Abstract
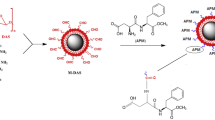
In our study, medium conditions were optimized for percent chemical oxygen demand (COD) reduction and drug removal from diclofenac sodium (DFS) solutions. Response surface methodology/central composite design was used for optimization. A. Campestris/Amberlite Styrene–divinylbenzene (XAD-4) biocomposite material was used as adsorbent. Four independent parameters (pH, initial concentration, interaction time and adsorbent amount) were chosen to optimize both % COD reduction and DFS removal. As a result of experiments, maximum 77% COD reduction and maximum 98% DFS removal were obtained at 4 pH, 225 mg/L initial concentration, 36 min and 0.69 adsorbent amount. Scanning electron microscope and Fourier transform infrared spectroscopy devices were used for characterization of adsorbent material. To identify the isotherm for the adsorption mechanism, the Langmuir, Freundlich, Temkin and Harkins–Jura isotherm equations were examined. The Freundlich isotherm had 96.2% regression coefficient (R2) and was linear, so had better fit compared to the other equations and the adsorption mechanism abided by the Freundlich isotherm. The results show that statistical optimization design was successfully applied to experiments and A. Campestris/Amberlite XAD-4 is an appropriate biocomposite adsorbent with specific affinity for % COD reduction and removal of DFS from aqueous solutions under optimal conditions.






Similar content being viewed by others
References
Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Kang, J.; Price, W.E.: Removal and fate of micropollutants in a sponge-based moving bed bioreactor. Biol. Technol. 159, 311–319 (2014)
Domaradzka, D.; Guzik, U.; Wojcieszyńska, D.: Biodegradation and biotransformation of polycyclic non-steroidal anti-inflammatory drugs. Rev. Environ. Sci. Bio/Technol. 14, 229–239 (2015)
Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H.: Biological degradation of pharmaceuticals in municipal wastewater treatment: proposing a classification scheme. Water Res. 40, 1686–1696 (2006)
Balcı, B.; Erkuş, A.; Erkuş, F.Ş: Farmasötik bileşiklerin sucul ortamda bulunuşu ve etkileri. Res. J. Biol. Sci. 3, 13–19 (2010)
Alvarino, T.; Suarez, S.; Lema, J.M.; Omil, F.: Understanding the removal mechanisms of PPCPs and the influence of main technological parameters in anaerobic UASB and aerobic CAS reactors. J. Hazard. Mater. 278, 506–513 (2014)
Daneshvar, A.; Svanfelt, J.; Kronberg, L.; Weyhenmeyer, G.A.: Winter accumulation of acidic pharmaceuticals in a Swedish river. Environ. Sci. Pollut. Res. 17, 908–916 (2010)
Sharma, H.R.; Trivedi, R.C.; Akolkar, P.; Gupta, A.: Micropollutants levels in macroinvertebrates collected from drinking water sources of Delhi, India. Int. J. Environ. Stud. 60(2), 99–110 (2003)
Barbieri, M.; Carrera, J.; Ayora, C.; Sanchez-Vila, X.; Licha, T.; Nödler, K.; Osorio, V.; Peres, S.; Köck-Schulmeyer, M.; Lopez-deAlda, M.; Barcelo, D.: Formation of diclofenac and sulfamethoxazole reversible transformation products in aquifer material under denitrifying conditions: batch experiments. Sci. Total Environ. 426, 256–63 (2012)
Nas, B.; Dolu, T.; Ateş, H.; Argun, M.E.; Yel, E.: Treatment alternatives for micropollutant removal in wastewater. Selçuk Üniversitesi Mühendislik Fakültesi Dergisi 5(2), 133–143 (2017)
Caban, M.; Lis, E.; Kumirska, J.; Stepnowski, P.: Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS (SIM) method based on Speedisk extraction disks and DIMETRIS derivatization. Sci. Total Environ. 538, 402–411 (2015)
Maia, G.S.; de Andrade, J.R.; da Silva, M.G.C.; Vieira, M.G.A.: Adsorption of diclofenac sodium onto commercial organoclay: kinetic, equilibrium and thermodynamic study. Powder Technol. 345, 140–150 (2019)
Bhadra, B.N.; Seo, P.W.; Jhung, S.H.: Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem. Eng. J. 301, 27–34 (2016)
Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, A.; Jarosova, B.: EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 47(17), 6475–6487 (2013)
Chen, J.B.; Gao, H.W.; Zhang, Y.L.; Zhang, Y.; Zhou, X.F.; Lı, Ch.Q.; Gao, H.P.: Developmental toxicity of diclofenac and elucidation of gene regulation in zebrafish (Danio rerio). Sci. Rep. 4, 4841 (2014)
Memmert, U.; Peither, A.; Burri, R.; Weber, K.; Schmidt, T.; Sumpter, J.P.H.; A. : Diclofenac: new data on chronic Toxicity and bioconcentration in fish. Environ. Toxicol. Chem. 32(2), 442–452 (2013)
Yerüstü Su Kalitesi Yönetmeliği (YSKY) RG Tarihi:10.8.2016, R.G. Sayısı:29797 Çevre ve Şehircilik Bakanlığı, Ankara
Kim, I.; Tanaka, H.: Photodegradation characteristics of PPCPs in water with UV treatment. Environ. Int. 35(5), 793–802 (2009)
Erkuş, A.; Başıbüyük, M.; Erkuş, F.Ş: The examination of paracetamol and diclofenac removal in activated sludge systems under different operating conditions. Int. J. Ecosyst. Ecol. Sci. 5, 315–320 (2015)
Da Silva, T.H.; Furtado, R.X.D.S.; Zaiat, M.; Azevedo, E.B.: Tandem anaerobic-aerobic degradation of ranitidine, diclofenac, and simvastatin in domestic sewage. Sci. Total Environ. 721, 137589 (2020)
Suarez, S.; Lema, J.M.; Omil, F.: Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour. Technol. 100(7), 2138–2146 (2009)
Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D.: Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 42(14), 3601–3610 (2008)
Boyd, G.R.; Zhang, S.; Grimm, D.A.: Naproxen removal from water by chlorination and biofilm processes. Water Res. 39, 668–676 (2005)
Esplugas, S.; Bila, D.M.; Gustavo, L.; Krause, T.; Dezotti, M.: Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 149, 631–642 (2007)
Souza, F.S.; Da Silva, V.V.; Rosin, C.K.; Hainzenreder, L.; Arenzon, A.; Pizzolato, T.; Féris, L.A.: Determination of pharmaceutical compounds in hospital wastewater and their elimination by advanced oxidation processes. J. Environ. Sci. Health Part A 53(3), 213–221 (2018)
Vergili, I.: Application of nano filtration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources. J. Environ. Manag. 127, 177–187 (2013)
Alvarez, T.S.; Munoz, M.; Zazo, A.J.; Casas, A.J.; García, J.: Synthesis of high surface area carbon adsorbents prepared from pine sawdust-Onopordum acanthium L. for nonsteroidal anti-inflammatory drugs adsorption. J. Environ. Manag. 183, 294–305 (2016)
Franco, A.M.; De Carvalho, C.B.; De Bonetto, M.M.; Soares, R.D.P.; Féris, L.A.: Diclofenac removal from water by adsorption using activated carbon in batch mode and fixed-bed column: isotherms, thermodynamic study and breakthrough curves modeling. J. Clean. Prod. 181, 145–154 (2018)
Bhatnagar, A.; Minocha, A.K.: Conventional and non-conventional adsorbents for removal of pollutants from water—a review. Indian J. Chem. Technol. 13, 203–217 (2006)
Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Wang, H.; Li, H.A.: facile band alignment of polymeric carbon nitride isotype heterojunctions for enhanced photocatalytic tetracycline degradation. Environ. Sci. Nano. 5(11), 2604–2617 (2018)
Vona, A.; Martino, F.; Garcia-Ivars, J.; Picó, Y.; Mendoza-Roca, J.A.; Iborra-Clar, M.I.: Comparison of different removal techniques for selected pharmaceuticals. J. Water Process Eng. 5, 48–57 (2015)
Ahmed, M.J.; Hameed, B.H.: Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: a review. Ecotoxicol. Environ. Saf. 149, 257–266 (2018)
Rad, L.R.; Irani, M.; Barzegar, R.: Adsorptive removal of acetaminophen and diclofenac using NaX nanozeolites synthesized by microwave method. Korean J. Chem. Eng. 32(8), 1606–1612 (2015)
Shirmardi, M.; Alavi, N.; Lima, E.C.; Takdastan, A.; Mahvi, A.H.; Babaei, A.A.: Removal of atrazine as an organic micro-pollutant from aqueous solutions: a comparative study. Process. Saf. Environ. Prot. 103, 23–35 (2016)
Suna, K.; Shia, Y.; Wang, X.; Li, Z.: Sorption and retention of diclofenac on zeolite in the presence of cationic surfactant. J. Hazard. Mater. 323, 584–592 (2017)
Jin, X.; Zheng, M.; Sarkar, B.; Naidu, R.; Chen, Z.: Characterization of bentonite modified with humic acid for the removal of Cu(II) and 2,4-dichlorophenol from aqueous solution. Appl. Clay. Sci. 134, 89–94 (2016)
Rakic, V.; Rajic, N.; Dakovic, A.; Auroux, A.: The adsorption of salicylic acid, acetylsalicylic acid and atenolol from aqueous solutions onto natural zeolites and clays: clinoptilolite, bentonite and kaolin. Micropor. Mesopor. Mater. 166, 185–194 (2013)
Yendluri, R.; Otto, D.P.; De Villiers, M.M.; Vinokurov, V.; Lvov, Y.M.: Application of halloysite clay nanotubes as a pharmaceutical excipient. Int. J. Pharm. 521(1–2), 267–273 (2017)
Leng, L.; Yuan, X.; Zeng, G.; Shao, J.; Chen, X.; Wu, Z.; Peng, X.: Surface characterization of rice husk bio-char produced by liquefaction and application for cationic dye (Malachite green) adsorption. Fuel 155, 77–85 (2015)
Umar, M.S.; Jennings, P.; Urmee, T.: Sustainable electricity generation from oil palm biomass wastes in Malaysia: an industry survey. Energy 67, 496–505 (2014)
Ince, M.; Ince, O.K.; Yonten, V.; Karaaslan, N.M.: Nickel, lead, and cadmium removal using a low-cost adsorbent-banana peel. At. Spectrosc. 37(3), 125–130 (2016)
Portinho, R.; Zanella, O.; Feris, L.A.: Grape stalk application for caffeine removal through adsorption. J. Environ. Manag. 202, 178–187 (2017)
Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.: Adsorptive removal of antibiotics from water and wastewater: progress and challenges. Sci. Total Environ. 532, 112–126 (2015)
Walcarius, A.; Mercier, L.: Mesoporous organosilica adsorbents: nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 20, 4478–4511 (2010)
Delgado, L.F.; Charles, P.; Glucina, K.; Morlay, C.: The removal of endocrine disrupting compounds, pharmaceutically activated compounds and cyanobacterial toxins during drinking water preparation using activated carbon—a review. Sci. Total Environ. 435, 509–525 (2012)
Hasan, Z.; Jhung, S.H.: Removal of hazardous organics from water using metalorganic frameworks (MOFs): plausible mechanisms for selective adsorptions. J. Hazard. Mater. 283, 329–339 (2015)
Manyangadze, M.; Chikuruwu, N.H.M.; Narsaiah, B.T.; Chakra, C.S.; Radhakumari, M.; Danha, G.: Enhancing adsorption capacity of nano-adsorbents via surface modification: a review. S. Afr. J. Chem. Eng. 31, 25–32 (2020)
Zhao, W.; Chen, I.W.; Huang, F.: Toward large-scale water treatment using nanomaterials. Nano Today 27, 11–27 (2019)
Gengec, E.; Kobya, M.; Demirbas, E.; Akyol, A.; Oktor, K.: Optimization of baker’s yeast wastewater using response surface methodology by electrocoagulation. Desalination 286, 200–209 (2012)
Myers, R.H.; Montgomery, D.C.: Response surface methodology: process and product optimization using designed experiments. Wiley, New York (1995)
Yonten, V.; Tanyol, M.; Yildirim, N.; Yildirim, N.C.; Ince, M.: Optimization of Remazol Brilliant Blue R dye removal by novel biosorbent P. eryngii immobilized on Amberlite XAD-4 using response surface methodology. Desalin. Water Treat. 57(33), 15592–15602 (2020)
Dharmadhikari, D.M.; Vanerkar, A.P.; Barhate, N.M.: Chemical oxygen demand using closed microwave digestion system. Environ. Sci. Technol. 39, 6198–6201 (2005)
Hiew, B.Y.Z.; Lee, L.Y.; Lai, K.C.; Gan, S.; Thangalazhy-Gopakumar, S.; Pan, G.T.; Yang, T.C.K.: Adsorptive decontamination of diclofenac by three-dimensional graphene-based adsorbent: response surface methodology, adsorption equilibrium, kinetic and thermodynamic studies. Environ. Res. 168, 241–253 (2019)
Şimşek, Y.: Sulu Çözeltiden Bakır (II) Adsorpsiyon Sürecinin Optimizasyonunda Yüzey Yanıt Metodolojisinin Uygulanması. Akademik Platform Mühendislik ve Fen Bilimleri Dergisi 6(3), 182–191 (2018)
Brdjanovic, D.; van Loosdrecht, M.C.; Versteeg, P.; Hooijmans, C.M.; Alaerts, G.J.; Heijnen, J.J.: Modeling COD, N and P removal in a full-scale WWTP Haarlem Waarderpolder. Water Res. 34, 846–858 (2000)
Yazdanbakhsh, A.R.; Mohammadi, A.S.; Sardar, M.; Godini, H.; Almasian, M.: COD removal from synthetic wastewater containing azithromycin using combined coagulation and a Fenton-like process. Environ. Eng. Manag. J. 13(12), 2929–2936 (2014)
Yonten, V.; Alp, H.; Yildirim, N.; Yildirim, N.C.; Ogedey, A.: Investigation of optimum conditions for efficient COD reduction in synthetic sulfamethazine solutions by Pleurotus eryngii var. ferulae using response surface methodology. J. Taiwan Inst. Chem. Eng. 80, 349–355 (2017)
Khellouf, M.; Chemini, R.; Salem, Z.; Khodja, M.; Zeriri, D.: Parametric study of COD reduction from textile processing wastewater using adsorption on cypress cone-based activated carbon: an analysis of a Doehlert response surface design. Arab. J. Sci. Eng. 44(12), 10079–10086 (2019)
Teğin, İ; Akdeniz, S.: Amberlit XAD-4 Polimerinin Biyosorbent Katkı Malzemesi Badem Kabuğu Kullanılarak Sulu Çözeltiden Cd Giderilmesi. Akademik Platform Mühendislik ve Fen Bilimleri Dergisi 5(2), 1–14 (2017)
Yargıç, A.S.; Yarbay Şahin, R.Z.; Özbay, N.; Önal, E.: Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. J. Clean. Prod. 88, 152–159 (2015)
García, A.V.; Santonja, M.R.; Sanahuja, A.B.; Selva, M.D.C.G.: Characterization and degradation characteristics of poly (ε-caprolactone)-based composites reinforced with almond skin residues. Polym. Degrad. Stabil. 108, 269–279 (2014)
Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C.: Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: kinetic and equilibrium studies. Chem. Eng. J. 260, 291–299 (2015)
Jacques, R.A.; Lima, E.C.; Dias, S.L.; Mazzocato, A.C.; Pavan, F.A.: Yellow passion-fruit shell as biosorbent to remove Cr(III) and Pb(II) from aqueous solution. Sep. Purif. Technol. 57(1), 193–198 (2007)
Korkmaz, K.: Yeni bir gıda atığı kullanarak sulu çözeltiden biyosorpsiyon metoduyla bazı kirliliklerin giderimi. Master's thesis, Batman Üniversitesi Fen Bilimleri Enstitüsü (2019)
Dos Santos, G.E.D.S.; Ide, A.H.; Duarte, J.L.S.; McKay, G.; Silva, A.O.S.; Meili, L.: Adsorption of anti-inflammatory drug diclofenac by MgAl/layered double hydroxide supported on Syagrus coronata biochar. Powder Technol. 364, 229–240 (2020)
Ozturk, D.; Dagdas, E.; Fil, B.F.; Bashir, M.J.K.: Central composite modeling for electrochemical degradation of paint manufacturing plant wastewater: one-step/two-response optimization. Environ. Technol. Innov. 5, 5–6 (2020). https://doi.org/10.1016/j.eti.2020.101264
Melgoza, B.; León-Santiesteban, H.H.; López-Medina, R.; Tomasini, A.: Naproxen sorption by non-viable rhizopus oryzae biomass. Water Air Soil Pollut. 231, 30 (2020)
Rosset, M.; Sfreddo, L.W.; Hidalgo, G.E.N.; Perez-Lopez, O.W.; Féris, L.A.: Adsorbents derived from hydrotalcites for the removal of diclofenac in wastewater. Appl. Clay Sci. 175, 150–158 (2019)
Dos Santos, J.M.; Pereira, C.R.; Foletto, E.L.; Dotto, G.L.: Alternative synthesis for ZnFe2O4/chitosan magnetic particles to remove diclofenac from water by adsorption. Int. J. Biol. Macromol. 131, 301–308 (2019)
Zhao, Y.; Liu, F.; Qin, X.: Adsorption of diclofenac onto goethite: adsorption kinetics and effects of pH. Chemosphere 180, 373–378 (2017)
Larous, S.; Meniai, A.H.: Adsorption of diclofenac from aqueous solution using activated carbon prepared from olive Stones. J. Hydrog. Energy 41, 10380–10390 (2016)
Darajeh, N.; Idris, A.; Masoumi, H.R.F.; Nourani, A.; Truong, P.; Sairi, N.A.: Modeling BOD and COD removal from palm oil mill secondary effluent in floating wetland by Chrysopogon zizanioides (L.) using response surface methodology. J. Environ. Manag. 181, 343 (2016)
Kousha, M.; Daneshvar, E.; Dopeikar, H.; Taghavi, D.; Bhatnagar, A.: Box–Behnken design optimization of Acid Black 1 dye biosorption by different brown macroalgae. Chem. Eng. J. 179, 158–168 (2012)
Erguven, G.; Yildirim, N.: Efficiency of some bacteria for chemical oxygen demand reduction of synthetic chlorsulfuron solutions under agiated culture conditions. Cell. Mol. Biol. 62, 92 (2016)
Freundlich, H.M.F.: Over the adsorption in solution. J. Phys. Chem. 57(385471), 1100–1107 (1906)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40(9), 1361–1403 (1918)
Temkin, M.; Pyzhev, V.: Kinetics of ammonia synthesis on promoted iron catalysts. Acta Phys. Chem. URSS 12, 327–356 (1940)
Jura, G.; Harkins, W.D.: Surfaces of solids. XIV. A unitary thermodynamic theory of the adsorption of vapors on solids and of insoluble flims on liquid subphases. J. Am. Chem. Soc. 68(10), 1941–1952 (1946)
Antunes, M.; Esteves, V.I.; Guégan, R.; Crespo, J.S.; Fernandes, A.N.; Giovanela, M.: Removal of diclofenac sodium from aqueous solution by Isabel grape bagasse. Chem. Eng. J. 192, 114–121 (2012)
Jauris, I.M.; Matos, C.F.; Saucier, C.; Lima, E.C.; Zar-bin, A.J.G.; Fagan, S.B.; Machado, F.M.; Zanella, I.: Adsorption of sodium diclofenac on graphene: a combined experimental and theoretical study. Phys. Chem. Chem. Phys. 18(3), 1526–1536 (2016). https://doi.org/10.1039/c5cp05940b
Kaur, M.; Datta, M.: Diclofenac sodium adsorption onto montmorillonite: adsorption equilibrium studies and drug release kinetics. Adsorpt. Sci. Technol. 32(5), 365–387 (2014)
Larous, S.; Meniai, A.H.: Adsorption of Diclofenac from aqueous solution using activated carbon prepared from olive stones. Int. J. Hydrogen Energy 41(24), 10380–10390 (2016)
Jodeh, S.; Abdelwahab, F.; Jaradat, N.; Warad, I.; Jodeh, W.: Adsorption of diclofenac from aqueous solution using Cyclamen persicum tubers based activated carbon (CTAC). J. As-Soc. Arab Uni. Basic Appl. Sci. 20(1), 32 (2016)
Wu, L.; Du, C.; He, J.; Yang, Z.; Li, H.: Effective adsorption of diclofenac sodium from neutral aqueous solution by low-cost lignite activated cokes. J. Hazard. Mater. 384, 121284 (2020)
Malhotra, M.; Suresh, S.; Garg, A.: Tea waste derived activated carbon for the adsorption of sodium diclofenac from wastewater: adsorbent characteristics, adsorption isotherms, kinetics, and thermodynamics. Environ. Sci. Pollut. Res. 25(32), 32210–32220 (2018)
de Luna, M.D.G.; Budianta, W.; Rivera, K.K.P.; Arazo, R.O.: Removal of sodium diclofenac from aqueous solution by adsorbents derived from cocoa pod husks. J. Environ. Chem. Eng. 5(2), 1465–1474 (2017)
Acknowledgements
The authors would like to sincerely express their gratitude to Yuzuncu Yil University (Faculty of Engineering Research Laboratories) for their analytical assistance in carrying out our laboratory studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özgüven, A., Yönten, V. & Kıvanç, M.R. The Utilization of a Statistical Program for Chemical Oxygen Demand Reduction and Diclofenac Sodium Removal from Aqueous Solutions via Agaricus campestris/Amberlite Styrene Divinylbenzene Biocomposite. Arab J Sci Eng 47, 441–454 (2022). https://doi.org/10.1007/s13369-021-05667-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05667-8