Abstract
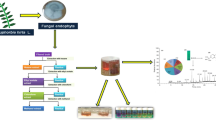
The present study aimed at screening nine soil fungi isolated from Puttaparthi, India for their antioxidant and antibacterial activities. Isolate (SSS-8), with notable antioxidant and antibacterial activities was identified as Periconia sp. using ITS gene sequence and morphology. The crude culture extract of SSS-8 obtained by growing the fungus in Potato Dextrose Broth (PDB) for 14 days at 30 °C and 120 rpm, exhibited DPPH free radical scavenging potential of about 89.62% at 1 mg/mL concentration. SSS-8 also showed promising antibacterial activity against bacteria Staphylococcus aureus, Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA) and moderate activity against Enterococcus faecalis using disk diffusion method at 25 mg/mL concentration. Chemical profiling of the crude culture extract of SSS-8 was carried out using gas chromatography-mass spectrometry (GC–MS). Some of the major compounds identified such as 2,4-di-tert-butylphenol, 1-tetracosanol, 2-phenylethanol, p-menthan-3-one are known to be antibacterial compounds and compounds such as methyl 3,4-dihydroxybenzoate, 1-octadecene, 2,4-di-tert-butylphenol, 1-tetracosanol are potential antioxidative agents. In conclusion, soil fungus Periconia sp. (SSS-8) could be explored as an effective producer of antioxidant and antibacterial compounds for utility in pharmaceutical and nutraceutical applications.




Similar content being viewed by others
References
Xu, L.L.; Cao, F.; Tian, S.S.; Zhu, H.J.: Alkaloids and polyketides from the soil fungus Aspergillus terreus and their antibacterial activities. Chem. Nat. Compd. 53, 1212–1215 (2017). https://doi.org/10.1007/s10600-017-2243-5
Bérdy, J.: Bioactive microbial metabolites. J. Antibiot. (Tokyo) 58, 1–26 (2005). https://doi.org/10.1038/ja.2005.1
Sharma, D.; Pramanik, A.; Kumar, P.: Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D. Don. Biotech 6, 1–14 (2016). https://doi.org/10.1007/s13205-016-0518-3
Feofilova, E.P.: The kingdom fungi : heterogeneity of physiological and biochemical properties and relationships with plants, animals and prokaryotes. Review 37, 124–137 (2001)
Mohamed, H.F.: Molecular analysis and anticancer properties of two identified isolates, Fusarium solani and Emericella nidulans isolated from Wady El–Natron soil in Egypt against Caco–2 (ATCC) cell line. Asian Pac J Trop Biomed 2, 863–869 (2012). https://doi.org/10.1016/S2221-1691(12)60244-5
Abdel-Aziz, M.S.; Ghareeb, M.A.; Saad, A.M.; Refahy, L.A.; Hamed, A.A.: Chromatographic isolation and structural elucidation of secondary metabolites from the soil-inhabiting fungus Aspergillus fumigatus 3T-EGY. Acta Chromatogr 30, 243–249 (2018). https://doi.org/10.1556/1326.2017.00329
Strobel, G.A.: Endophytes as sources of bioactive products. Microbes Infect 5, 535–544 (2003). https://doi.org/10.1016/S1286-4579(03)00073-X
Pham-Huy, L.A.; He, H.; Pham-Huy, P.C.: Free radicals, antioxidants in disease and health. Int J Biomed Sci 4, 89–96 (2008)
Behera, B.C.; Verma, N.; Sonone, A.; Makhija, U.: Determination of antioxidative potential of lichen Usnea ghattensis in vitro. LWT Food Sci Technol 39, 80–85 (2006). https://doi.org/10.1016/j.lwt.2004.11.007
Patil, M.P.; Patil, R.H.; Maheshwari, V.L.: Biological activities and identification of bioactive metabolite from endophytic Aspergillus flavus L7 Isolated from Aegle marmelos. Curr Microbiol 71, 39–48 (2015). https://doi.org/10.1007/s00284-015-0805-y
Gunasekaran, R.; Janakiraman, D.; Rajapandian, S.G.K.; Appavu, S.P.; Namperumalsamy Venkatesh, P.; Prajna, L.: Periconia species - an unusual fungal pathogen causing mycotic keratitis. Indian J Med Microbiol 39, 36–40 (2021). https://doi.org/10.1016/j.ijmmb.2020.10.006
Markovskaja, S.; Kačergius, A.: Morphological and molecular characterisation of Periconia pseudobyssoides sp. nov. and closely related P. byssoides. Mycol Prog 13, 291–302 (2014). https://doi.org/10.1007/s11557-013-0914-6
Zhang, D.; Tao, X.; Chen, R.; Liu, J.; Li, L.; Fang, X.; Yu, L.; Dai, J.: Pericoannosin A, a polyketide synthase-nonribosomal peptide synthetase hybrid metabolite with new carbon skeleton from the endophytic fungus periconia sp. Org Lett 17, 4304–4307 (2015). https://doi.org/10.1021/acs.orglett.5b02123
Teles, H.L.; Sordi, R.; Silva, G.H.; Castro-Gamboa, I.; da Silva Bolzani, V.; Pfenning, L.H.; de Abreu, L.M.; Costa-Neto, C.M.; Young, M.C.; Araújo, Â.R.: Aromatic compounds produced by Periconia atropurpurea, an endophytic fungus associated with Xylopia aromatica. Phytochemistry 67, 2686–2690 (2006). https://doi.org/10.1016/j.phytochem.2006.09.005
Shin DS, Oh MN, Yang HC, Oh KB (2005) Biological characterization of periconicins, bioactive secondary metabolites, produced by Periconia sp. OBW 15
Subba Rao, N.; John Devadas, D.: Fluoride incidence in groundwater in an area of Peninsular India. Environ Geol 45, 243–251 (2003). https://doi.org/10.1007/s00254-003-0873-3
Waksman, S.A.: A method for counting the number of fungi in the soil. J Bacteriol 7, 339–33941 (1922)
Arora, D.S.; Chandra, P.: Antioxidant activity of Aspergillus fumigatus. ISRN Pharmacol 2011, 1–11 (2011). https://doi.org/10.5402/2011/619395
Brand-Williams, W.; Cuvelier, M.E.; Berset, C.: Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28, 25–30 (1995). https://doi.org/10.1016/S0023-6438(95)80008-5
Kumar, R.; Sukhvinder, S.; Purewal, S.: Phenolic content, antioxidant potential and DNA damage protection of pearl millet (Pennisetum glaucum) cultivars of North Indian region. J Food Meas Charact 11, 126–133 (2017). https://doi.org/10.1007/s11694-016-9379-z
Balouiri, M.; Sadiki, M.; Ibnsouda, S.K.: Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6, 71–79 (2016). https://doi.org/10.1016/j.jpha.2015.11.005
Monggoot, S.; Pichaitam, T.; Tanapichatsakul, C.; Pripdeevech, P.: Antibacterial potential of secondary metabolites produced by Aspergillus sp., an endophyte of Mitrephora wangii. Arch Microbiol 200, 951–959 (2018). https://doi.org/10.1007/s00203-018-1511-5
Zhang, Y.J.; Zhang, S.; Liu, X.Z.; Wen, H.A.; Wang, M.: A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett Appl Microbiol (2010). https://doi.org/10.1111/j.1472-765X.2010.02867.x
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols. pp. 315–322. Elsevier
Cantrell, S.A.; Hanlin, R.T.; Emiliano, A.: Periconia variicolor sp. nov., a new species from Puerto Rico. Mycologia 99, 482–487 (2007). https://doi.org/10.1080/15572536.2007.11832573
Arora, D.S.; Chandra, P.: In vitro antioxidant potential of some soil fungi: screening of functional compoundsand their purification from penicillium citrinum. Appl Biochem Biotechnol 165, 639–651 (2011). https://doi.org/10.1007/s12010-011-9282-3
Gao, W.; Chai, C.; He, Y.; Li, F.; Hao, X.; Cao, F.; Gu, L.; Liu, J.; Hu, Z.; Zhang, Y.: Periconiastone a, an antibacterial ergosterol with a pentacyclo heptadecane system from Periconia sp. TJ403-rc01. Org Lett 21, 8469–8472 (2019). https://doi.org/10.1021/acs.orglett.9b03270
Kim, S.; Shin, D.S.; Lee, T.; Oh, K.B.: Periconicins, two new fusicoccane diterpenes produced by an endophytic fungus Periconia sp. with antibacterial activity. J Nat Prod 67, 448–450 (2004). https://doi.org/10.1021/np030384h
Zhu, Y.-J.; Zhou, H.-T.; Hu, Y.-H.; Tang, J.-Y.; Su, M.-X.; Guo, Y.-J.; Chen, Q.-X.; Liu, B.: Antityrosinase and antimicrobial activities of 2-phenylethanol, 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem 124, 298–302 (2011). https://doi.org/10.1016/j.foodchem.2010.06.036
Haiyan, G.; Lijuan, H.; Shaoyu, L.; Chen, Z.; Ashraf, M.A.: Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi J Biol Sci 23, 524–530 (2016). https://doi.org/10.1016/j.sjbs.2016.02.020
Aissaoui, N.; Mahjoubi, M.; Nas, F.; Mghirbi, O.; Arab, M.; Souissi, Y.; Hoceini, A.; Masmoudi, A.S.; Mosbah, A.; Cherif, A.; Klouche-Khelil, N.: Antibacterial potential of 2,4-Di-tert-butylphenol and calixarene-based prodrugs from thermophilic Bacillus licheniformis isolated in Algerian hot spring. Geomicrobiol J 36, 53–62 (2019). https://doi.org/10.1080/01490451.2018.1503377
Tsuda, T.; Watanabe, M.; Ohshima, K.; Yamamoto, A.; Kawakishi, S.; Osawa, T.: Antioxidative components isolated from the seed of tamarind (Tamarindus indica L.). J Agric Food Chem 42, 2671–2674 (1994). https://doi.org/10.1021/jf00048a004
Yoon, M.; Jeong, T.; Park, D.; Ming-zhe, X.; Hyun-Woo, O.; Kyoung-Bin, S.; Woo Song, L.; Ho-Yong, P.: Antioxidant effects of quinoline alkaloids and 2,4-Di-tert-butylphenol isolated from Scolopendra subspinipes. Biol Pharm Bull 29, 735–739 (2006)
Tonisi, S.; Okaiyeto, K.; Hoppe, H.; Mabinya, L.V.; Nwodo, U.U.: Chemical constituents, antioxidant and cytotoxicity properties of Leonotis leonurus used in the folklore management of neurological disorders in the Eastern Cape, South Africa. 3 Biotech 10, 1–14 (2020). https://doi.org/10.1007/s13205-020-2126-5
Faridha Begum, I.; Mohankumar, R.; Jeevan, M.; Ramani, K.: GC–MS analysis of bio-active molecules derived from Paracoccus pantotrophus FMR19 and the antimicrobial activity against bacterial pathogens and MDROs. Indian J. Microbiol. 56, 426–432 (2016). https://doi.org/10.1007/s12088-016-0609-1
Acknowledgements
The authors dedicate this work to Bhagawan Sri Sathya Sai Baba, Founder Chancellor of the Sri Sathya Sai Institute of Higher Learning. The authors acknowledge UGC-SAP (DRS) and DST-FIST, Government of India for the infrastructural support to the Department of Biosciences, SSSIHL, Prasanthi Nilayam. Skanda S is thankful to UGC, New Delhi, for the award of UGC-BSR fellowship. The authors thank Dr Pradeep BE, Department of Biosciences, Sri Sathya Sai Institute of Higher Learning, for providing the bacterial cultures used in the study.
Author information
Authors and Affiliations
Contributions
SS carried out the laboratory work and drafted the manuscript; VBS supervised the study design and reviewed the manuscript. The authors approved the submission of the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
Skanda S and Vijayakumar B S declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Skanda, S., Vijayakumar, B.S. Antioxidant and antibacterial potential of crude extract of soil fungus Periconia sp. (SSS-8). Arab J Sci Eng 47, 6707–6714 (2022). https://doi.org/10.1007/s13369-021-06061-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-06061-0