Abstract
Thermal decomposition of plastics by pyrolysis into oil is a successful way of treating wastes. Nevertheless, the production of carbon nanotubes (CNTs) from wastes improves the feasibility of the waste management process. An experimental setup was developed to study the influence of different heating rates on the produced oil by pyrolysis of waste polypropylene (WPP), and the influence of using foamed nickel on the produced CNTs as a function of operating temperature and heating rate. Different heating rates of 5, 10, and 20 °C/min were examined, as well as the different carbon vapor deposition (CVD) temperatures of 600, 700, and 800 °C were studied. It has been found that increasing the heating rate from 5 to 20 °C/min increases the oil yield from 59.3 to 71%, but on the other hand it decreases the quality of the oil. It has been also found that increasing the heating rate decreases the quality of CNTs, i.e., uniform CNTs with small diameter and small wall thickness, and as well as the quantity. The physical properties of the produced CNTs have been improved by increasing the CVD temperature; however, the quantity of CNTs decreased. The highest yield of CNTs produced was 43.12% at the lowest CVD temperature and heating rate examined, i.e., 600 and 5 °C/min, respectively. The optimum heating rate and CVD temperature for the pyrolysis of waste polypropylene to achieve the highest quality of CNTs with moderate production of 39.34%, is the lowest heating rate examined, i.e., 5 °C/min, with a moderate CVD temperature of 700 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The continuous scientific and technological progress has led to increased demand and energy depletion worldwide. The US consumed 98 quadrillion Btu (British thermal units) of energy in 2017, about 17% of total world energy consumption. The increase in energy consumption produces a continuous decrease in fossil fuel sources and increased environmental damage resulting from gas emissions [1]. It is expected that the reserve of fossil fuels will be phased out after 2042 [2]. Therefore, there is a high necessity to search for new environmentally friendly energy sources. Municipal Solid Waste (MSW) consists of the daily use of many commodities and their disposals, such as packaging, appliances, paint, bottles, and furniture [3]. The production of MSW became two billion tons/year, and it is expected to range to 3 billion tons in 2025 [4]. The most significant component of MSW is plastic waste, used in many applications because of its low cost, high durability, and low weight. The continuous increase in the production rate of plastic products poses a significant threat due to the absence of adequate mechanisms for treating these large quantities of waste and safely disposing it [5]. Polypropylene (PP) is the second most common plastic material globally after polyethylene (PE), and it is also one of the most common fractions of plastic trash that ends up in landfills [6]. It was found that municipal solid waste contains about 24.3% of polypropylene waste [7]. Polypropylene is used in many applications because of its mechanical properties, good chemical resistance, easy processing, and low density. Polypropylene is utilized in medical bags, food packaging, computer components, pipes, the automotive industry, office folders, furniture, general containers, and storage boxes [8]. Table s1 in the supplementary material shows the plastic waste ranking [9]. The accumulation of plastic waste in landfills adversely affects the environment because it needs a considerable period to decompose [10].
It is notoriously difficult to recycle this large amount of plastic waste. Thus, it is necessary to search for a better option of disposal of this waste when it is difficult to reuse, avoiding landfill accumulation [11, 12]. Therefore, researchers made many efforts to get rid of this waste and make the best use of it. The researchers used these wastes as feedstock in many applications to produce more essential products, including the heat treatment process. Pyrolysis can give an alternative waste control option to reduce the range of landfills and save gases, liquids, and even produce valuable Carbon Nanotubes (CNTs) from the thermal decomposition of the waste plastic [13]. The gas generated from the pyrolysis of plastic has a high calorific value as it contains hydrogen, methane, ethane, propane and butane. The liquid produced can be used as a substitute conventional fuel [14]. Many studies have proven that the generated gases can be used in various applications, such as heating, power generation or transportation. Zhao et al. [15] synthesized mesoporous Fe-SBA- 15 and used it in the thermal decomposition of polypropylene to obtain light hydrocarbon oils. Auxilio et al. [16] used catalysts to produce oil from plastic waste with more convenient properties for biofuels.
There are other ways to improve the properties of the resulting oil for use as biofuels by hydrogenating the oil produced from pyrolysis. The properties of the produced oil are matched to the diesel fuel properties [17]. The carbon nanotubes mechanical, electrical and thermal properties are excellent as the CNTs can be used in many applications such as civil engineering applications, aerospace and automotive [18]. Wen et al. [19] used nanosheets of carbon produced from waste plastic in supercapacitors with high efficiency. Also, Borsodi et al. [20] added CNTs into a matrix of commercial LDPE to improve its properties. The pyrolysis process also has many environmental benefits as it aids in reducing the rate of climate variation and depleting fossils and toxins that harm humans. Therefore, this technology is considered economically and environmentally beneficial because it consumes a small amount of energy and a low amount of harmful emissions to the environment from the direct burning of plastic waste [21, 22].
The production of CNTs from plastic waste using the chemical vapor deposition (CVD) method is done through the pyrolysis of hydrocarbons, i.e., waste plastics, to convert the hydrocarbon into gas and then collect the CNTs deposited on the surface of the applied catalyst. Catalyst development is a critical factor in creating excellent quality and quantity of CNTs, as the catalyst has a significant role in forming CNTs. Most catalysts depend on the participation of nickel as it is considered an excellent catalyst to obtain CNTs, due to its high ability to break C-H bonds and C–C bonds [23].
Waste polypropylene (PP) was used by Mishra et al. [24] to create multi-walled carbon nanotubes utilizing a single step of chemical vapor deposition (CVD) and nickel powder as a catalyst between 600, 700, and 800 °C, and it has been found that the pyrolysis of plastic at 800 °C gives the purest form of CNTs. The effects of several metal oxides, MgO, CaO, and TiO2, and mixed oxide CaTiO3, on the effectiveness of a bimetallic NiMo catalyst during the formation of CNTs from waste polypropylene were studied by Modekwe et al. [25, 26], and it has been found that the quality and quantity of produced CNTs depend significantly on the type of catalyst used and its stability.
Shen et al. [27] studied using polypropylene as a carbon source to get multi-walled carbon nanotubes (MWCNTs) using a combination of bimetallic Ni–Al catalysts. It is observed that this combination increased the yield and improved the characteristics of MWCNTs products. Yao et al. [28] and Yang et al. [29] used Ni–Fe as a catalyst with different Ni:Fe ratios to get CNTs by pyrolysis of plastics. It is noticed that the catalyst composition affects the quantity and quality of CNTs, and it is also noticed that the characteristics of CNTs improved significantly when using Ni/Fe molar ratio of 3. Veksha et al. [30] carried out the MWCNTs from a non-condensable gas output from the pyrolysis process of different types of packaging plastic waste using Ni-Ca as a catalyst at 700 °C. Acomb et al. [31] studied the effect of copper, iron, nickel, and cobalt at 800 °C on hydrogen gas and CNTs using low-density polyethylene. It is concluded that the most significant quantity of CNTs and hydrogen by nickel and iron, a low amount of CNTs deposited on the surface of copper, and optimum quality of CNTs by cobalt catalyst.
The use of powder catalysts became limited in most applications because it requires a large amount of energy, time, and effort to prepare it. Besides, it needs special reactors to operate the catalyst [32]. Therefore, it is necessary to use a cheap and easy catalyst to overcome these shortcomings. So, this research aims to maximize the amount of CNTs coming from the thermal cracking of waste polypropylene using a cheap and easy-to-use catalyst, e.g., commercial foamed nickel. Foamed nickel has a large surface area to mass ratio, enhancing and increasing carbon's deposited mass compared to nickel's mass.
Yao et al. [33] employed Fe–Ni catalysts with polypropylene plastic to produce carbon materials. The impacts of different catalytic temperatures, i.e. 600, 700, and 800 °C, on the physicochemical properties of obtained carbon compounds were studied. It is concluded that a catalysis temperature of more than 700 °C was required to successfully produce carbon nanotubes, with subsequent temperature increases affecting yield rather than thermal stability or graphitic degree. Carbon deposition on an iron alumina catalyst was studied by Acomb et al. [34] at temperatures of 700, 800, and 900 °C.
A rise in catalyst temperature resulted in a larger carbon nanotubes and hydrogen yield. Liu et al. [35] used catalytic pyrolysis of PP and the subsequent catalytic decomposition of pyrolysis gases over a nickel catalyst to get MWCNTs and hydrogen. The effect of decomposition temperature (500–800 °C) on the performance of the reaction system was studied. The yield of MWCNT and hydrogen concentration increased as the decomposition temperature increased, and the maximum was obtained at 700 °C.
It can be concluded from the above literature survey that most of the previous studies used a single-stage reactor during the catalytic conversion of WPP into oil or CNTs, due to the complexity of the conversion process. In addition, most of the previous studies focused on the effect of the heating rate on the amount and quality of the oil produced [33, 34] irrespective of the quality and quantity of the CNTs produced. Therefore, in this work, an experimental setup has been developed using a double-stage reactors to study the effect of different heating rates on the produced oil and CNTs. Also, the influence of using a commercial foamed nickel as a catalyst on the yield of CNTs from the uncondensed oil as a function of the operating temperature and heating rate is studied. Nickel foam was used as a catalyst in this research due to its availability, cheapness, and large contact area to mass ratio. Different heating rates of 5, 10, and 20 °C/min were examined, as well as different carbon vapor deposition (CVD) temperatures of 600, 700, and 800 °C were studied.
2 Experimental Work
2.1 Preparation of WPP and Nickel Foam
In this study, the raw material used for pyrolysis was waste polypropylene (WPP), collected from Henkel Corporation for Plastic Waste Collection, Egypt [36]. For the thermal gravimetric (TG) analysis and the pyrolysis tests, the WPP was washed, dried, and then shredded into smaller particles and filtered through a mesh of size 500 µm. Nickel foam (NF) was chosen as a catalyst because of its commercial availability and good pore characteristics, and bulk density properties. The physical properties of the nickel foam are presented in Table s2. Nickel foam has a large specific surface area to mass ratio and a homogeneous three-dimensional network structure, which has an excellent ability to attract CNTs by the CVD method. The mass of the used nickel foam catalyst was constant, i.e. 0.15 g in all performed experiments. The catalyst was first oxidized in a tubular furnace at 500 °C in stable air for 30 min, then left for one h at 600 °C at a flow rate of 100 mL/min of H2 and N2, such that the percentage of hydrogen to nitrogen is 40/60. Then, the furnace temperature was reduced to operating CVD temperature with a nitrogen flow rate of 60 mL/min. The amount of carbon deposited on the catalyst, i.e., the carbon yield %, is calculated as follows:
where MTotal is the total mass of the deposited carbon and catalyst after the CVD process, MNF is the mass of the nickel foam catalyst before reaction.
2.2 Experimental Setup and Procedure
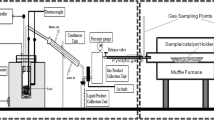
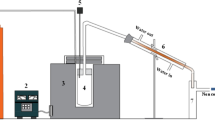
The experimental setup consists of a pyrolysis reactor, gas condenser, and catalytic reactor, as shown in Fig. 1. The reactors are horizontal tube reactors, such that the tubes of the reactors are made of stainless steel with a length of 80 cm and an inner diameter of 4 cm. Nitrogen is passed through the reactors with a 60 mL/min flow rate during the reaction process for expelling air and operating under an oxygen-free environment.
The first reactor is the pyrolysis reactor, a tube furnace with a heating power of 2 kW. The pyrolysis reactor is charged by 5 gm of WPP and heated to 500 °C at a heating rate of 5 °C/min, 10, and 20 °C/min. The exit of the reactor is connected to a condenser at room temperature to condense the gaseous products. The condensed liquid is collected, and the uncondensed gases are passed to the second reactor, i.e., the catalytic reactor, where the nickel foam (NF) catalyst is located. The NF is kept at different temperatures in the catalytic reactor, i.e., 600, 700, and 800 °C, to examine the influence of varying CVD temperatures on the deposition of CNTs. The catalytic reactor is heated first to the desired temperature. Then the pyrolysis reactor turned on and heated the WPP to 500 °C and kept at that temperature for 30 min until the reaction was completed. Both reactors are turned off when the reaction is completed, and the system is left to cool down to room temperature; and during that cooling process, nitrogen continues to flow. Finally, the collected liquid and deposited coke in the pyrolysis reactor are weighed, and the gas yield is obtained by mass balance [37]. The mass percentage of the products were computed for oil, char, and the uncondensed gases as follows:
2.3 Characterization
2.3.1 Waste Polypropylene (WPP)
To investigate the thermal decomposition behavior of WPP, thermogravimetric (TG) and differential thermal (DT) analysis measurements were taken using a thermogravimetric analyzer (SDT Q600 USA). In a pure nitrogen atmosphere, WPP was heated from room temperature to 1000 °C at a rate of 10 °C/min.
2.3.2 Oil
Gas chromatography-mass spectroscopy (GC–MS) was used to evaluate qualitatively the oil coming out from the pyrolysis process. The analyses were performed using a GC system 7890A/5975C inert MS with triple Axis to match the chromatogram peaks, USA. Helium was used as the carrier gas. Peaks with a high probability, quality of more than 80%, were identified using the Wiley Registry/NIST Library.
2.3.3 Characterization of Catalysts
A Quanta FEG 250 scanning electron microscope (SEM, Quanta FEG250, FEI, USA) was used to examine the microstructures and morphologies of CNTs deposited on the surface of the NF catalyst. A high-resolution transmission electron microscope (TEM), i.e., JEOL JEM-2100, Japan, was used to image the NF catalyst. The samples were ultrasonically suspended in ethanol, and the droplets of suspension were deposited on a copper grid to obtain TEM species. The identification and crystalline phase were analyzed by X-ray diffraction (PANalytical Empyrean, Netherlands) using CuKa radiation and the range of recorded data in 2θ from 5 to 80°. The samples were measured using Witec Confocal Raman microscope (WITec GmbH, Germany), alpha 300R, with excitation laser recording from 500 to 3000 cm−1.
3 Experimental Results
3.1 TG Analysis of WPP
TG and DTG curves for the pyrolysis of waste polypropylene using nitrogen gas with a heating rate of 10 °C/min are shown in Fig. 2. It can be seen from Fig. 2 that the maximum weight loss was at 465 °C due to the thermal decomposition of PP. The DTG curve shows that the thermal decomposition starts at 410 °C, and the maximum decomposition rate occurs at 468 °C. The pyrolysis process ends completely by turning WPP into gases at a temperature of 485 °C [38].
3.2 Oil Analysis
3.2.1 Mass Percentage of Products
The optimum reaction temperature for high oil production with low density and low amount of wax was stated to be 500 °C [39]. Further cracking to produce low-range hydrocarbons is likely to happen if the residence time of volatiles within the heating zone is increased. A comparison of the products from the pyrolysis of WPP at 500 °C with various heating rates such as 5, 10, and 20 °C/min is shown in Table 1. By decreasing the heating rate, the oil yield decreases while the gas yield increases, and vice versa. That is because, under low heating rates, dissociation is first caused by random scission, which results in dissociation of the carbon–carbon bond, which needs less dissociation energy and produces short polymer chains, followed by b-scission. The heating time, i.e., the residence time of WPP increases at low heating rates, which subsequently raises the proportion of low-chain hydrocarbons (C1–C16) [40], increasing the gas production rate. However, decreasing the residence time by increasing the heating rate prevented low chain hydrocarbons produced from cracking to crack further. As a result, fewer gaseous products with higher chain hydrocarbons were made, increased oil yield, and increased wax formation in the oil phase [41].
3.2.2 GC–MS analysis of Oil Produced
GC–MS analysis of the oil obtained from the pyrolysis of WPP at different heating rates of 5, 10, and 20 °C/min has been performed, and the weight percentage of the oil obtained is shown in Table s3, while the weight percentage of some of the light components of the oil, i.e., C9H18, C10H20, and C12H26 is presented in Table 2. It can be concluded that the high percentage of light hydrocarbons occurs at the low heating rate and decreases with high heating rates [42]. The weight percentage of light hydrocarbons C9H18, C10H20, and C12H26 at a heating rate of 5 °C/min is 31.16, 3.25, and 38.5%, respectively, which is higher than at a heating rate of 20 °C/min., i.e., 9,25, 1.47, and 34.96%, respectively, as can be seen in Table 2. It was also observed that there were many similar compounds, such as Dodecane, Heptane, 4-methyl, and Cyclopentene [43]. Some compounds that contain fluorine, such as C21H35F7O2, silica, such as C9H15Si, and bromine, such as C54H108Br2, were found, but in low quantities, this is due to the impurities in the WPP sample [41].
The weight percentage of the carbon number in the hydrocarbon as a function of the heating rate for the pyrolytic oil is shown in Table 2. The values shown in Table 2 are based on the GC–MS analysis. It can be concluded that in the case of a low heating rate of 5 °C/min, the percentage of light hydrocarbons ranging from C5 to C9 was approximately 41.8%, while in case of the higher heating rates of 10, and 20 °C/min, the percentage decreases to 34.1 and 20.5%, respectively, which is in line with the conclusions of López et al. [42]. However, the percentage of heavy hydrocarbons ≥ C15 was approximately 1.4% at a heating rate of 5 °C/min, while in case of the higher heating rates of 10, and 20 °C/min, the percentage increases to 2.2 and 10.8%, respectively, which is in line with the conclusions of Ahmad et al. [44].
3.3 CNTs Properties
3.3.1 Influence of the Deposited CNTs on the Surface of the Catalyst Ni Foam
The SEM images of the Ni foam before and after the CVD process are shown in Fig. 3. The surface of the Ni foam before the CVD process is shown in Fig. 3a and b, which is a three-dimensional porous structure. Also, it can be concluded that the Ni foam surface is smooth, especially before the CVD process. However, the surface changes to a rough surface because of carbon deposition on the Ni foam after the CVD process with no change in the three-dimensional structure, as shown in Fig. 3c and d. The Ni foam three-dimensional structure retained its shape during the CVD process, which indicates that the Ni foam has high strength. It can be concluded based on Fig. 3e and f that the deposited carbon particles on the Ni foam are multiwall carbon nanotubes. Figure 3f is an image of the deposited carbon nanotubes on the Ni foam, and it is taken using TEM; the diameter of the tubes varies between 11.61 nm and 22.5 nm, while the thickness of the tube is 2.35 nm.
3.3.2 Influence of the CVD Temperature on the Deposited CNTs
The SEM and TEM images of the deposited CNTs on the surface of the nickel foam catalyst after the CVD process for non-condensable gases produced from the pyrolysis of WPP are shown in Fig. 4a–f. The images shown are in case of a pyrolysis heating temperature of 500 °C at a heating rate of 5 °C/min and the CVD temperature varies between 600, 700, and 800 °C. It can be concluded that the diameter of the deposited CNTs decreases, and the thickness of the CNT walls by increasing the CVD temperature [45], as shown in Fig. 4a–f. It is found that the mean diameter of the CNTs at a CVD temperature of 600 °C is 55 nm, and at a CVD temperature of 700 °C is 30 nm, and the diameter drops to 26 nm at a temperature of 800 °C. Chen et al. [46] concluded that the diameter of CNTs is affected by the size of Ni crystals. They found that Ni particles were broken down into smaller particles at high temperatures. The size of nickel particles decreases as the temperature increases from 600 to 800 °C, resulting in a decrease in CNT diameter. It can also be concluded based on Figs. 4 a–f that increasing the CVD temperature assists in producing CNTs with a uniform diameter [47].
The production of the CNTs as a function of the CVD temperature is presented in Fig. 5a. It is noted that the production of the CNTs decreased from 43.12 to 35.67% with the increase in CVD temperature from 600 to 800 °C. It can be concluded that increasing the CVD temperature increases the quality of the CNTs, i.e., narrow, and thin tubes are produced and decreased the production yield.
3.3.3 Influence of the CVD Temperature on the Graphitization of the Deposited CNTs
The XRD forms of the NF catalysts before and after the CNTs deposition on the catalyst's surface at different CVD temperatures, i.e., 600, 700, and 800 °C, are shown in Fig. 4g. The reflections that appeared at 2θ = 44.33°, 51.66°, and 76.2° are defined to be metallic Ni (1 1 1), (2 0 0), and (2 2 0), as found in all the samples. Additionally, it shows that the graphite peaks (0 0 2) appeared at 2θ = 26.4°, indicating the formation of CNTs [48]. It can be observed from Fig. 4g that the height of the carbon peaks increases with the CVD temperature, which indicates an increase in the graphitization degree. It can be concluded that increasing the CVD temperature improves the graphitization degree of the synthesized CNTs [32].
Raman analysis has been used to assess the crystallinity, graphitization degree, and structure of CNTs synthesized over the Ni foam catalyst. The Raman spectra of the CNTs that are synthesized at different CVD temperatures, i.e., 600, 700, and 800 °C, and at a heating rate of 5 °C/min, are shown in Fig. 4h. Three basic patterns occur at 1346 cm−1 (D band), 1590 cm−1 (G band), and 2695 cm−1 (2D band). The D-band expresses CNTs with structural defects, the G-band expresses the regularity of the graphitic carbon crystal structure, and the 2d-band indicates the pureness of the CNTs [49]. The ratio between the height of the D-peak, ID, and the G-peak, IG, is used to determine the quality and crystallization of the carbon nanotubes [50]. The ID/IG is 1.09, 0.98, and 0.879 at CVD temperatures of 600, 700, and 800 °C, respectively.
As the CVD temperature increases, the ID/IG decreases, indicating higher quality and crystallization of the synthesized CNTs. Also, the 2D peak at a CVD temperature of 600 °C is lower than at 700 and 800 °C, which indicates that the synthesized CNTs produced at high temperatures are purer than the CNTs produced at low temperatures.
3.3.4 Influence of the Heating Rate on the Deposited CNTs
The SEM and TEM images for the CNTs developed on the surface of nickel foam at a CVD temperature of 700 °C are shown in Fig. 6a–f. The CNTs are formed from the non-condensable gasses produced from the pyrolysis of WPP at 500 °C and different heating rates of 5, 10, and 20 °C/min. It is found that the mean diameter of the CNTs at a heat rate of 5 °C/min is 30 nm, and the mean diameter at a heat rate of 10 °C/min is 74 nm, while the mean diameter at a heat rate of 20 °C/min is 145 nm. Increasing the heating rate from 5 to 20 °C/min increases the diameter of the CNTs and the wall thickness, while the inner diameter decreases until it becomes clogged, as shown in Fig. 6a–f. It is also noted that the rate of CNTs production decreased from 39.34, 23.12% to 11.02% with the increase in heating rate from 5, 10 °C/min, to 20 °C/min, respectively, as shown in Fig. 5b. This phenomenon can be explained by the fact that a low heating rate leads to a longer residence time, which allows more cracking and thus obtaining; (1) a more significant amount of light hydrocarbon fractions, (2) increasing the rate of produced methane, and (3) decreasing the flowrate of uncondensed gases [51]. Thus, longer residence time helps depose carbon on the catalyst's surface with higher quantity and quality. While increasing the heating rate decreases the residence time, which reduces the chances of more cracking and obtaining; (1) a low amount of light hydrocarbon fractions, (2) decreasing the rate of produced methane, and (3) rapid generation of uncondensed gases, which decreases the quantity and quality of the deposited CNTs [41, 52].
The SEM and TEM images of the CNTs produced from the pyrolysis of WPP at a heating rate of 5 °C/min a, b, 10 °C/min c, d and 20 °C/min e, f, g XRD forms of the NF catalysts before and after CNTS deposition, h The Raman spectra of the CNTs and at a CVD temperature of 700 °C and different heating rates
The XRD analysis of the synthesized CNTs produced from the pyrolysis of WPP at heating rates of 5, 10, and 20 °C/min and CVD temperature of 700 °C is presented in Fig. 6g. It can be seen that the carbon peak decreases with the heating rate, which indicates that the graphitization degree decreases with the heating rate.
The Raman spectra of the CNTs that are synthesized at different pyrolysis heating rates of 5, 10, and 20 °C/min and at a constant CVD temperature of 700 °C, are shown in Fig. 6h. The ID/IG values decreased from 1.072, 1.06, and 0.98, as the heat rates decreased from 20, 10 °C/min, and 5 °C/min, respectively. It can be concluded that decreasing the heating rate improves the purity, crystallization, and graphitization degree of synthesized CNTs.
4 Discussion of Results
The influence of the Heating Rate (HR) and the Chemical Vapor Deposition (CVD) temperature on the quality and quantity of Carbon Nanotubes (CNTs) produced by pyrolysis of WPP is summarized in Fig. 7. It can be concluded from the performed analysis that increasing the Chemical Vapor Deposition (CVD) temperature increases the quality and decreases the quantity of the produced CNTs, while reducing the CVD temperature decreases the quality and increases the quantity. The Heating Rate (HR) of the pyrolysis process of WPP decreases the quality and the quantity of produced CNTs, and vice versa. Therefore, it can have concluded based on Fig. 7 that the highest quality can be obtained by decreasing the HR of the pyrolysis process and increasing the CVD temperature. In contrast, the highest quantity can be obtained by decreasing the HR and decreasing the CVD Temperature. However, the optimum heating rate and CVD temperature for the pyrolysis of waste polypropylene to achieve the average quality and quantity of the produced CNTs, is 5 °C/min, i.e., the lowest heating rate, and 700 °C (moderate CVD temperature), respectively. Further research will be conducted to correlate CNTs yield as a function of the heating rate and the CVD temperature.
5 Conclusions
This research aims to improve the quality and quantity of the CNTs produced from the pyrolysis of waste polypropylene (WPP) using nickel foam as a catalyst due to its availability, cheapness and large contact area to mass ratio. The influence of different heating rates on the produced oil, as well as the influence of using foamed nickel on the produced CNTs as a function of operating temperature and heating rate are examined in this research. Different heating rates of 5, 10, and 20 °C/min were examined, as well as different carbon vapor deposition (CVD) temperatures of 600, 700, and 800 °C were studied. It has been found that,
-
(1)
Increasing the heating rate from 5 to 20 °C/min increases the oil yield from 59.3 to 71%, but on the other hand it decreases the quality of the oil.
-
(2)
Decreasing the heating rate increases the quality as well as the quantity of the produced CNTs.
-
(3)
The physical properties of the CNTs have been improved by increasing the CVD temperature, however, the quantity of CNTs decreased.
-
(4)
The highest yield of CNTs produced was 43.12% at the lowest CVD temperature and heating rate examined, i.e., 600 °C and 5 °C/min, respectively.
-
(5)
The optimum heating rate and CVD temperature for the pyrolysis of waste polypropylene to achieve the highest quality of CNTs with a moderate production of 39.34%, is the lowest heating rate examined, i.e., 5 °C/min, with a moderate CVD temperature of 700 °C.
References
Qi, P.; Chang, G.; Wang, H.; Zhang, X.; Guo, Q.: Production of aromatic hydrocarbons by catalytic co-pyrolysis of microalgae and polypropylene using HZSM-5. J. Anal. Appl. Pyrolysis 136, 178–185 (2018)
Capuano, L.: International energy outlook 2018 (IEO2018). US Energy Inf. Adm. (EIA): Wash. DC USA 2018, 21 (2018)
Mileski, J. Mitigation strategies for municipal solid waste generation in Lincoln, (2021)
Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N.: The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: review of the state of art and future research directions. Prog. Energy Combust. Sci. 71, 1–80 (2019)
Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S.: Catalytic pyrolysis of plastic waste: a review. Process Safety Environ. Protect. 102, 822–838 (2016)
Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M.: Current state and future prospects of plastic waste as source of fuel: a review. Renew. Sustain. Energy Rev. 50, 1167–1180 (2015)
Pramanik, H.; Gaurh, P.: Production and characterization of pyrolysis oil using waste polyethylene in a semi batch reactor. Indian J. Chem. Technol. (IJCT) 25(4), 336–344 (2019)
Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; McDonald, A.G.: Evaluation of pyrolysis process parameters on polypropylene degradation products. J. Anal. Appl. Pyrolysis 109, 272–277 (2014)
Saleem, J.; Riaz, M.A.; Gordon, M.: Oil sorbents from plastic wastes and polymers: a review. J. Hazard. Mater. 341, 424–437 (2018)
Zhang, Y.; Duan, D.; Lei, H.; Villota, E.; Ruan, R.: Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl. Energy 251, 113337 (2019)
Wu, S.L.; Kuo, J.H.; Wey, M.Y.: Thermal degradation of waste plastics in a two-stage pyrolysis-catalysis reactor over core-shell type catalyst. J. Anal. Appl. Pyrolysis 142, 104641 (2019)
Ragaert, K.; Delva, L.; Van Geem, K.: Mechanical and chemical recycling of solid plastic waste. Waste Manag. 69, 24–58 (2017)
de Paula, F.G.; de Castro, M.C.; Ortega, P.F.; Blanco, C.; Lavall, R.L.; Santamaría, R.: High value activated carbons from waste polystyrene foams. Microporous Mesoporous Mater. 267, 181–184 (2018)
Akubo, K.; Nahil, M.A.; Williams, P.T.: Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J. Energy Inst. 92(1), 195–202 (2019)
Zhao, Y.; Wang, W.; Jing, X.; Gong, X.; Wen, H.; Deng, Y.: Catalytic cracking of polypropylene by using Fe-SBA-15 synthesized in an acid-free medium for production of light hydrocarbon oils. J. Anal. Appl. Pyrolysis 146, 104755 (2020)
Auxilio, A.R.; Choo, W.L.; Kohli, I.; Srivatsa, S.C.; Bhattacharya, S.: An experimental study on thermo-catalytic pyrolysis of plastic waste using a continuous pyrolyser. Waste Manag. 67, 143–154 (2017)
Mangesh, V.L.; Padmanabhan, S.; Tamizhdurai, P.; Narayanan, S.; Ramesh, A.: Combustion and emission analysis of hydrogenated waste polypropylene pyrolysis oil blended with diesel. J. Hazard. Mater. 386, 121453 (2020)
Peng, H.; Li, Q.; Chen, T.: Industrial applications of carbon nanotubes. William Andrew, New York (2016)
Wen, Y.; Kierzek, K.; Chen, X.; Gong, J.; Liu, J.; Niu, R.; Mijowska, E.; Tang, T.: Mass production of hierarchically porous carbon nanosheets by carbonizing “real-world” mixed waste plastics toward excellent-performance supercapacitors. Waste Manag. 87, 691–700 (2019)
Borsodi, N.; Szentes, A.; Miskolczi, N.; Wu, C.; Liu, X.: Carbon nanotubes synthetized from gaseous products of waste polymer pyrolysis and their application. J. Anal. Appl. Pyrolysis 120, 304–313 (2016)
Ahamed, A.; Veksha, A.; Yin, K.; Weerachanchai, P.; Giannis, A.; Lisak, G.: Environmental impact assessment of converting flexible packaging plastic waste to pyrolysis oil and multi-walled carbon nanotubes. J. Hazard. Mater. 390, 121449 (2020)
Shen, Y.; Gong, W.; Zheng, B.; Gao, L.: Ni–Al bimetallic catalysts for preparation of multiwalled carbon nanotubes from polypropylene: influence of the ratio of Ni/Al. Appl. Catal. B 181, 769–778 (2016)
Wang, J.; Shen, B.; Lan, M.; Kang, D.; Wu, C.: Carbon nanotubes (CNTs) production from catalytic pyrolysis of waste plastics: the influence of catalyst and reaction pressure. Catal. Today 351, 50–57 (2020)
Mishra, N.; Das, G.; Ansaldo, A.; Genovese, A.; Malerba, M.; Povia, M.; Ricci, D.; Di Fabrizio, E.; Di Zitti, E.; Sharon, M.; Sharon, M.: Pyrolysis of waste polypropylene for the synthesis of carbon nanotubes. J. Anal. Appl. Pyrolysis 94, 91–98 (2012)
Modekwe, H.U.; Mamo, M.A.; Moothi, K.; Daramola, M.O.: Effect of different catalyst supports on the quality, yield and morphology of carbon nanotubes produced from waste polypropylene plastics. Catalysts 11(6), 692 (2021)
Modekwe, H.U.; Mamo, M.; Moothi, K.; Daramola, M.O.: Synthesis of bimetallic NiMo/MgO catalyst for catalytic conversion of waste plastics (polypropylene) to carbon nanotubes (CNTs) via chemical vapour deposition method. Mater. Today Proc. 38, 549–552 (2021)
Shen, Y.; Gong, W.; Zheng, B.; Gao, L.: Ni–Al bimetallic catalysts for preparation of multiwalled carbon nanotubes from polypropylene: Influence of the ratio of Ni/Al. Appl. Catal. B Environ. 181, 769–778 (2016)
Yao, D.; Wu, C.; Yang, H.; Zhang, Y.; Nahil, M.A.; Chen, Y.; Williams, P.T.; Chen, H.: Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on Ni-Fe bimetallic catalyst. Energy Convers. Manag. 148, 692–700 (2017)
Yang, W.; Cao, L.; Li, W.; Du, X.; Lin, Z.; Zhang, P.: Carbon nanotube prepared by catalytic pyrolysis as the electrode for supercapacitors from polypropylene wasted face masks. Ionics, 1–12 (2022)
Veksha, A.; Yin, K.; Moo, J.G.S.; Oh, W.D.; Ahamed, A.; Chen, W.Q.; Weerachanchai, P.; Giannis, A.; Lisak, G.: Processing of flexible plastic packaging waste into pyrolysis oil and multi-walled carbon nanotubes for electrocatalytic oxygen reduction. J. Hazard. Mater. 387, 121256 (2020)
Acomb, J.C.; Wu, C.; Williams, P.T.: The use of different metal catalysts for the simultaneous production of carbon nanotubes and hydrogen from pyrolysis of plastic feedstocks. Appl. Catal. B Environ. 180, 497–510 (2016)
Feng, M.; Luo, Z.H.; Cao, G.P.; Lu, H.: Tunable growth of carbon nanotubes forests on nickel foam as structured support for palladium catalyst toward polystyrene hydrogenation. J. Taiwan Inst. Chem. Eng. 97, 119–127 (2019)
Yao, D.; Li, H.; Dai, Y.; Wang, C.H.: Impact of temperature on the activity of Fe-Ni catalysts for pyrolysis and decomposition processing of plastic waste. Chem. Eng. J. 408, 127268 (2021)
Acomb, J.C.; Wu, C.; Williams, P.T.: Effect of growth temperature and feedstock: catalyst ratio on the production of carbon nanotubes and hydrogen from the pyrolysis of waste plastics. J. Anal. Appl. pyrolysis 113, 231–238 (2015)
Liu, J.; Jiang, Z.; Yu, H.; Tang, T.: Catalytic pyrolysis of polypropylene to synthesize carbon nanotubes and hydrogen through a two-stage process. Polym. Degrad. Stability 96(10), 1711–1719 (2011)
Henkel and Plastic Bank open first collection centers in Egypt, https://www.henkel.com, Last visited 16.7.2022
Mabood, F.; Shah, J.; Jan, M.R.: Catalytic conversion of waste low density polyethylene into valuable products. J. Chem. Soc. Pakistan 32(5), 574–581 (2010)
Ng, H.M.; Saidi, N.M.; Omar, F.S.; Ramesh, K.; Ramesh, S; Bashir, S.: Thermogravimetric analysis of polymers. Encycloped. Polym. Sci. Technol. pp.1–29 (2002)
Singh, R.K.; Ruj, B.: Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel 174, 164–171 (2016)
Xue, Y.; Johnston, P.; Bai, X.: Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 142, 441–451 (2017)
Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P.: Impact of fast and slow pyrolysis on the degradation of mixed plastic waste: product yield analysis and their characterization. J. Energy Inst. 92(6), 1647–1657 (2019)
López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A.: Pyrolysis of municipal plastic wastes: influence of raw material composition. Waste Manag. 30(4), 620–627 (2010)
Yan, G.; Jing, X.; Wen, H.; Xiang, S.: Thermal cracking of virgin and waste plastics of PP and LDPE in a semibatch reactor under atmospheric pressure. Energy Fuels 29(4), 2289–2298 (2015)
Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W.: Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy 12(7), 663–671 (2015)
Jarrah, N.A.; van Ommen, J.G.; Lefferts, L.: Mechanistic aspects of the formation of carbon-nanofibers on the surface of Ni foam: a new microstructured catalyst support. J. Catal. 239(2), 460–469 (2006)
Chen, D.; Christensen, K.O.; Ochoa-Fernández, E.; Yu, Z.; Tøtdal, B.; Latorre, N.; Monzón, A.; Holmen, A.: Synthesis of carbon nanofibers: effects of Ni crystal size during methane decomposition. J. Catal. 229(1), 82–96 (2005)
Gong, J.; Liu, J.; Wan, D.; Chen, X.; Wen, X.; Mijowska, E.; Jiang, Z.; Wang, Y.; Tang, T.: Catalytic carbonization of polypropylene by the combined catalysis of activated carbon with Ni2O3 into carbon nanotubes and its mechanism. Appl. Catal. A Gen. 449, 112–120 (2012)
Zhang, K.; Xia, X.; Deng, S.; Zhong, Y.; Xie, D.; Pan, G.; Wu, J.; Liu, Q.; Wang, X.; Tu, J.: Nitrogen-doped sponge Ni fibers as highly efficient electrocatalysts for oxygen evolution reaction. Nano-micro Lett. 11(1), 1–11 (2019)
Aboul-Enein, A.A.; Awadallah, A.E.: Impact of Co/Mo ratio on the activity of CoMo/MgO catalyst for production of high-quality multi-walled carbon nanotubes from polyethylene waste. Mater. Chem. Phys. 238, 121879 (2019)
Moo, J.G.S.; Veksha, A.; Oh, W.D.; Giannis, A.; Udayanga, W.C.; Lin, S.X.; Ge, L.; Lisak, G.: Plastic derived carbon nanotubes for electrocatalytic oxygen reduction reaction: Effects of plastic feedstock and synthesis temperature. Electrochem. Commun. 101, 11–18 (2019)
Tao, P.; Ma, X.B.; Chen, D.Z.; Wang, H.: Pyrolysis of waste plastics: Effect of heating rate on product yields and oil properties. In: Advanced Materials Research, pp. 1–10. Trans Tech Publ, Switzerland (2013)
Hujuri, U.; Ghoshal, A.K.; Gumma, S.: Temperature-dependent pyrolytic product evolution profile for polypropylene. J. Appl. Polym. Sci. 119(4), 2318–2325 (2011)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eldahshory, A.I., Emara, K., Abd-Elhady, M.S. et al. High Quality and Maximizing the Production of CNTs from the Pyrolysis of Waste Polypropylene. Arab J Sci Eng 48, 8655–8666 (2023). https://doi.org/10.1007/s13369-022-07206-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07206-5