Abstract
Background
HIV-associated neurocognitive disorders (HAND) is hypothesized to be a result of myeloid cell-induced neuro-inflammation in the central nervous system that may be initiated in the periphery, but the contribution of peripheral T cells in HAND pathogenesis remains poorly understood.
Methods
We assessed markers of T cell activation (HLA-DR + CD38+), immunosenescence (CD57 + CD28-), and immune-exhaustion (TIM-3, PD-1 and TIGIT) as well as monocyte subsets (classical, intermediate, and non-classical) by flow cytometry in peripheral blood derived from individuals with HIV on long-term stable anti-retroviral therapy (ART). Additionally, normalized neuropsychological (NP) composite test z-scores were obtained and regional brain volumes were assessed by magnetic resonance imaging (MRI). Relationships between proportions of immune phenotypes (of T-cells and monocytes), NP z-scores, and brain volumes were analyzed using Pearson correlations and multiple linear regression models.
Results
Of N = 51 participants, 84.3% were male, 86.3% had undetectable HIV RNA < 50 copies/ml, median age was 52 [47, 57] years and median CD4 T cell count was 479 [376, 717] cells/uL. Higher CD4 T cells expressing PD-1 + and/or TIM-3 + were associated with lower executive function and working memory and higher CD8 T cells expressing PD-1+ and/or TIM-3+ were associated with reduced brain volumes in multiple regions (putamen, nucleus accumbens, cerebellar cortex, and subcortical gray matter). Furthermore, higher single or dual frequencies of PD-1 + and TIM-3 + expressing CD4 and CD8 T-cells correlated with higher CD16 + monocyte numbers.
Conclusions
This study reinforces evidence that T cells, particularly those with immune exhaustion phenotypes, are associated with neurocognitive impairment and brain atrophy in people living with HIV on ART. Relationships revealed between T-cell immune exhaustion and inflammatory in CD16+ monocytes uncover interrelated cellular processes likely involved in the immunopathogenesis of HAND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV-associated neurological complications including structural and functional brain alterations as well as neurocognitive disorders present a serious concern for people living with HIV (PLWH). Structural magnetic resonance imaging (sMRI) studies have indicated widespread atrophy in a number of brain regions as well as cerebellar and total subcortical gray matter volume differences indicating brain abnormalities in HIV-positive individuals(Kallianpur et al. 2013; Ma et al. 2021; Nir et al. 2019) Lower CD4 + T cell levels have been previously associated with smaller hippocampal and thalamic volumes in those on ART and lower putamen volumes in those not on antiretroviral therapy (ART). Brain atrophy in both gray and white matter regions is associated with cognitive decline and can result despite long-term stable use of ART(Nir et al. 2021/01; Nir et al. 2019). HIV-associated neurocognitive disorder, also known as HAND has a prevalence that is estimated to be as high as 30–50% and encompasses a spectrum of neurocognitive disease in PLWH(Heaton et al. 2011; Tozzi et al. 2007). Neurocognitive impairment seen in individuals treated early with ART is predominantly mild and characterized by decreased information processing speed and memory deficits, however gray matter volume has been shown to accurately discriminate between HAND patients, PLWH but no neurocognitive impairment, and control(Schantell et al. 2021; Tozzi et al. 2007). Despite mild symptomatology, HAND impacts daily functioning and is associated with increased morbidity and mortality (Benedict et al. 2000; Morgan et al. 2012). To date, there are no clinically proven therapies for HAND for individuals already on stable, virally suppressive ART.
In autopsy studies of people with HIV, histological evaluation has demonstrated a high degree of residual chronic inflammation in brain tissue and increased presence of macrophages and microglial cells despite ART(Anthony et al. 2005). Associations of clinical measures of HAND to blood and cerebrospinal fluid (CSF) markers of monocyte/macrophage activation (e.g., neopterin, sCD163 and sCD14) suggest the importance of the myeloid compartment in the pathogenesis of HAND (Agsalda-Garcia et al. 2017; Brown 2015; Kusao et al. 2012; Ndhlovu et al. 2015; Rappaport and Volsky 2015; Williams et al. 2014). Other biomarkers such as HIV DNA, autoantibodies, and inflammatory cytokines present in the CSF as well as alterations in T-cell abundance and CD4/CD8 T-cell ratios in the peripheral blood have been associated with altered neurocognitive functioning in PLWH but are still unable to be used for diagnostic purposes(Abassi et al. 2017; Gougeon et al. 2017; Koffler et al. 2018; Vassallo et al. 2016). These biomarker associations, however, fail to account for the association of HAND to alterations in T cell specific immunological markers. T cell markers associated with HAND include low nadir CD4 T cell counts, low CD4/CD8 T cell ratios, increased frequencies of activated CD4 and CD8 T cells measured both in the periphery and in the CSF, higher levels of the T cell chemo-attractant CXCL10, and presence of CD8 T cells expressing IFN-γ in the CSF (Ellis et al. 2011; Mehla et al. 2012; Schrier et al. 2015; Vassallo et al. 2016). Furthermore, recent studies have identified the prevalence and persistence of HIV in freshly isolated CSF derived CD4 T cells in ART suppressed individuals with HIV (Farhadian et al. 2022). These T cell specific associations suggest that T cells may play a larger role in the pathogenesis of HAND than is currently recognized.
T cell exhaustion, is characterized by the upregulation of negative checkpoint receptors (NCRs) and has recently been of major interest in HIV cure research as it is linked to the persistence of the latent HIV reservoir (Chew et al. 2016; Fromentin et al. 2016). PD-1 is an NCR that is upregulated in chronic viral infections and is associated with suppression of T cells in parenchymal tissues as well as T cell exhaustion (Manenti et al. 2022; Said et al. 2010). Higher levels of PD-1 expression on CD4 T cells is associated with worsening neurocognitive impairment in people living with HIV (Grauer et al. 2015; Said et al. 2010). Furthermore, PD-1 expression has been found to be upregulated on T cells in other neuroinflammatory conditions and resulting in a reduction of Th1 cells; however, the exact mechanisms associated with CNS injury are still unclear (Manenti et al. 2022). Less is known about the impact of NCRs and T cell exhaustion on non-infectious chronic complications of HIV including HAND. In this study, we sought to assess the relationship of primary (PD-1) and secondary tier (TIGIT and TIM-3) NCRs on T cells to measures of brain integrity (NP impairment and to regional brain volumes) in PLWH. We report that T cell immune exhaustion is associated with worse cognition and decreased brain volumes.
Methods
Study participants
This retrospective study was conducted utilizing entry data and specimens from the Hawaii Aging with HIV Cohort-Cardiovascular Disease (HAHC-CVD) study, a 5-year natural history study investigating the role of chronic HIV infection in the development of cardiovascular disease (CVD) among PLWH on suppressive ART. Inclusion criteria required documented HIV-positive status, age ≥ 40 years, and use of combination ART ≥ 3 months. Patients who were institutionalized or with active malignancy, infection or AIDS-defining illness at the time of enrollment were excluded. Due to the use of MRI in this study, the following exclusion criteria were employed: (i) uncontrolled major affective disorder; (ii) active psychosis; (iii) loss of consciousness > 5 min; (iv) pregnancy or breast feeding; (v) factors that would preclude MRI (e.g. claustrophobia); and (vi) any past or present condition (central nervous system infection, brain trauma, stroke, substance abuse, ect.) deemed by the evaluating physician to introduce confounding variables. Extensive HIV immunologic assessments were available from this cohort and further details have been previously described (Shikuma et al. 2012). A subset of this cohort underwent NP testing and brain MRI. IRB approval was obtained from the University of Hawaii Human Studies Program (CHS #16,476), and all participants provided written informed consent prior to enrollment into the HAHC-CVD study. The study was conducted in accordance with the Declaration of Helsinki and adhered to the guidelines of the University of Hawaii IRB. At the time of entry into the cohort, all participants gave written informed consent to allow use of clinical data and banked specimens for future studies related to HIV and its complications. All banked specimens and data collected from participants were anonymized and de-identified prior to analysis.
Neuropsychological testing
Participants completed a comprehensive 80-minute NP testing battery at the time of study entry. NP testing was conducted by trained psychometrists at the University of Hawaii Clinics at Kakaako with quality assurance of testing procedures provided by a neuropsychologist (Robert H. Paul Ph.D.). Participants were tested in a quiet room to minimize distractions and provided breaks as needed. The NP battery involved a series of tests that were selected to assess the function of specific cognitive domains (learning and memory, psychomotor speed, working memory, and executive function) known to be sensitive to HIV-related cognitive dysfunction as shown in Table 1. As previously described, raw NP test scores were z-transformed by use of age, gender and education-adjusted normative data. Composite, domain specific z-scores (psychomotor speed [NPZpm]; learning and memory [NPZlrn-mem]; executive functioning [NPZef]; working memory [NPZwm]) were derived by averaging z-scores of the appropriate individual tests. A global z score (NPZ-global) was the arithmetic mean of z-scores on 14 NP tests(Chai et al. 2018; Kallianpur et al. 2016b; Umaki et al. 2013). Normative data were adapted from the Multicenter AIDS Cohort Study, which included PLWH and HIV-seronegative individuals (Valcour and Paul 2006). Diagnosis of HAND were not made in this study.
Neuroimaging
Neuroimaging was performed as previously described (Kallianpur et al. 2016a). Briefly, MRIs were conducted on a 3.0-Tesla Philips Medical Systems Achieva scanner equipped with an 8-channel head coil (InVision Imaging, Honolulu). For each participant, a high-resolution anatomical volume was acquired with a sagittal T1-weighted 3D turbo field echo (T1W 3D TFE) sequence (echo time TE/repetition time TR = 3.2 ms/6.9 ms; flip angle 8°; slice thickness 1.2 mm with no gap; in-plane resolution 1.0 mm2; field of view 256 × 256 mm2). To obtain regional brain volumes, structural MRI data were processed with FreeSurfer (version 5.0, https://surfer.nmr.mgh.harvard.edu/) in a procedure that includes skull-stripping (Segonne et al. 2004), intensity normalization (Sled et al. 1998), Talairach transformation, subcortical white matter and deep gray matter segmentation (Fischl 2004; Fischl et al. 2002), and cortical gray/white matter boundary and pial surface reconstruction (Dale et al. 1999). Quality assurance of processed data was performed visually, and cortical surfaces and subcortical segmentations were checked prior to statistical analysis. FreeSurfer’s estimate of intracranial volume (ICV) is a reliable measure for regional brain volume normalization (Buckner et al. 2004).
PBMC isolation and flow cytometry assay
PBMC isolation and flow cytometry assays were performed as previously reported (Chew et al. 2016). In brief, whole blood was drawn into EDTA tubes at the time of study entry and cells were processed for peripheral blood mononuclear cells (PBMCs) isolation within one hour of collection and cryopreserved. Banked cryopreserved PBMCs were thawed and washed with warmed AIM-V liquid media (Invitrogen). For detection of T cell activation, senescence, and exhaustion thawed cryopreserved PBMCs were stained with AARD to exclude nonviable cells and for surface expression of CD3, CD28, CD4, CD38, HLA-DR, CD8, CD57, PD-1, TIM-3, and TIGIT. Data were acquired on a custom 4-laser BD LSRFortessa Cell Analyzer and all compensation and gating analyses were performed in FlowJo analytical software to quantitate CD4 and CD8 T cells expressing activation (CD38 and HLADR), senescence (CD28 and CD57), and exhaustion (PD-1, TIM-3, TIGIT) markers. Data are expressed as the percentage of T cells expressing these markers. For monocyte immunophenotyping, PBMCs were surface-stained with CD3, CD14, CD16, CD56, CD19, CD20, HLA-DR antibodies, and with Live/Dead fixable yellow dead cell stain. Data were acquired on a custom 4-laser BD LSRFortessa Cell Analyzer and all compensation and gating analyses were performed in FlowJo analytical software (gating strategy previously published(Shikuma et al. 2014). Percentages of classical, intermediate, and non-classical monocyte subsets were determined based on CD14 and CD16 staining and absolute numbers of each subset were calculated from WBC and monocyte percent obtained from available clinical CBC performed on each participant from the same blood draw.
Statistical analysis
Demographic, clinical, and immunologic information were summarized by median (interquartile range) values for continuous variables and frequency (percentage) for categorical variables. Correlation between immunologic parameters, neurocognitive receptor domains, and brain volumes were assessed by Pearson’s correlation. Multivariable linear regression analyses were used to assess the association of exhausted T cells with NP z-scores and brain volumes. Statistical analyses were performed using IBM SPSS version 22 software and statistical significance was set at a p-value < 0.05. Continuous variables with skewed distributions were log-10 transformed prior to analyses.
Results
Baseline characteristics of participants
A total of 51 participants enrolled into the HAHC-CVD cohort had available NP performance testing and brain imaging and were included in this analysis. The demographic, clinical, and immunologic characteristics of the participants are summarized in Table 2. The majority of participants were male with a median age of 51 years. Participants were mostly of Caucasian ethnicity and had a median current CD4 T cell count of 479 [376, 717] cells/mm3. The median nadir CD4 count was much lower at 92.0 [33.0, 198.25] cells/mm3. Undetectable plasma HIV viral load, defined as HIV RNA 50 copies/ml, was noted in 86.3% of the participants. Overall NP performance in our population was within normal limits, with slightly worse performance on learning and memory subdomains.
Relationship between NCR-expressing T cells and clinical parameters, T cell immune activation and senescence
CD4 and CD8 T cells were evaluated by flow-cytometry for single and double expression of three known NCRs: PD-1, TIM-3, and TIGIT (Table 2). Pearson correlations of single and double NCR-expressing CD8 and CD4 T cells with other T cell parameters (current CD4 T cell count, CD4 CD8 T cell ratio, nadir CD4 count, % activated T cells, and % senescent T cells) are shown in Table 3. Higher percentages of CD4 and CD8 T cells single positive for TIGIT or PD-1 correlated with lower current CD4 T cell counts and CD4/CD8 T cell ratios. Higher frequencies of TIGIT or PD-1 single positive CD4 T cells, but not with CD8 T cells, correlated with lower nadir CD4 T cell counts. Higher frequencies of PD-1 single positive CD4 T cells and TIM-3 single positive CD8 T cells correlated with higher frequencies of senescent (CD57 + CD28-) CD8 T cells. Higher frequencies of CD4 and CD8 T cells single positive for PD-1 and TIGIT correlated with higher frequencies of activated (CD38 + HLA-DR+) CD8 T cells. There were no correlations found between CD4 T cells single positive for TIM-3 with other T cell parameters.
Higher percentages of CD4 and CD8 T cells double positive for PD-1, TIM-3, and/or TIGIT correlated with lower CD4 T cell counts, lower CD4 CD8 T cell ratios, and higher percentages of activated CD8 T cells. Higher percentages of CD8 T cells double positive for PD-1 and TIM-3, as well as with CD4 T cells double positive for PD-1 and TIGIT, correlated with lower nadir CD4 T cell counts. Higher percentages of CD4 T cells double positive for PD-1 and TIM-3 correlated with higher percentages of senescent CD4 T cells. Higher percentages of CD4 T cells double positive for PD-1 and TIM-3 and CD8 T cells double positive for TIM-3 and TIGIT correlated with higher percentages of senescent CD8 T cells.
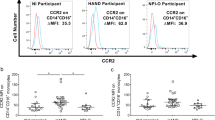
Relationship of NCR-expressing T cells and CD16+ monocyte subpopulations
Proportions of monocyte subpopulations were evaluated by flow-cytometry based on CD14 and CD16 expression. Correlations between single and dual NCR-expressing CD8 and CD4 T cells and monocyte subpopulation counts are shown in Table 4. Higher frequencies of CD4 T cells single positive for TIM-3 correlated with higher counts of intermediate (CD14 + + CD16+) monocytes. Higher percentages of
both CD4 and CD8 T cells single positive for PD-1 correlated with higher counts of non-classical (CD14+/lowCD16++) monocytes.
Higher frequencies of CD4 T cells double positive for PD-1 and TIM-3 correlated with higher counts of both intermediate and non-classical monocytes. Higher frequencies of CD4 T cells double positive for TIM-3 and TIGIT correlated with higher counts of intermediate monocytes. No significant associations were found with activated and senescent T cells with monocyte subpopulations.
Relationship of NCR-expressing CD4 T cells and NP performance
Significant correlations with NP test scores were found for NCR double positive CD4 T cells. No relationships with NP performance were observed with any NCR markers on CD8 T cells. Higher frequencies of CD4 T cells double positive for TIM-3 and PD-1 correlated with lower executive function (r=-0.427, p = 0.013) and working memory (r=-0.352, p = 0.045). CD4 T cells double positive for TIM-3 and TIGIT correlated with slower psychomotor speed (r=-0.350, p = 0.050). In multivariable linear regression models (Table 5) controlling for nadir CD4 count, higher frequencies of CD4 T cells double positive for PD-1 and TIM-3 remained associated with lower executive function (p < 0.001) and working memory (p = 0.009), with an association trend with overall NP performance (NPZ-Global; p = 0.056). In addition, higher frequencies of CD4 T cells double positive for TIM-3 and TIGIT remained associated with slower psychomotor speed (p = 0.018).
Relationship of T cells and regional brain volumes
In multivariable linear regression models (Table 6) controlling for age, nadir CD4 T cell count and intracranial volume, only CD8 T cells single and double positive for PD-1, TIM-3, and/or TIGIT were associated with regional brain volumes. Interestingly, no volumetric associations were found for NCRs on CD4 T cells. Higher frequencies of CD8 T cells single positive for PD-1 were associated with smaller volumes of the putamen, nucleus accumbens, cerebellar cortex, and subcortical gray matter. Higher frequencies of CD8 T cells double positive for PD-1 and TIM-3 were associated with smaller putamen and nucleus accumbens. Higher frequencies of CD8 T cells double positive for PD-1 and TIGIT were associated with only smaller volumes of the nucleus accumbens.
Correlations between monocyte subpopulations, NP performance, and brain volumes
Higher frequency of total monocyte correlated with lower psychomotor speed (r = -0.347, p = 0.04), while higher frequency of non-classical monocyte correlated with lower executive function (r = − 0.424, p = 0.022) and is shown in Supplemental Table 1. Higher frequencies of non-classical monocyte correlated with lower putamen volume (r = -0.378, p = 0.048), while higher total non-classical monocyte count correlated with lower cerebellar cortical (r = -0.430, p = 0.022) and lower putamen volumes (r= -0.385, p = 0.043) as shown in Supplemental Table 1. When adjusted for nadir CD4 count, only the following associations remained significant by multivariable linear regression: higher frequency of total monocyte with lower psychomotor speed (β = -0.367, p = 0.048) and higher frequency of non-classical monocyte with lower executive function (β = -0.466, p = 0.019) as shown in Supplemental Table 2.
Discussion
Our study describes the novel relationship between dual expressing PD-1/TIM-3 CD4 and CD8 T cells with neurocognitive impairment and brain atrophy, respectively, and highlights NCR-expressing T cells as a potential novel target that may reverse cognitive impairment in HIV among PLWH on ART. In addition, our data suggests that a close relationship exists between NCR-expressing T cells and circulating monocyte subpopulations in the context of HIV.
As expected, our study found that higher frequencies of CD4 and CD8 T cells single and double positive for PD-1, TIM-3, and/or TIGIT supports previous findings showing the relationship between lower clinical measures of immune function, such as lower current CD4 count, CD4/CD8 T cell ratio, and nadir CD4 T cell count and HIV cognitive impairment(Fenwick et al. 2019). Importantly, parameters of dysregulated immune function, particularly the CD4/CD8 T cell ratio, have been linked to non-AIDS comorbidities, including subclinical atherosclerosis, lower renal function, muscle wasting, and to worsening cognitive function (Bernal Morell et al. 2016; Fenwick et al. 2019; Grauer et al. 2015; Serrano-Villar et al. 2014a, b) and that the exhausted T cell population may serve as a more accurate functional marker of HIV-associated comorbidities beyond the central nervous system.
Poorer executive function, working memory performance, decreased psychomotor speed, and poor everyday functioning have been identified in PLWH despite potent ART (Heaton et al. 2011; Koffler et al. 2018). Our study findings relating NCR-expressing CD4 T cell subpopulations to these subdomains suggests that they directly or indirectly affect cognitive impairment. By contrast, NCR expression on CD8 T cells are associated with smaller volumes of the putamen, nucleus accumbens, cerebellar cortex, and subcortical gray matter; areas known to be impacted by HIV despite ART(Ances and Hammoud 2014; Holt et al. 2012; Kallianpur et al. 2013, 2020). Grey matter volumes in cortical and subcortical regions have also been used to classify those with HAND with a high sensitivity and specificity further supporting an association between regional brain volumes and NP functioning in PLWH(Schantell et al. 2021). Studies have shown that brain regions responsible for executive functioning predominantly involves the prefrontal cortex, basal ganglia and thalamus(Royall et al. 2002). Working memory, on the other hand, primarily involves the fronto-parietal brain region and, more recently, subcortical regions have previously reported correlations between frailty characteristics and reduced volume in HIV(Chai et al. 2018; Kallianpur et al. 2016b). In the current study, we only identified one weakly positive correlation between cerebellar cortex ICV and executive functioning, but did not find any correlation between other brain volumes and NP z-scores (Supplemental Table 2). This was likely due to the small sample size of our cohort.
Review of the literature has revealed scant studies on the role of NCR-expressing T cells in the pathogenesis of HAND. Among PLWH with mild or severe neurocognitive alterations, one study has found increased PD-1 expression on memory CD4 + T cells, with the highest levels on effector memory T cells (Grauer et al. 2015). Interestingly, a recent mouse model tauopathy dementia showed that PD-1/PD-L1 checkpoint blockade led to increased immunomodulatory monocyte-derived macrophages and improved cognitive performance(Rosenzweig et al. 2019).
The relationships linking NCR-expressing CD4 T cells with NP performance and NCR-expressing CD8 T cells with brain volumes found in our study remain an enigma. This discordant relationship may be secondary to differences in the key function of these cells– i.e., CD4 T cells are more involved in pro-inflammatory cytokine production which may mediate neuropsychological dysfunction without cell loss, whereas CD8 T cell’s cytotoxic function may mediate neuronal loss resulting in structural changes (Subra and Trautmann 2019).
We found some correlations between NCR-expressing T cells and various monocyte subsets (Table 4). Ramello et al. reported that autologous monocytes co-cultured with CD4 or CD8 tumor-induced senescent (TIS)-T cells result in higher production of pro-inflammatory cytokines from monocyte/macrophages including TNF-α, IL-1β, and IL-6 (Ramello et al. 2014). It is worth noting that cell-to-cell interaction was required for the modulation of the monocyte/macrophages and that this was mediated by TIM-3 and CD40L (Ramello et al. 2014). It is possible that this interaction may be bi-directional as it is also known that monocyte/macrophages are effective antigen-presenting cells capable of activating both CD4 and CD8 T cells to effector phenotypes through the expression of antigen via HLA surface proteins(Ances and Hammoud 2014).
Significant correlations were also noted between monocytes, neurocognitive domains, and brain volumes. On multivariable linear regression adjusted for CD4 count, higher frequency of total monocyte was associated with lower psychomotor speed, while higher frequency of non-classical monocyte was associated with lower executive function. Similar to peripheral T cell exhaustion, peripheral monocytes likely play a role in CNS function since these cells have the ability to cross the blood-brain barrier. Previous studies have reported similar associations between monocytes and brain function (Clifford 2017; Fischer-Smith et al. 2001; Kusao et al. 2012; Ndhlovu et al. 2015; Shikuma et al. 2014; Veenstra et al. 2019; Williams et al. 2014).
This study has several limitations, including its cross-sectional nature, small sample size, and lack of an HIV-seronegative control for monocyte and T-cell NCR experiments. This study was not designed to establish the diagnosis of HAND. We performed several statistical analyses between variables. Due to sample size limitations and the exploratory nature of this manuscript, we did not plan on applying multiplicity corrections to our analyses. Additionally, our experiments focused on PBMCs and may not necessarily reflect immune dysregulation within the CNS, as we did not perform cerebrospinal fluid analyses. The strengths of this study, however, include the availability of NP performance and MRI data in an otherwise immunologically well-characterized cohort of HIV infected individuals on ART. Future directions include the use of a larger cohort to strengthen the statistical power, including monocyte and T-cell analyses among HIV-seronegative controls, and repeating the NP assessments in the context of newer ‘test and treat’ strategies using integrase-based ART regimens.
In conclusion, we report that dual expressing PD-1/TIM-3 CD4 and CD8 T cells are associated with neurocognitive impairment and brain atrophy, respectively. Results from this study highlight a potential novel interaction of NCR-expressing T cells with circulating blood monocytes, and a possible role of the pro-inflammatory consequence of this interaction on the development of HAND and brain atrophy in PLWH on ART. Novel immunotherapy targeting PD-1 and TIM-3 may be considered as treatment modalities for HAND.
Data availability
All supporting data for this study are available from the corresponding author upon reasonable request.
References
Abassi M, Morawski BM, Nakigozi G, Nakasujja N, Kong X, Meya DB, Robertson K, Gray R, Wawer MJ, Sacktor N, Boulware DR (2017) Cerebrospinal Fluid Biomarkers and HIV-Associated Neurocognitive Disorders in HIV- Infected Individuals in Rakai, Uganda. Journal of neurovirology 23
Agsalda-Garcia MA, Sithinamsuwan P, Valcour VG, Chalermchai T, Tipsuk S, Kuroda J, Nakamura C, Ananworanich J, Zhang G, Schuetz A, Slike BM, Shiramizu B, Group SS (2017) Brief report: CD14 + enriched peripheral cells secrete cytokines unique to HIV-Associated Neurocognitive disorders. J Acquir Immune Defic Syndr 74:454–458
Ances BM, Hammoud DA (2014) Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS 9:545–551
Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2005) Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 64:529–536
Benedict RH, Mezhir JJ, Walsh K, Hewitt RG (2000) Impact of human immunodeficiency virus type-1-associated cognitive dysfunction on activities of daily living and quality of life. Arch Clin Neuropsychol 15:535–544
Bernal Morell E, Serrano Cabeza J, Munoz A, Marin I, Masia M, Gutierrez F, Cano A (2016) The CD4/CD8 ratio is inversely Associated with Carotid Intima-Media thickness progression in human immunodeficiency virus-infected patients on antiretroviral treatment. AIDS Res Hum Retroviruses 32:648–653
Brown A (2015) Understanding the MIND phenotype: macrophage/microglia inflammation in neurocognitive disorders related to human immunodeficiency virus infection. Clin Transl Med 4:7
Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ (2004) A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage 23:724–738
Chai WJ, Abd Hamid AI, Abdullah JM (2018) Working Memory From the Psychological and Neurosciences Perspectives: A Review. Frontiers in Psychology 9
Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, Liegler T, Mitchell BI, Hecht FM, Ostrowski M, Shikuma CM, Hansen SG, Maurer M, Korman AJ, Deeks SG, Sacha JB, Ndhlovu LC (2016) TIGIT Marks Exhausted T Cells, correlates with Disease Progression, and serves as a target for Immune Restoration in HIV and SIV infection. PLoS Pathog 12:e1005349
Clifford DB (2017) HIV Associated Neurocognitive Disorder. Current opinion in infectious diseases 30
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194
Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I, Group C (2011) CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 25:1747–1751
Farhadian SF, Lindenbaum O, Zhao J, Corley MJ, Im Y, Walsh H, Vecchio A, Garcia-Milian R, Chiarella J, Chintanaphol M, Calvi R, Wang G, Ndhlovu LC, Yoon J, Trotta D, Ma S, Kluger Y, Spudich S (2022) HIV viral transcription and immune perturbations in the CNS of people with HIV despite ART. JCI Insight 7.
Fenwick C, Joo V, Jacquier P, Noto A, Banga R, Perreau M, Pantaleo G (2019) T-cell exhaustion in HIV infection. Immunol Rev 292
Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Régulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J (2001) CNS invasion by CD14+/CD16 + peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection - PubMed. J Neurovirol 7
Fischl B (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, Killian M, Epling L, Hoh R, Sinclair E, Hecht FM, Bacchetti P, Deeks SG, Lewin SR, Sekaly RP, Chomont N (2016) CD4 + T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog 12:e1005761
Gougeon M-L, Poirier-Beaudouin B, Durant J, Lebrun-Frenay C, Saïdi H, Seffer V, Ticchioni M, Chanalet S, Carsenti H, Harvey-Langton A, Laffon M, Cottalorda J, Pradier C, Dellamonica P, Vassallo M (2017) HMGB1/anti-HMGB1 antibodies define a molecular signature of early stages of HIV-Associated Neurocognitive disorders (HAND). Heliyon 3.
Grauer OM, Reichelt D, Gruneberg U, Lohmann H, Schneider-Hohendorf T, Schulte-Mecklenbeck A, Gross CC, Meuth SG, Wiendl H, Husstedt IW (2015) Neurocognitive decline in HIV patients is associated with ongoing T-cell activation in the cerebrospinal fluid. Ann Clin Transl Neurol 2:906–919
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16
Holt JL, Kraft-Terry SD, Chang L (2012) Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol 18:291–302
Kallianpur KJ, Shikuma C, Kirk GR, Shiramizu B, Valcour V, Chow D, Souza S, Nakamoto B, Sailasuta N (2013) Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology 80:1792–1799
Kallianpur KJ, Gerschenson M, Mitchell BI, LiButti DE, Umaki TM, Ndhlovu LC, Nakamoto BK, Chow DC, Shikuma CM (2016a) Oxidative mitochondrial DNA damage in peripheral blood mononuclear cells is associated with reduced volumes of hippocampus and subcortical gray matter in chronically HIV-infected patients. Mitochondrion 28:8–15
Kallianpur KJ, Sakoda M, Gangcuangco LMA, Ndhlovu LC, Umaki T, Chow D, Wongjittraporn S, Shikuma CM (2016b) Frailty characteristics in chronic HIV patients are markers of White Matter Atrophy independently of age and depressive symptoms: a pilot study. Open Med J 3:138–152
Kallianpur KJ, Jahanshad N, Sailasuta N, Benjapornpong K, Chan P, Pothisri M, Dumrongpisutikul N, Laws E, Ndhlovu LC, Clifford KM, Paul R, Jagodzinski L, Krebs S, Ananworanich J, Spudich S, Valcour V, SEARCH010/RV254 Study Group (2020) Regional brain volumetric changes despite 2 years of treatment initiated during acute HIV infection - PubMed. AIDS. (London, England) 34
Koffler S, Mahone EM, Marcopulos BA, Johnson-Greene DE, Smith G (2018) Neuropsychology: Science and Practice, 3 edn. Oxford University Press
Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, Velasco VN, Marshall A, Whitenack N, Shikuma C, Valcour V (2012) Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci 24:71–80
Ma Q, Shi X, Chen G, Song F, Liu F, Zheng H, Shi Y, Cai D-C (2021) HIV-Associated Structural and functional brain alterations in homosexual males. Frontiers in Neurology 12.
Manenti S, Orrico M, Masciocchi S, Mandelli A, Finardi A, Furlan R (2022) PD-1/PD-L Axis in Neuroinflammation: New insights. Front Neurol 13:877936
Mehla R, Bivalkar-Mehla S, Nagarkatti M, Chauhan A (2012) Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J Neuroinflammation 9:239
Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, Ellis R, Grant I, Woods SP, Group HIVNRP (2012) Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr 61:341–348
Ndhlovu LC, D’Antoni ML, Ananworanich J, Byron MM, Chalermchai T, Sithinamsuwan P, Tipsuk S, Ho E, Slike BM, Schuetz A, Zhang G, Agsalda-Garcia M, Shiramizu B, Shikuma CM, Valcour V (2015) Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naive HIV-infected Thais. J Neuroimmunol 288:25–33
Nir TM, Jahanshad N, Ching CR, Cohen RA, Harezlak J, Schifitto G, Lam HY, Hua X, Zhong J, Zhu T, Taylor MJ, Campbell TB, Daar ES, Singer EJ, Alger JR, Thompson PM, Navia BA (2019) Progressive Brain Atrophy in Chronically Infected and Treated HIV + Individuals. Journal of neurovirology 25
Nir TM, Fouche J-P, Ananworanich J, Ances BM, Boban J, Brew BJ, Chaganti JR, Chang L, Ching CRK, Cysique LA, Ernst T, Faskowitz J, Gupta V, Harezlak J, Heaps-Woodruff JM, Hinkin CH, Hoare J, Joska JA, Kallianpur KJ, Kuhn T, Lam HY, Law M, Lebrun-Frénay C, Levine AJ, Mondot L, Nakamoto BK, Navia BA, Pennec X, Porges EC, Salminen LE, Shikuma CM, Surento W, Thames AD, Valcour V, Vassallo M, Woods AJ, Thompson PM, Cohen RA, Paul R, Stein DJ, Jahanshad N (2021) /01). Association of Immunosuppression and viral load with subcortical brain volume in an International Sample of people living with HIV. JAMA Network Open 4.
Ramello MC, Boari JT, Canale FP, Mena HA, Negrotto S, Gastman B, Gruppi A, Rodriguez EV, Montes CL (2014) Tumor-induced senescent T cells promote the secretion of pro-inflammatory cytokines and angiogenic factors by human monocytes/macrophages through a mechanism that involves Tim-3 and CD40L. Cell Death Dis 5:e1507
Rappaport J, Volsky DJ (2015) Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J Neurovirol 21:235–241
Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, Keren-Shaul H, Ben-Yehuda H, Weill-Raynal P, Cahalon L, Kertser A, Baruch K, Amit I, Weiner A, Schwartz M (2019) PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun 10
Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, Curt LaFrance W, Coffey J CE (2002) Executive control function. J Neuropsychiatry Clin Neurosci 14
Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy J-P, Douek DC, Haddad EK, Sekaly R-P (2010) Programmed death-1–induced interleukin-10 production by monocytes impairs CD4 + T cell activation during HIV infection. Nat Med 16:452–459
Schantell M, Taylor BK, Lew BJ, O’Neill JL, May PE, Swindells S, Wilson TW (2021) Gray matter volumes discriminate cognitively impaired and unimpaired people with HIV. NeuroImage: Clinical 31
Schrier RD, Hong S, Crescini M, Ellis R, Perez-Santiago J, Spina C, Letendre S, Group H (2015) Cerebrospinal fluid (CSF) CD8 + T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS ONE 10:e0116526
Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004) A hybrid approach to the skull stripping problem in MRI. NeuroImage 22:1060–1075
Serrano-Villar S, Perez-Elias MJ, Dronda F, Casado JL, Moreno A, Royuela A, Perez-Molina JA, Sainz T, Navas E, Hermida JM, Quereda C, Moreno S (2014a) Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS ONE 9:e85798
Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue PY, Van Natta ML, Meinert CL, Lederman MM, Hatano H, Jain V, Huang Y, Hecht FM, Martin JN, McCune JM, Moreno S, Deeks SG (2014b) HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8 + T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 10:e1004078
Shikuma CM, Seto T, Liang CY, Bennett K, DeGruttola V, Gerschenson M, Stein JH, Budoff M, Hodis HN, Delaney JA, Ogata-Arakaki D, Pramyothin P, Chow D (2012) Vitamin D levels and markers of arterial dysfunction in HIV. AIDS Res Hum Retroviruses 28:793–797
Shikuma CM, Chow DC, Gangcuangco LM, Zhang G, Keating SM, Norris PJ, Seto TB, Parikh N, Kallianpur KJ, Nakamoto BK, Nagamine LS, Ndhlovu LC, Barbour JD (2014) Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS ONE 9:e90330
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Subra C, Trautmann L (2019) Role of T lymphocytes in HIV Neuropathogenesis. Curr HIV/AIDS Rep 16:236–243
Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 45:174–182
Umaki TM, Gangcuangco LMA, Chow DC, Nakamoto BK, Marotz L, Kallianpur KJ, Shikuma CM (2013) Poorer neuropsychological performance increases risk for social services among HIV-infected individuals. HAWAI‘I JOURNAL OF MEDICINE & PUBLIC HEALTH, p 72
Valcour V, Paul R (2006) HIV infection and dementia in older adults. Clin Infect Diseases: Official Publication Infect Dis Soc Am 42
Vassallo M, Fabre R, Durant J, Lebrun-Frenay C, Joly H, Ticchioni M, DeSalvador F, Harvey-Langton A, Dunais B, Laffon M, Cottalorda J, Dellamonica P, Pradier C, Vassallo M, Fabre R, Durant J, Lebrun-Frenay C, Joly H, Ticchioni M, DeSalvador F, Harvey-Langton A, Dunais B, Laffon M, Cottalorda J, Dellamonica P, Pradier C (2016) A decreasing CD4/CD8 ratio over time and lower CSF-penetrating antiretroviral regimens are associated with a higher risk of neurocognitive deterioration, independently of viral replication. J Neurovirol 2016 23:223
Veenstra M, Byrd DA, Inglese M, Buyukturkoglu K, Williams DW, Fleysher L, Li M, Gama L, León-Rivera R, Calderon TM, Clements JE, Morgello S, Berman JW (2019) CCR2 on peripheral blood CD14 + CD16 + monocytes correlates with neuronal damage, HIV-Associated Neurocognitive disorders, and Peripheral HIV DNA: reseeding of CNS reservoirs? J Neuroimmune Pharmacol 14:120–133
Williams AM, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW (2014) Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res 12:85–96
Acknowledgements
We thank our study participants and community physicians and nurses. This work was partially presented at the 2016 International AIDS Society (IAS) conference held from July 18-22 nd in Durban, South Africa and the 2017 Conference for Retroviruses and Opportunistic Infections (CROI) held from February 13-16 th in Seattle, Washington. This study received funding support from R01HL095135, U54MD007584, U54MD007601, and U54GM138062.
Funding
This study received funding support from R01HL095135, U54MD007584, U54MD007601, U54GM138062, and K12HL143960.
Author information
Authors and Affiliations
Contributions
C.S., D.C., L.N., R.P., B.M., and K.K. contributed to the conception and design of the study. Data acquisition was performed by B.M., L.G., K.K., and R.P. Data analyses were led by B.M., L.G., and I.E. All of the authors critically reviewed the manuscript, interpreted the data, provided important intellectual content, and approved the final draft of the paper prior to submission.
Corresponding author
Ethics declarations
Competing interests
Lishomwa C. Ndhlovu is supported and reports grants from the NIH under award R01MH130197 and has received consulting fees from work as a scientific advisor for AbbVie, ViiV Healthcare, and Cytodyn and also serves on the Board of Directors of CytoDyn and has financial interests in Ledidi AS all for work outside of the submitted work. All of the other authors have no financial disclosure or conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitchell, B.I., Yazel Eiser, I.E., Kallianpur, K.J. et al. Dynamics of peripheral T cell exhaustion and monocyte subpopulations in neurocognitive impairment and brain atrophy in chronic HIV infection. J. Neurovirol. (2024). https://doi.org/10.1007/s13365-024-01223-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13365-024-01223-w