Abstract
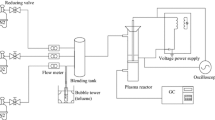
Removal of nitrogen oxides (NO\(_x\)) from a cycled adsorption–desorption and decomposition system was studied at ambient temperature, which allows low concentration and high flow rate emissions. This system exhibited excellent recyclability and showed high performance through repeated cycling, with 85.4% NO conversion after eight cycles. Different metal oxide supports on activated carbon were used to remove NO by nonthermal plasma assisted catalytic adsorption–decomposition. The Cu/AC showed a large adsorption capacity, and copper ions increased the decomposition of NO, resulting in high energy efficiency. FTIR and TPD results showed that NO adsorption on the catalyst surface states is mainly as nitrate. The textural characteristics of catalysts for cyclic operation were investigated, and the catalytic activity relied on O atoms, Cu atoms and \(\hbox {Cu}^{2+}\) on the catalyst surface. The discharge voltages and several frequencies of the electric source were also investigated. As injection power energy increased, the reactor plasma chemical reaction process was increased.
Similar content being viewed by others
References
Zhang, J.; Huang, Y.; Chen, X.: Selective catalytic oxidation of NO over iron and manganese oxides supported on mesoporous silica. J. Nat. Gas Chem. 7, 273–277 (2008)
Kim, H.H.; Ogata, A.; Futamura, S.: Oxygen partial pressure-dependent behavior of various catalysts for the total oxidation of VOCs using cycled system of adsorption and oxygen plasma. Appl. Catal. B 79, 356–367 (2008)
Zhao, D.; Li, X.; Shi, C.; et al.: Low-concentration formaldehyde removal from air using a cycled storage-discharge (CSD) plasma catalytic process. Chem. Eng. Sci. 66, 3922–3929 (2011)
Wang, F.; Tang, X.L.; Yi, H.H.; et al.: NO removal in the process of adsorption-NTP catalytic decomposition. RSC Adv. 4, 8502–8509 (2014)
Helen, H.Y.; Yang, R.T.: Removal of NO by reversible adsorption on Fe–Mn based transition metal oxides. Langmuir 17, 4997–5003 (2001)
Wang, M.X.; Huang, Z.H.; Takaaki, S.; et al.: NO removal by electrospun porous carbon nanofibers at room temperature. Chem. Eng. J. 170, 505–511 (2001)
Brandenberger, S.; KrÖcher, O.; Tissler, A.; et al.: The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by \({\rm NH}_{3}\). Appl. Catal. B 95, 348–357 (2010)
Wang, X.Q.P.; Ning, Y.; et al.: Adsorption of low concentration phosphine in yellow phosphorus off-gas by impregnated activated carbon. J. Hazard. Mater. 171, 588–593 (2009)
GB13223-2011. Emission standard of air pollutants for thermal power plants of China.
Xu, X.L.; Chen, Z.H.; Chen, W.K.; et al.: Theoretical study of NO dimer adsorption and dissociation on the CuCr\(_{2}\)O\(_{4}\)(100) surface. Chin. J. Struct. Chem. 27, 927–932 (2008)
Iwamoto, M.; Yoda, Y.; Yamazoe, N.; et al.: Study of metal oxide catalysts by temperature programmed desorption oxygen adsorption on various metal oxides. J. Phys. Chem. 82, 2564–2570 (1978)
Margarita, K.; Ahmet, S.V.: Cobalt supported on zirconia and sulfated zirconia I.: FT-IR spectroscopic characterization of the \({\rm NO}_{x}\) species formed upon NO adsorption and \({\rm NO}/{\rm O}_{2}\) coadsorption. J. Catal. 223, 352–363 (2004)
Wang, Q.; Park, S.Y.; et al.: Co/KxTi\(_{2}\)O\(_{5}\) catalysts prepared by ion exchange method for NO oxidation to NO\(_{2}\). Appl. Catal. B 79, 101–107 (2008)
Valanidou, L.; Theologides, C.; Zorpas, A.A.; et al.: A novel highly selective and stable Ag/MgO \(-\) CeO\(_{2}\)O\(_{3 }\)catalyst for the low-temperature ethanol-SCR of NO. Appl. Catal. B 107, 164–167 (2011)
Liu, J.; Wang, P.H.: Structural characterization of pan-based ACF\(({\rm {I}})\)-change of content and binding state of nitrogen. New Carbon Mater. 14, 48–52 (1999)
Guo, Z.C.; Xie, Y.S.; et al.: Catalytic oxidation of NO to \({\rm NO}_{2}\) on activated carbon. Energy Convers. Manag. 42, 2005–2018 (2001)
Isao, M.; Yozo, K.; Masukai, S.; et al.: Removal of SOx and NOx over activated carbon fibers. Carbon 38, 227–239 (2000)
Kijlstra, W.S.; Brands, D.S.; Poels, E.K.; et al.: Mechanism of the selective catalytic reduction of NO by \({\rm NH}_{3}\) over MnOx/Al\(_{2}\)O\(_{3}\). J. Catal. 171, 208–218 (1997)
Pena, D.A.; Uphade, B.S.; Smimiotis, P.G.: \({\rm TiO}_{2}\)-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with \({\rm NH}_{3}\): I. Evaluation and characterization of first row transition metals. J. Catal. 221, 421–431 (2004)
Machida, M.; Kurogi, D.; Kijima, T.: MnO x -\({\rm CeO}_{2}\) binary oxides for catalytic NOx sorption at low temperatures. Selective reduction of sorbed NOx. Chem. Mater. 12, 3165–3170 (2000)
Zhang, H.; Chu, W.; Xu, H.Y.; Zhou, J.: Plasma-assisted preparation of Fe-Cu bimetal catalyst for higher alcohols synthesis from carbon monoxide hydrogenation. Fuel 89, 3127–3131 (2010)
Oukacine, L.; Gitzhofer, F.; Abatzoglou, N.; Gravelle, D.: Application of the induction plasma to the synthesis of two dimensional steam methane reforming Ni/Al\(_{2}\)O\(_{3}\) catalyst. Sur. Coat. Technol. 201, 2046–2053 (2006)
Hua, W.; Jin, L.J.; He, X.F.; Liu, J.H.; Hu, H.Q.: Preparation of Ni/MgO catalyst for \({\rm CO}_{2}\) reforming of methane by dielectric-barrier discharge plasma. Catal. Commun. 11, 968–972 (2010)
Hueso, J.L.; Espinós, J.P.; Caballero, A.; Cotrinoa, J.; González-Elipea, A.R.: XPS investigation of the reaction of carbon with NO, \({\rm O}_{2}, {\rm N}_{2}\) and \({\rm H}_{2}{\rm O}\) plasmas. Carbon 45, 89–96 (2007)
Snyder, H.R.; Anderson, G.K.: Effect of air and oxygen content on the dielectric barrier discharge decomposition of chlorobenzene. IEEE Trans. Plasma Sci. 26, 1695–1699 (1998)
Zhao, G.B.; Janardhan Garikipati, S.V.B.; Hu, X.D.; et al.: The effect of gas pressure on NO conversion energy efficiency in nonthermal nitrogen plasma. Chem. Eng. Sci. 60, 1927–1937 (2005)
Kim, H.H.; Takashima, K.; Katsura, S.; et al.: Low-temperature NOx reduction processes using combined systems of pulsed corona discharge and catalysts. J. Phys. D Appl. Phys. 34, 604–613 (2001)
Indarto, A.: Partial oxidation of methane to methanol with nitrogen dioxide in dielectric barrier discharge plasma: experimental and molecular modeling. Plasma Sources Sci. Technol. 25, 025002 (2016)
Hur, M.; Lee, J.O.; Lee, J.Y.; Kang, W.S.; et al.: Abatement characteristics of \({\rm N}_{2}{\rm O}\) in low-pressure plasma reactor. Plasma Sources Sci. Technol. 25, 015008 (2016)
Thomas, C.M.; Kimberly, K.W.; Reece, R.J.: An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 28, 41–45 (2000)
Herron, J.; Green, D.: Chemical kinetics database and predictive schemes for nonthermal air plasma chemistry. Part II. Neutral species reactions. Plasma Chem. Plasma Process. 21, 459–481 (2001)
McLamon, C.R.; Mathur, V.K.: Nitrogen oxide decomposition by barrier discharge. Ind. Eng. Chem. Res. 39, 2779–2787 (2000)
Kanazawa, Seiji; Sumi, Tomoyoshi; Sato, Naruaki; et al.: Wide-range two-dimensional imaging of NO density profiles by LIF technique in a corona radical shower Reactor. IEEE Trans. Plasma Sci. 41, 200–205 (2005)
Francke, K.P.; Rudolph, R.; Miessner, H.: Design and operating characteristics of a simple and reliable DBD reactor for use with atmospheric air. Plasma Chem. Plasma Process. 23, 47–57 (2003)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Yi, H. & Tang, X. Removal of NO Using a Dielectric Barrier Discharge Reactor in a Cycled Adsorption–Desorption and Decomposition System. Arab J Sci Eng 42, 1463–1474 (2017). https://doi.org/10.1007/s13369-016-2344-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-016-2344-7