form. The positive values of hardness (η) and so negative values of back donation energy (ΔE back donation =−\(\frac{\upeta }{4}\)) is a good indication of electron donating and charge transfer from metal back to N,N'-HBTETA and N,N'-HBPDA molecules [79]. This enhances inhibitors binding to metal surface for preventing corrosion. Regarding the nucleophilicity index (ε), we found that the numerical values of N, N’-HBTETA are higher than N, N’-HBPDA. So, N, N’-HBTETA molecule possesses a high nucleophilic character. This is confirming that N, N’-HBTETA molecule is able to support electrons to steel surface. Other important parameter is fraction of transferred electrons where positive values (ΔN > 0) indicate the possibility of electrons to be donated from inhibitors to metal surface and the negative values (ΔN < 0) for the opposite state [80]. Our data revealed positive values of ΔN for N,N'-HBTETA and N,N'-HBPDA molecules in the neutral form, this show that they have a tendency to donate their electrons to steel surface with a priority of N,N'-HBTETA molecule over N,N'-HBPDA molecule. Recently the electro-accepting power (\({\upomega }^{+}=\frac{(\mathrm{I}+3\mathrm{A}{)}^{2}}{16(\mathrm{I}-\mathrm{A})}\) ) and the electro-donating (\({\upomega }^{-}=\frac{(3\mathrm{I}+\mathrm{A}{)}^{2}}{16(\mathrm{I}-\mathrm{A})}\)) were used to determine the ability of inhibitors to receive or to donate electrical charge, respectively [81]. Our data showed that ω − > ω + for N, N’-HBTETA and N, N’-HBPDA at all conditions. This means that the donating power predominates and reflects good binding to steel surface to protect it from corrosion.
3.9.2 Local Reactivity by Fukui Analysis
The previously calculated and discussed parameters were regards the whole molecular skeleton but in this section, we will determine the specific atoms that participates in donating or accepting electrons. The local reactivities for atoms were calculated using Fukui functions (\({{f}_{k}}^{-}\) for electrophilic attack, \({{f}_{k}}^{+}\) for nucleophilic attack) based on finite difference approximation method using the equations mentioned in literature [24, 82]. The calculated local atomic charges for N, N’-HBTETA and N, N’-HBPDA molecules by Mulliken Population analysis are collected in Table 7.
The Mulliken charges and the molecular electrostatic potential are collected in Fig. S4. Regarding HBPDA molecule, the most probable sites for electrons donating (highest values of \({{f}_{k}}^{-}\)) are localized on two oxygen atoms of the two carbonyl groups (C = O) > two oxygen atoms of the two hydroxyl groups (-OH) > one of nitrogen atoms of the N–H groups. This is confirming the importance of hetero atoms in donating electrons to steel surface and facilitate adsorption. Regarding HBTETA molecule, the highest values of \({{f}_{k}}^{-}\) are localized on two nitrogen atoms of the N–H groups localized on the middle of the molecular skeleton > two oxygen atoms of the two carbonyl groups (C=O) > two oxygen atoms of the two hydroxyl groups (-OH). Thi s is confirming the importance of hetero atoms in donating electrons to steel surface and facilitate adsorption. The extra two nitrogen atoms in N, N’-HBTETA molecule than N, N’-HBPDA molecule obtained the highest\({{f}_{k}}^{-}\) values, this supports the experimental inhibition efficiency ranking N, N’-HBTETA > N, N’-HBPDA. The probable sites for electrons accepting (highest values of \({{f}_{k}}^{+}\)) are mostly located on carbons of one of the aromatic moieties for both N, N’-HBTETA and N, N’-HBPDA molecules.
3.9.3 Monte Carlo Simulations
For HBTETA and HBPDA systems on steel surface and the inhibition mechanism, the MC simulations were investigated under consideration of the experimental condition like the protonation and solvation processes. Figure 12 collects the snapshots of the adsorption of N, N’-HBTETA and N, N’-HBPDA molecules onto Fe (110).
It is obvious that the inhibitor molecules obtain approximately planner structure with a small deviation of one aromatic moiety. This approximately planner structure facilitates the parallel adsorption on Fe (110) surface to cover more surface area and enhance protection [83]. Figure 12 shows that N, N’-HBTETA and N, N’-HBPDA molecules had been adsorbed closely to Fe surface and most of active sites participate in the adsorption process.
Table 8 records the calculated adsorption energy and so binding energies for N, N’-HBTETA and N, N’-HBPDA molecules in different forms and phases. The high negative values of the adsorption energies indicate the spontaneous tendency for adsorption.
We can find that the Ebin values for N, N’-HBTETA and N, N’-HBPDA were 193.655 and 245.464, respectively, for the neutral gas phase. These indicate that the N, N’-HBTETA molecule has extra adsorption ability on Fe surface than the N, N’-HBPDA molecule. The priority of N, N’-HBTETA is due to the long-chain structure than N, N’-HBPDA molecule beside the presence of two extra nitrogen atoms as adsorption sites. This finding supports the previously discussed experimental and quantum studies.
3.9.4 Mechanism of Action of the Corrosion Inhibitors
According to the previously discussed electrochemical and computational data, we can propose the following corrosion mechanism as summarized in Fig. 13. Because of the prepared benzamides molecules are characterized by several active sites like the hetero atom and π clouds from the aromatic moieties, these molecules are preferred for adsorption on carbon steel surfaces more than corrosion particles and the aggressive solution to form a highly protective thin film. The LUMO and HOMO distributions of the studied benzamides molecules assessed from the computational data could be considered as the most probable sites for donation of electrons via coordination to the carbon steel surface and receive electrons via back donation. HOMO sites are distributed on the middle of the molecular skeleton of the two molecules. So, the nitrogen atom is donator of electrons to empty d-orbital of Fe to form coordination bonds to facilitate adsorption via chemical adsorption. On the other hand, one of the terminal aromatic moieties gained the LUMO character and ready for accepting electrons from carbon steel surface to strength the adsorption. Upon protonation of the nitrogen atom by the action of acidic environment, physical adsorption could be occurred by the electrostatic attraction between the positively charges hetero atoms and the negatively charged adsorbed chloride ion on carbon steel surface and also back donation to these sites on the inhibitors [84].
A comparison between our investigated inhibitors and other published inhibitors for steel in HCl is listed in Table S1. It is clear from the comparison table that our investigated compounds are higher in efficiency and more in protection ability.
4 Conclusions
Concerning our research, two branched aromatic amine compounds based on propylenediamine (PDA) and triethylenetetramine (TETA) were prepared as new corrosion inhibitors of carbon steel in 1 M HCl solution. By adding the obtained amino inhibitors, N, N`-bis (p-hydroxybenzoyl) propanediamine (N, N’-HBPDA) and N, N`-bis (p-hydroxybenzoyl) triethylenetetramine (N, N’-HBTETA), the efficiency of inhibition process on steel interface in acid solution was enhanced with increase the inhibitor ratio. The presence of alcoholic, phenolic, aliphatic and aromatic linkages containing amino inhibitors are assured by FTIR spectroscopy as well as 1H NMR spectroscopy. Indeed, organic mixtures of oxygenated composites like carbonyl, carboxylate and alcohol groups making it conceivable to stabilize the composition of the corrosion inhibitor. The potentiodynamic polarization measurements revealed that the amino inhibitors have good adsorption impact. The adsorbed molecules on metal follow the Langmuir adsorption isotherm. The obtained diagrams of SEM and XRD analyses are compatible with the electrochemical measurements. The computational calculation is in a good agreement and support to the electrochemical data. The quantum data confirm that the efficiency depends mainly on the molecular and geometrical structures of N, N’-HBTETA and N, N’-HBPDA molecules, and MC simulation confirms their binding to Fe (110) surface.
References
Chauhan, D.S.; Verma, C.; Quraishi, M.A.: Molecular structural aspects of organic corrosion inhibitors: experimental and computational insights. J. Mol. Struct. 1227, 129374 (2021). https://doi.org/10.1016/j.molstruc.2020.129374
Senthilkumar, G.; Umarani, C.; Ramachandran, A.: Investigation on corrosion inhibition effect of N-[4-(1,3-benzo[d]thiazol-2-ylcarbamoyl)phenyl]quinoline-6-carboxamide as a novel organic inhibitor on mild steel in 1N HCl at different temperatures: experimental and theoretical study. J. Indian Chem. Soc. 98, 100079 (2021). https://doi.org/10.1016/j.jics.2021.100079
Carranza, M.S.S.; Reyes, Y.I.A.; Gonzales, E.C.; Arcon, D.P.; Franco, F.C.: Electrochemical and quantum mechanical investigation of various small molecule organic compounds as corrosion inhibitors in mild steel. Heliyon. 7, e07952 (2021). https://doi.org/10.1016/j.heliyon.2021.e07952
Cui, L.; Hang, M.; Huang, H.; Gao, X.: Experimental study on multi-component corrosion inhibitor for steel bar in chloride environment. Constr. Build. Mater. 313, 125533 (2021). https://doi.org/10.1016/j.conbuildmat.2021.125533
Dong, B.; Liu, W.; Zhang, T.; Chen, L.; Fan, Y.; Zhao, Y.; Yang, W.; Banthukul, W.: Corrosion failure analysis of low alloy steel and carbon steel rebar in tropical marine atmospheric environment: outdoor exposure and indoor test. Eng. Fail. Anal. 129, 105720 (2021). https://doi.org/10.1016/j.engfailanal.2021.105720
Rani, B.E.A.; Basu, B.B.J.: green inhibitors for corrosion protection of metals and alloys: an overview. Int. J. Corros. 2012, 1–15 (2012). https://doi.org/10.1155/2012/380217
Goni, L.K.M.O.; Mazumder, M.A.J.; Quraishi, M.A.; Rahman, M.M.: Bioinspired heterocyclic compounds as corrosion inhibitors: a comprehensive review. Chem. An Asian J. 16, 1324–1364 (2021). https://doi.org/10.1002/asia.202100201
Farag, A.A.; Ismail, A.S.; Migahed, M.A.: Environmental-friendly shrimp waste protein corrosion inhibitor for carbon steel in 1 M HCl solution. Egypt. J. Pet. 27, 1187–1194 (2018). https://doi.org/10.1016/j.ejpe.2018.05.001
Abbas, M.A.; Zakaria, K.; El-Shamy, A.M.; Abedin, S.Z.E.: Utilization of 1-butylpyrrolidinium chloride ionic liquid as an eco-friendly corrosion inhibitor and biocide for oilfield equipment: combined weight loss, electrochemical and SEM studies. Zeitschrift für Phys. Chemie. 235, 377–406 (2021). https://doi.org/10.1515/zpch-2019-1517
Bedair, M.A.; Alosaimi, E.H.; Melhi, S.: A study of the inhibitive effect for corrosion of steel in 1.0 M HCl using a new nonionic surfactant based on coumarin moiety: chemical, electrochemical and quantum mechanics calculations. J. Adhes. Sci. Technol. (2021). https://doi.org/10.1080/01694243.2021.2018864
Farag, A.A.; Ismail, A.S.; Migahed, M.A.: Inhibition of carbon steel corrosion in acidic solution using some newly polyester derivatives. J. Mol. Liq. 211, 915–923 (2015). https://doi.org/10.1016/j.molliq.2015.08.033
Hassan, A.; Heakal, B.; Younis, A.; Bedair, M.; El-Billy, Z.; Mohamed, M.: Synthesis of some triazole Schiff base derivatives and their metal complexes under microwave irradiation and evaluation of their corrosion inhibition and biological activity. Egypt. J. Chem. (2019). https://doi.org/10.21608/ejchem.2019.10834.1699
Abdelsalam, M.M.; Bedair, M.A.; Hassan, A.M.; Heakal, B.H.; Younis, A.; Elbialy, Z.I.; Badawy, M.A.; Fawzy, H.E.-D.; Fareed, S.A.: Green synthesis, electrochemical, and DFT studies on the corrosion inhibition of steel by some novel triazole Schiff base derivatives in hydrochloric acid solution. Arab. J. Chem. 15, 103491 (2022). https://doi.org/10.1016/j.arabjc.2021.103491
Sığırcık, G.; Yildirim, D.; Tüken, T.: Synthesis and inhibitory effect of N, N’-bis(1-phenylethanol)ethylenediamine against steel corrosion in HCl Media. Corros. Sci. 120, 184–193 (2017). https://doi.org/10.1016/j.corsci.2017.03.003
El Azzouzi, M.; Aouniti, A.; Tighadouin, S.; Elmsellem, H.; Radi, S.; Hammouti, B.; El Assyry, A.; Bentiss, F.; Zarrouk, A.: Some hydrazine derivatives as corrosion inhibitors for mild steel in 10 M HCl: weight loss, electrochemichal, SEM and theoretical studies. J. Mol. Liq. 221, 633–641 (2016). https://doi.org/10.1016/j.molliq.2016.06.007
Haldhar, R.; Kim, S.-C.; Prasad, D.; Bedair, M.A.; Bahadur, I.; Kaya, S.; Dagdag, O.; Guo, L.: Papaver somniferum as an efficient corrosion inhibitor for iron alloy in acidic condition: DFT, MC simulation, LCMS and electrochemical studies. J. Mol. Struct. 1242, 130822 (2021). https://doi.org/10.1016/j.molstruc.2021.130822
Bedair, M.; Metwally, M.; Soliman, S.; Al-Sabagh, A.; Salem, A.; Mohamed, T.: Extracts of mint and tea as green corrosion inhibitors for mild steel in hydrochloric acid solution. Al-Azhar Bull. Sci. 26, 1–14 (2015). https://doi.org/10.21608/absb.2015.23766
Tang, B.; Li, D.; Fu, F.; Xu, Y.; Yu, G.; Zhang, J.: A strategy for cleaner pickling: effect, mechanism, and evaluation method of a complex-inhibitor in hydrochloric acid medium. Ind. Eng. Chem. Res. 51, 2615–2621 (2012). https://doi.org/10.1021/ie201538m
Finšgar, M.; Jackson, J.: Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros. Sci. 86, 17–41 (2014). https://doi.org/10.1016/j.corsci.2014.04.044
Furtado, L.B.; Nascimento, R.C.; Guimarães, M.J.O.C.; Henrique, F.J.F.S.; Rocha, J.C.; Seidl, P.R.; Gomes, J.A.C.P.: Cleaner corrosion inhibitors using Peumus boldus Molina formulations in oil well acidizing fluids: gravimetric, electrochemical and DFT studies. Sustain. Chem. Pharm. 19, 100353 (2021). https://doi.org/10.1016/j.scp.2020.100353
Rashid, K.H.; Khadom, A.A.: 3-Methoxypropyl-amine as corrosion inhibitor for X80 steel in simulated saline water. J. Mol. Liq. 319, 114326 (2020). https://doi.org/10.1016/j.molliq.2020.114326
Pakiet, M.; Kowalczyk, I.H.; Leiva Garcia, R.; Akid, R.; Brycki, B.E.: Influence of different counterions on gemini surfactants with polyamine platform as corrosion inhibitors for stainless steel AISI 304 in 3 M HCl. J. Mol. Liq. 268, 824–831 (2018). https://doi.org/10.1016/j.molliq.2018.07.120
El-Lateef, H.M.A.; Soliman, K.A.; Al-Omair, M.A.; Adam, M.S.S.: A combination of modeling and experimental approaches to investigate the novel nicotinohydrazone Schiff base and its complexes with Zn(II) and ZrO(II) as inhibitors for mild-steel corrosion in molar HCl. J. Taiwan Inst. Chem. Eng. 120, 391–408 (2021). https://doi.org/10.1016/j.jtice.2021.03.036
Bedair, M.A.; Soliman, S.A.; Bakr, M.F.; Gad, E.S.; Lgaz, H.; Chung, I.-M.; Salama, M.; Alqahtany, F.Z.: Benzidine-based Schiff base compounds for employing as corrosion inhibitors for carbon steel in 1.0 M HCl aqueous media by chemical, electrochemical and computational methods. J. Mol. Liq. 317, 114015 (2020). https://doi.org/10.1016/j.molliq.2020.114015
Soliman, S.A.A.; Metwally, M.S.S.; Selim, S.R.R.; Bedair, M.A.A.; Abbas, M.A.: Corrosion inhibition and adsorption behavior of new Schiff base surfactant on steel in acidic environment: experimental and theoretical studies. J. Ind. Eng. Chem. 20, 4311–4320 (2014). https://doi.org/10.1016/j.jiec.2014.01.038
Li, X.-L.; Xie, B.; Feng, J.-S.; Lai, C.; Bai, X.-X.; Li, T.; Zhang, D.-L.; Mou, W.-Y.; Wen, L.; Gu, Y.-T.: 2-Pyridinecarboxaldehyde-based Schiff base as an effective corrosion inhibitor for mild steel in HCl medium: experimental and computational studies. J. Mol. Liq. 345, 117032 (2021). https://doi.org/10.1016/j.molliq.2021.117032
Carey, F.A.; Sundberg, R.J.: Advanced Organic Chemistry, Part A: Structure and Mechanisms. Springer, US, Boston, MA (2007)
Barmatov, E.; Hughes, T.: Degradation of a schiff-base corrosion inhibitor by hydrolysis, and its effects on the inhibition efficiency for steel in hydrochloric acid. Mater. Chem. Phys. 257, 123758 (2021). https://doi.org/10.1016/j.matchemphys.2020.123758
Qu, Q.; Jiang, S.; Bai, W.; Li, L.: Effect of ethylenediamine tetraacetic acid disodium on the corrosion of cold rolled steel in the presence of benzotriazole in hydrochloric acid. Electrochim. Acta. 52, 6811–6820 (2007). https://doi.org/10.1016/j.electacta.2007.04.114
Hashim, N.Z.N.; Kassim, K.; Mohd, Y.: (E)-N1-(4-chlorobenzylidene)-N4-phenylbenzene-1,4-diamine as mild steel corrosion inhibitor in 1M HCl. APCBEE Proc. 3, 239–244 (2012). https://doi.org/10.1016/j.apcbee.2012.06.076
Ma, L.; Lu, W.; Yang, D.; Shen, J.; Gao, Z.; Zhang, S.; Liao, Q.: Dithiocarbamate modified glucose as a novel eco-friendly corrosion inhibitor for copper in sodium chloride media. Sustain. Chem. Pharm. 22, 100488 (2021). https://doi.org/10.1016/j.scp.2021.100488
Agrawal, Y.K.; Talati, J.D.; Shah, M.D.; Desai, M.N.; Shah, N.K.: Schiff bases of ethylenediamine as corrosion inhibitors of zinc in sulphuric acid. Corros. Sci. 46, 633–651 (2004). https://doi.org/10.1016/S0010-938X(03)00174-4
Xu, C.; Jin, W.L.; Wang, H.L.; Wu, H.T.; Huang, N.; Li, Z.Y.; Mao, J.H.: Organic corrosion inhibitor of triethylenetetramine into chloride contamination concrete by eletro-injection method. Constr. Build. Mater. 115, 602–617 (2016). https://doi.org/10.1016/j.conbuildmat.2016.04.076
Sathiyanarayanan, S.; Marikkannu, C.; Palaniswamy, N.: Corrosion inhibition effect of tetramines for mild steel in 1M HCl. Appl. Surf. Sci. 241, 477–484 (2005). https://doi.org/10.1016/j.apsusc.2004.07.050
Okeniyi, J.O.; Akinlabi, E.T.; Ikotun, J.O.; Akinlabi, S.A.; Okeniyi, E.T.: Data on triethylenetetramine effect on steel-rebar corrosion-rate in concrete immersed in 0.5 M H 2 SO 4. Chem. Data Collect. 15–16, 238–243 (2018). https://doi.org/10.1016/j.cdc.2018.06.005
Liu, Y.; Dang, Z.; Xu, Y.; Xu, T.: Pyrite passivation by triethylenetetramine: an electrochemical study. J. Anal. Methods Chem. 2013, 1–8 (2013). https://doi.org/10.1155/2013/387124
Li, Q.; Gao, G.; Wang, R.; Zhang, S.; An, S.; Wang, L.: Role of 1-methylimidazole in regulating the CO2 capture performance of triethylenetetramine-based biphasic solvents. Int. J. Greenh. Gas Control. 108, 103330 (2021). https://doi.org/10.1016/j.ijggc.2021.103330
Pietrocola, F.; Castoldi, F.; Zischka, H.; Kroemer, G.: Extending the mode of action of triethylenetetramine (trientine): autophagy besides copper chelation. J. Hepatol. 73, 970–972 (2020). https://doi.org/10.1016/j.jhep.2020.05.046
Lu, J.; Chan, Y.-K.; Poppitt, S.D.; Cooper, G.J.S.: Determination of triethylenetetramine (TETA) and its metabolites in human plasma and urine by liquid chromatography–mass spectrometry (LC–MS). J. Chromatogr. B. 859, 62–68 (2007). https://doi.org/10.1016/j.jchromb.2007.09.001
Li, Z.; Chang, P.-H.; Jiang, W.-T.: Mechanisms of Cu2+, triethylenetetramine (TETA), and Cu-TETA sorption on rectorite and its use for metal removal via metal-TETA complexation. J. Hazard. Mater. 373, 187–196 (2019). https://doi.org/10.1016/j.jhazmat.2019.03.085
Zhou, C.-Y.; Zhao, J.; Wu, Y.-B.; Yin, C.-X.; Pin, Y.: Synthesis, characterization and studies on DNA-binding of a new Cu(II) complex with N1, N8-bis(l-methyl-4-nitropyrrole-2-carbonyl)triethylenetetramine. J. Inorg. Biochem. 101, 10–18 (2007). https://doi.org/10.1016/j.jinorgbio.2006.07.011
Onopchenko, A.; Harrison, J.J.; Chan, C.Y.: The reaction of phthalic anhydride with diethylenetriamine and triethylenetetramine. A literature correction. Bull. Chem. Soc. Jpn. 71, 717–721 (1998). https://doi.org/10.1246/bcsj.71.717
Dassault Systems Materials Studio, BIOVIA, 5005 Wateridge Vista Drive, San Diego, CA 92121, USA, (2017)
Perdew, J.P.; Burke, K.; Ernzerhof, M.: Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
Perdew, J.P.; Wang, Y.: Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B. 45, 13244–13249 (1992). https://doi.org/10.1103/PhysRevB.45.13244
Abbas, M.A.; Bedair, M.A.; El-Azabawy, O.E.; Gad, E.S.: Anticorrosion effect of ethoxylate sulfanilamide compounds on carbon steel in 1 M hydrochloric acid: electrochemical and theoretical studies. ACS Omega 6, 15089–15102 (2021). https://doi.org/10.1021/acsomega.1c01274
Alarfaji, S.S.; Ali, I.H.; Bani-Fwaz, M.Z.; Bedair, M.A.: Synthesis and assessment of two malonyl dihydrazide derivatives as corrosion inhibitors for carbon steel in acidic media: experimental and theoretical Studies. Molecules 26, 3183 (2021). https://doi.org/10.3390/molecules26113183
Adsorption Locator Module Built in Materials Studio, BIOVIA, San Diego, CA, USA, (2017)
Abuelela, A.M.; Bedair, M.A.; Zoghaib, W.M.; Wilson, L.D.; Mohamed, T.A.: Molecular structure and mild steel/HCl corrosion inhibition of 4,5-dicyanoimidazole: vibrational, electrochemical and quantum mechanical calculations. J. Mol. Struct. 1230, 129647 (2021). https://doi.org/10.1016/j.molstruc.2020.129647
Rajarajan, P.N.; Jeganathan, P.; Rajeswari, K.; Sumathy, N.; Devi, A.U.: GC-MS, FTIR and 1H, 13C NMR structural analysis and identification of secondary metabolites from seawater bacterial population. Asian J. Pharm. Pharmacol. 5, 1038–1043 (2019). https://doi.org/10.31024/ajpp.2019.5.6.9
Elsharif, A.M.; Abubshait, S.A.; Abdulazeez, I.; Abubshait, H.A.: Synthesis, characterization and corrosion inhibition studies of polyunsaturated fatty acid derivatives on the acidic corrosion of mild steel: experimental and computational studies. J. Mol. Liq. 319, 114162 (2020). https://doi.org/10.1016/j.molliq.2020.114162
Yadav, D.K.; Quraishi, M.A.: Application of some condensed uracils as corrosion inhibitors for mild steel: gravimetric, electrochemical, surface morphological, UV–visible, and theoretical investigations. Ind. Eng. Chem. Res. 51, 14966–14979 (2012). https://doi.org/10.1021/ie301840y
Sappani, H.K.; Karthikeyan, S.: 4-Chloro-2-((furan-2-ylmethyl) amino)-5-sulfamoylbenzoic acid (FSM) and N-(Isopropylcarbamoyl)-4-( m -tolylamino) pyridine-3-sulfonamide (TSM) as potential inhibitors for mild steel corrosion in 1 N H 2 SO 4 medium. Part I. Ind. Eng. Chem. Res. 53, 3415–3425 (2014). https://doi.org/10.1021/ie401956y
Nady, H.; El-Rabiei, M.M.; Samy, M.: Corrosion behavior and electrochemical properties of carbon steel, commercial pure titanium, copper and copper–aluminum–nickel alloy in 3.5% sodium chloride containing sulfide ions. Egypt. J. Pet. 26, 79–94 (2017). https://doi.org/10.1016/j.ejpe.2016.02.008
Abbas, M.A.; Arafa, E.I.; Gad, E.S.; Bedair, M.A.; El-Azabawy, O.E.; Al-Shafey, H.I.: Performance assessment by experimental and Theoretical approaches of newly synthetized benzyl amide derivatives as corrosion inhibitors for carbon steel in 1.0 M hydrochloric acid environment. Inorg. Chem. Commun. 143, 109758 (2022). https://doi.org/10.1016/j.inoche.2022.109758
Gopiraman, M.; Selvakumaran, N.; Kesavan, D.; Karvembu, R.: Adsorption and corrosion inhibition behaviour of N-(phenylcarbamothioyl)benzamide on mild steel in acidic medium. Prog. Org. Coat. 73, 104–111 (2012). https://doi.org/10.1016/j.porgcoat.2011.09.006
Chauhan, D.S.; Quraishi, M.A.; Jafar Mazumder, M.A.; Ali, S.A.; Aljeaban, N.A.; Alharbi, B.G.: Design and synthesis of a novel corrosion inhibitor embedded with quaternary ammonium, amide and amine motifs for protection of carbon steel in 1 M HCl. J. Mol. Liq. 317, 113917 (2020). https://doi.org/10.1016/j.molliq.2020.113917
Zakaria, K.; Abbas, M.A.; Bedair, M.A.: Herbal expired drug bearing glycosides and polysaccharides moieties as green and cost-effective oilfield corrosion inhibitor: electrochemical and computational studies. J. Mol. Liq. 352, 118689 (2022). https://doi.org/10.1016/j.molliq.2022.118689
Vranda Shenoy, K.; Venugopal, P.P.; Reena Kumari, P.D.; Chakraborty, D.: Effective inhibition of mild steel corrosion by 6-bromo-(2,4-dimethoxyphenyl)methylidene]imidazo [1,2-a]pyridine-2-carbohydrazide in 05 M HCl: insights from experimental and computational study. J. Mol. Struct. 1232, 130074 (2021). https://doi.org/10.1016/j.molstruc.2021.130074
Gangan, A.; ElSabbagh, M.; Bedair, M.A.; Ahmed, H.M.; El-Sabbah, M.; El-Bahy, S.M.; Fahmy, A.: Influence of pH values on the electrochemical performance of low carbon steel coated by plasma thin SiOxCy films. Arab. J. Chem. 14, 103391 (2021). https://doi.org/10.1016/j.arabjc.2021.103391
Barbouchi, M.; Benzidia, B.; Aouidate, A.; Ghaleb, A.; El Idrissi, M.; Choukrad, M.: Theoretical modeling and experimental studies of Terebinth extracts as green corrosion inhibitor for iron in 3% NaCl medium. J. King Saud Univ. - Sci. 32, 2995–3004 (2020). https://doi.org/10.1016/j.jksus.2020.08.004
Ashmawy, A.; Said, R.; Naguib, I.; Yao, B.; Bedair, M.: Anticorrosion study for brass alloys in heat exchangers during acid cleaning using novel Gemini surfactants based on Benzalkonium tetrafluoroborate. ACS Omega 7, 17849–17860 (2022). https://doi.org/10.1021/acsomega.2c01119
Majeed, M.N.; Yousif, Q.A.; Bedair, M.A.: Study of the corrosion of nickel-chromium alloy in an acidic solution protected by nickel nanoparticles. ACS Omega 7, 29850–29857 (2022). https://doi.org/10.1021/acsomega.2c02679
Bedair, M.A.; El-Sabbah, M.M.B.; Fouda, A.S.; Elaryian, H.M.: Synthesis, electrochemical and quantum chemical studies of some prepared surfactants based on azodye and Schiff base as corrosion inhibitors for steel in acid medium. Corros. Sci. 128, 54–72 (2017). https://doi.org/10.1016/j.corsci.2017.09.016
Anupama, K.K.; Ramya, K.; Shainy, K.M.; Joseph, A.: Adsorption and electrochemical studies of Pimenta dioica leaf extracts as corrosion inhibitor for mild steel in hydrochloric acid. Mater. Chem. Phys. 167, 28–41 (2015). https://doi.org/10.1016/j.matchemphys.2015.09.013
El-Sabbah, M.M.B.; Bedair, M.A.; Abbas, M.A.; Fahmy, A.; Hassaballa, S.; Moustafa, A.A.: Synergistic effect between natural honey and 0.1 M KI as green corrosion inhibitor for steel in acid medium. Zeitschrift für Phys. Chemie. 233, 627–649 (2019). https://doi.org/10.1515/zpch-2018-1208
Elaryian, H.M.; Bedair, M.A.; Bedair, A.H.; Aboushahba, R.M.; Fouda, A.E.-A.S.: Synthesis, characterization of novel coumarin dyes as corrosion inhibitors for mild steel in acidic environment: experimental, theoretical, and biological studies. J. Mol. Liq. 346, 118310 (2022). https://doi.org/10.1016/j.molliq.2021.118310
Bahgat Radwan, A.; Sliem, M.H.; Okonkwo, P.C.; Shibl, M.F.; Abdullah, A.M.: Corrosion inhibition of API X120 steel in a highly aggressive medium using stearamidopropyl dimethylamine. J. Mol. Liq. 236, 220–231 (2017). https://doi.org/10.1016/j.molliq.2017.03.116
Popova, A.: Temperature effect on mild steel corrosion in acid media in presence of azoles. Corros. Sci. 49, 2144–2158 (2007). https://doi.org/10.1016/j.corsci.2006.10.020
Gürten, A.A.; Keleş, H.; Bayol, E.; Kandemirli, F.: The effect of temperature and concentration on the inhibition of acid corrosion of carbon steel by newly synthesized Schiff base. J. Ind. Eng. Chem. 27, 68–78 (2015). https://doi.org/10.1016/j.jiec.2014.11.046
Wang, C.; Zou, C.; Cao, Y.: Electrochemical and isothermal adsorption studies on corrosion inhibition performance of β-cyclodextrin grafted polyacrylamide for X80 steel in oil and gas production. J. Mol. Struct. 1228, 129737 (2021). https://doi.org/10.1016/j.molstruc.2020.129737
Bedair, M.A.; Soliman, S.A.; Metwally, M.S.: Synthesis and characterization of some nonionic surfactants as corrosion inhibitors for steel in 1.0 M HCl (experimental and computational study). J. Ind. Eng. Chem. 41, 10–22 (2016). https://doi.org/10.1016/j.jiec.2016.07.005
Bilgili, S.; Atac, A.; Bardak, F.: Theoretical and experimental investigation of the spectroscopic features of and interionic interactions in 1-hexyl-3-methylimidazolium chloride, 1-hexyl-3-methylimidazolium tetrafluoroborate and 1-hexyl-3-methylimidazolium hexafluorophosphate ionic liquid. J. Mol. Liq. 301, 112468 (2020). https://doi.org/10.1016/j.molliq.2020.112468
Mostafa, M.A.; Ashmawy, A.M.; Reheim, M.A.M.A.; Bedair, M.A.; Abuelela, A.M.: Molecular structure aspects and molecular reactivity of some triazole derivatives for corrosion inhibition of aluminum in 1 M HCl solution. J. Mol. Struct. 1236, 130292 (2021). https://doi.org/10.1016/j.molstruc.2021.130292
Bedair, M.A.; Abuelela, A.M.; Zoghaib, W.M.; Mohamed, T.A.: Molecular structure, tautomer’s, reactivity and inhibition studies on 6-Methyl-2-thiouracil for mild steel corrosion in aqueous HCl (1.00 M): experimental and theoretical studies. J. Mol. Struct. 1244, 130927 (2021). https://doi.org/10.1016/j.molstruc.2021.130927
Badr, E.A.; Bedair, M.A.; Shaban, S.M.: Adsorption and performance assessment of some imine derivatives as mild steel corrosion inhibitors in 1.0 M HCl solution by chemical, electrochemical and computational methods. Mater. Chem. Phys. 219, 444–460 (2018). https://doi.org/10.1016/j.matchemphys.2018.08.041
Awad, M.K.; Metwally, M.S.; Soliman, S.A.; El-Zomrawy, A.; Bedair, M.A.: Experimental and quantum chemical studies of the effect of poly ethylene glycol as corrosion inhibitors of aluminum surface. J. Ind. Eng. Chem. 20, 796–808 (2014). https://doi.org/10.1016/j.jiec.2013.06.009
Abbas, M.A.; Bedair, M.A.: Adsorption and computational studies for evaluating the behavior of silicon based compounds as novel corrosion inhibitors of carbon steel surfaces in acidic media. Zeitschrift für Phys. Chemie. 233, 225–254 (2019). https://doi.org/10.1515/zpch-2018-1159
Bedair, M.A.: The effect of structure parameters on the corrosion inhibition effect of some heterocyclic nitrogen organic compounds. J. Mol. Liq. 219, 128–141 (2016). https://doi.org/10.1016/j.molliq.2016.03.012
Gebril, M.A.; Bedair, M.A.; Soliman, S.A.; Bakr, M.F.; Mohamed, M.B.I.: Experimental and computational studies of the influence of non-ionic surfactants with coumarin moiety as corrosion inhibitors for carbon steel in 1.0 M HCl. J. Mol. Liq. 349, 118445 (2022). https://doi.org/10.1016/j.molliq.2021.118445
Abdelshafi, N.S.; Ibrahim, M.A.; Badran, A.-S.; Halim, S.A.: Experimental and theoretical evaluation of a newly synthesized quinoline derivative as corrosion inhibitor for iron in 1.0 M hydrochloric acid solution. J. Mol. Struct. 1250, 131750 (2022). https://doi.org/10.1016/j.molstruc.2021.131750
Bedair, M.A.; Soliman, S.A.; Hegazy, M.A.; Obot, I.B.; Ahmed, A.S.: Empirical and theoretical investigations on the corrosion inhibition characteristics of mild steel by three new Schiff base derivatives. J. Adhes. Sci. Technol. 33, 1139–1168 (2019). https://doi.org/10.1080/01694243.2019.1582889
Bedair, M.A.; Fouda, A.S.; Ismail, M.A.; Mostafa, A.: Inhibitive effect of bithiophene carbonitrile derivatives on carbon steel corrosion in 1 M HCl solution: experimental and theoretical approaches. Ionics (Kiel). 25, 2913–2933 (2019). https://doi.org/10.1007/s11581-018-2811-0
Chaouiki, A.; Chafiq, M.; Al-Moubaraki, A.H.; Bakhouch, M.; El Yazidi, M.; Gun Ko, Y.: Electrochemical behavior and interfacial bonding mechanism of new synthesized carbocyclic inhibitor for exceptional corrosion resistance of steel alloy: DFTB, MD and experimental approaches. Arab. J. Chem. 15, 104323 (2022). https://doi.org/10.1016/j.arabjc.2022.104323
Acknowledgements
The authors are greatly thankful to the Egyptian Petroleum Research Institute (EPRI) and Al-Azhar University for funding and support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MAA contributed to visualization, methodology, experimental, writing for the original draft, revision, coordination, data curation, investigation, formal analysis, validation and review and editing. EIA contributed to visualization, methodology, experimental and writing. MAB contributed to visualization, methodology, quantum chemical calculations, writing for the original draft, revision, coordination, data curation, investigation, formal analysis, validation and review and editing. ASI contributed to visualization, methodology, writing for the original draft, revision and coordination. OEEA contributed to visualization, methodology, experimental and revision. SAB contributed to visualization, methodology, experimental and revision. HIAS contributed to conceptualization, methodology, visualization, validation, writing—review and editing and supervision.
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbas, M.A., Arafa, E.I., Bedair, M.A. et al. Synthesis, Characterization, Thermodynamic Analysis and Quantum Chemical Approach of Branched N, N′-bis(p-hydroxybenzoyl)-Based Propanediamine and Triethylenetetramine for Carbon Steel Corrosion Inhibition in Hydrochloric Acid Medium. Arab J Sci Eng 48, 7463–7484 (2023). https://doi.org/10.1007/s13369-022-07520-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07520-y