Abstract
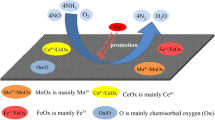
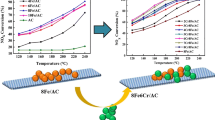
FM/ACFN and Ce-doped CFM/ACFN low-temperature catalysts are prepared by an impregnation method that takes polyacrylonitrile-based activated carbon fiber modified with nitric acid as the carrier. The catalysts are characterized by X-ray diffraction, scanning electron microscopy, Fourier-transform infrared spectroscopy, and thermogravimetric analysis. The effects of temperature, oxygen, and sulfur dioxide on the adsorption and removal of NO by catalyst are studied by laboratory gas distribution. Results show that the addition of metal oxide can increase the ability of chemical adsorption of NO by ACFN by 2.5%, and the ability of catalytic reduction of NO can be increased by up to 14%. Under the condition of oxygen and ammonia as reducing agent at 250 °C, the ability of metal-oxide-loaded ACFN to catalyze the reduction of NO can reach up to 68%. The addition of Ce does not completely inhibit the decrease of the ability of the catalyst in treating NO under sulfur-containing conditions, but it can maintain the catalyst’s reducing ability at a relatively stable level, and the presence of SO2 will reduce the redox capacity of ACFN itself.
Graphic Abstract


















Similar content being viewed by others
References
Can F, Courtois X, Royer S et al (2012) An overview of the production and use of ammonia in NSR + SCR coupled system for NOx, reduction from lean exhaust gas. Catal Today 197(1):144–154
Adapa S, Gaur V, Verma N (2006) Catalytic oxidation of NO by activated carbon fiber (ACF). Chem Eng J 116(1):25–37
Mochida I, Shirahama N, Kawano S et al (2000) NO oxidation over activated carbon fiber (ACF). Part 1. Extended kinetics over a pitch based ACF of very large surface area. Fuel 79(14):1713–1723
Yoshikawa M, Yasutake A, Mochida I (1998) Low-temperature selective catalytic reduction of NOx, by metal oxides supported on active carbon fibers. Appl Catal A Gener 173:239–245
Huang J, Tong Z, Huang Y et al (2008) Selective catalytic reduction of NO with NH3, at low temperatures over iron and manganese oxides supported on mesoporous silica. Appl Catal B 78(3–4):309–314
Wu Z, Jin R, Wang H et al (2009) Effect of ceria doping on SO resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal Commun 10(6):935–939
Junior MAA, Matsushima JT, Rezende MC et al (2017) Production and characterization of activated carbon fiber from textile PAN fiber. J Aerosp Technol Manag 9(4):423–430
Gao ZM, Yue WU, Mei T (1996) NO Reduction by surface oxygen-containing groups on active carbons. Chem Res Chin Univ 17(6):961–964
Raymundo-Pinero E, Cazorla-Amoros D, Linares-Solano A (2003) The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibres. Carbon. 41(10):1925–1932
Guo J, Lua AC (2000) Preparation of activated carbons from oil-palm-stone chars by microwave induced Carbon dioxide activation. Carbon 38(14):1985–1993
Guo Y, Zhao J, Zhang H et al (2005) Use of rice husk-based porous carbon for adsorption of Rhodamine B from aqueous solutions. Dyes Pigm 66(2):123–128
Yoon KS, Ryu SK (2010) Removal of NO using surface modified activated carbon fiber (ACF) by impregnation and heat-treatment of propellant waste. Korean J Chem Eng 27(6):1882–1886
Qi G, Yang RT, Chang R (2004) MnOx-CeO mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH at low temperatures. Appl Catal B 51(2):93–106
Pena DA, Uphade BS, Reddy EP et al (2004) Identification of surface species on titania-supported manganese, chromium, and copper oxide low-temperature SCR catalysts. J Phys Chem B. 108(28):9927–9936
Guan B, Lin H, Cheng Q et al (2010) Synergistic reduction of NOx from diesel engine exhaust by non-thermal plasma facilitated NH3-SCR. J Eng Thermophys 31(10):1767–1771
Zeng Zheng, Pei Lu, Li Caiting et al (2012) Selective catalytic reduction (SCR) of NO by urea loaded on activated carbon fibre (ACF) and CeO2/ACF at 30 & #xB0;C: The SCR mechanism. Environ Technol Lett 33(11):7
Wang Y, Li X, Zhan L et al (2016) Effect of SO2 on activated carbon honeycomb supported CeO2–MnOx catalyst for NO removal at low temperature. Ind Eng Chem Res 54(8):150211102045005
Yoshimura Y, Yasuda H, Sato T et al (2001) Sulfur-tolerant Pd-Pt/Yb-USY zeolite catalysts used to reformulate diesel oils. Appl Catal A 207(1):303–307
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, B., Liu, W., Liang, F. et al. NO Adsorption and Removal at Low Temperature by Adsorption Catalyst (Ce–Fe–Mn/ACFN). Catal Lett 149, 3119–3131 (2019). https://doi.org/10.1007/s10562-019-02867-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02867-8