Abstract
Persistent inflammation is described in people with HIV (PWH) on antiretroviral treatment (ART). Early ART initiation is associated with reduced inflammation. We aimed to evaluate neuroinflammation, using translocator protein (TSPO) [11C]PBR28 PET neuroimaging in PWH who initiated ART during acute HIV (aPWH) versus chronic HIV infection (cPWH) versus a control population. This was a cross-sectional, observational study. All participants underwent [11C]PBR28 PET-CT neuroimaging. Using a two-tissue compartment model, total volume of distribution (VT) and distribution volume ratios (DVR) using cortical grey matter as a pseudo-reference region at 20 regions of interest (ROIs) were calculated. Differences in VT and DVR were compared between groups using the Kruskall-Wallis test. Seventeen neuro-asymptomatic male PWH on ART (9 aPWH, 8 cPWH) and 8 male control participants (CPs) were included. Median (interquartile range, IQR) age was 40 (30, 46), 44 (41, 47) and 21 (20, 25) years in aPWH, cPWH and CPs, respectively. Median (IQR) CD4 (cells/µL) and CD4:CD8 were 687 (652, 1014) and 1.37 (1.24, 1.42), and 700 (500, 720) and 0.67 (0.64, 0.82) in aPWH and cPWH, respectively. Overall, no significant difference in VT and DVR were observed between the three groups at any ROIs. cPWH demonstrated a trend towards higher mean VT compared with aPWH and CPs at most ROIs. No significant differences in neuroinflammation, using [11C]PBR28 binding as a proxy, were identified between cPWH, aPWH and CPs. A trend towards lower absolute [11C]PBR28 binding was seen amongst aPWH and CPs, suggesting early ART may mitigate neuroinflammation.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
With the widespread implementation of modern antiretroviral treatment (ART), the prognosis and life expectancy for people with HIV has significantly improved (Hogg et al. 1998; May et al. 2014). However, people with HIV on virologically suppressive ART remain at increased risk of non-AIDS co-morbidities including cognitive impairment (Robertson et al. 2007; Schouten et al. 2014; Simioni et al. 2010). The underlying mechanisms are likely multifactorial; persistent microglial activation (Anthony et al. 2005; Minagar et al. 2002) and neuroinflammation in people with HIV on virological suppressive ART (Edén et al. 2016) is postulated to be a contributor.
The 18kDa Translocator protein (TSPO), located on the outer mitochondrial membrane, is highly expressed in activated microglia (Banati et al. 2004; Liu et al. 2014) and is associated with neuroinflammation (Liu et al. 2014). Synthetic radiolabelled ligands that selectively bind to TSPO have been developed and can be utilised to image dynamic microglial activation in the human brain in vivo, using positron emission tomography (PET) imaging (Stephenson et al. 1995). The first-generation TSPO radiotracer, [11C]-PK11195 was limited by low blood–brain barrier penetration and high nonspecific binding. Second-generation radiotracers such as [11C]PBR28 and [11C]DPA-713 have improved blood–brain barrier penetration and signal-to-noise ratios, however binding affinity is dependent on single-nucleotide polymorphism rs6971, whereby genotypic testing must be carried out to ascertain whether an individual is a low-, medium- or high-affinity binder.
TSPO PET brain imaging has been used to investigate neuroinflammation in people with HIV (Boerwinkle et al. 2020; Coughlin et al. 2014; Garvey et al. 2014; Hammoud et al. 2005; Vera et al. 2016; Wiley et al. 2006), with conflicting results. While higher TSPO binding in people with HIV has generally been reported compared with persons without HIV, the precise anatomical locations have varied between studies. These inconsistencies are likely related to (1) different methodologies used to quantify TSPO radiotracer uptake, (2) cohort differences (inclusion or exclusion of people with HIV with cognitive impairment), (3) differences in criteria for defining cognitive impairment (Nightingale et al. 2021) and (4) small sample sizes.
Strategies to reduce persistent immune activation and inflammation include the early initiation of ART, soon after HIV acquisition (Pace & Frater 2014). Early ART initiation may partly mitigate the effects uncontrolled HIV replication has on the central nervous system and thereby reduce the persistent neuroinflammation which has been observed in ART-treated individuals. Early evidence supports a reduction in cerebrospinal fluid biomarkers of inflammation when ART is initiated during acute HIV infection (Hellmuth et al. 2019; Oliveira et al. 2017), however, long-term data on brain parenchymal inflammation and neurological sequelae are lacking.
The aim of this study was to evaluate neuroinflammation, measured using TSPO [11C]PBR28 radiotracer binding, in people with HIV who initiated ART during acute versus chronic HIV infection, and compared with control individuals, using volume of distribution (VT) as a primary outcome and distribution volume ratio (DVR) as a secondary outcome.
Methods
All participants underwent structural cerebral magnetic resonance imaging (MRI) and cerebral positron emission tomography-computed tomography (PET-CT) imaging with [11C]PBR28 ligand at the Imanova Centre for Imaging Sciences, London, UK.
Participants living with HIV
Individuals attending HIV outpatient services at Imperial College Healthcare NHS Trust, London, UK, were invited to enrol into the study. Eligible participants were adult males ≥ 18 years of age, receiving ART for ≥ 3 months with plasma HIV RNA < 50 copies/mL, high affinity binders to [11C] PBR28 on TSPO genotypic testing and in good health. Exclusion criteria included significant neurological comorbidities, alcohol or recreational drug use disorder, contraindication to arterial cannulation, lumbar puncture or magnetic resonance (MR) imaging, body mass index > 30 kg/m2 and participation in another research study involving ionising radiation such that the subject would be exposed to a cumulative dose of > 10 mSv in the previous 12 months. Participants with HIV were assigned into two cohorts: participants who initiated ART during acute HIV infection were defined as people who had started ART within three months of confirmed primary HIV infection, based on one of the following six criteria: a) positive HIV-1 serology within a maximum of 24 weeks of a documented negative HIV-1 serology test result (can include point of care test (POCT) using blood for both tests), b) a positive p24 antigen result and a negative HIV antibody test, c) negative antibody test with either detectable HIV RNA or proviral DNA, d) Recent Infection Testing Algorithm (RITA) test reported as “Incident” confirming the HIV-1 antibody avidity is consistent with recent infection (within the preceding 16 weeks), e) weakly reactive or equivocal 4th generation HIV antibody-antigen test and f) equivocal or reactive antibody test with < 4 bands on western blot. Participants who initiated ART during chronic HIV infection were defined as people who had started ART more than six months after known or presumed date of HIV acquisition.
The studies were approved by UK Research Ethics Committees (REC) (reference numbers 16/LO/0096 and 12/LO/1570). Permission was obtained from the UK Administration of Radioactive Substances Advisory Committee (ARSAC) (reference numbers: RPC 630/3764/34269 and 630/3764/ 29,163) for the administration of [11C] PBR28. All participants provided written informed consent prior to commencing any study procedures.
Control participants
Brain [11C] PBR28 PET-CT imaging data for the control participants were obtained from participants enrolled into either a human pharmacological blocking study to determine brain [11C] PBR28 binding potential in vivo(Owen et al. 2014) or enrolled into a brain [11C] PBR28 PET-CT imaging database for healthy volunteers supported by GlaxoSmithKline plc. The eligibility criteria to enrol into the pharmacological blocking study and the GlaxoSmithKline plc healthy volunteers’ database were similar to the criteria for the studies for persons with HIV.
Brain magnetic resonance imaging (MRI)
T1-weighted whole brain structural images were obtained on a Siemens MAGNETOM® Verio 3.0 Tesla magnetic resonance scanner (Siemens Healthineers, Munich, Germany).
Brain [11C] PBR28 positron emission tomography-computed tomography (PET-CT) imaging
-
1
Radiotracer synthesis
[11C] PBR28 was produced on site at the Imanova Centre for Imaging Sciences immediately before use, according to local standard operating procedures. Quality assurance assessments were made using validated procedures in accordance with good manufacturing practices before injection to ensure the manufactured [11C] PBR28 met the prerequisite specifications
-
2
Arterial blood sampling and processing
Following skin infiltration with 1% lidocaine as local anaesthetic, a cannula was inserted into the participant’s radial artery to enable regular arterial blood sampling throughout the cerebral PET-CT imaging procedure.
-
3
PET imaging data acquisition
The cerebral [11C] PBR28 PET-CT images were acquired using a Biograph 6 PET-CT scanner (Siemens Healthcare). Participants were injected with an intravenous bolus of [11C] PBR28 over 20 s at the beginning of the 90-min 3D-mode of dynamic PET acquisition; injected activities ranged from 120.45 to 374.45 MBq.
-
4
Blood data processing
Arterial blood was sampled to enable generation of an arterial plasma input function. A continuous sampling system (ABSS Allogg, Mariefred, Sweden) was used to measure whole blood activity each second for the first 15 min of each scan. Discrete arterial blood samples were manually withdrawn at 5, 10, 15, 20, 30, 50, 70 and 90 min time points after the scan commenced to measure whole blood and plasma activity. Samples obtained at 5, 10, 20, 30, 50, 70 and 90 min time points after the scan commenced were analysed using high-performance liquid chromatography (HPLC) to determine the fraction of parent radioactivity in the arterial plasma. The first three discrete blood samples were used to calibrate the continuous blood data, then the continuous and discrete datasets were used to form a whole blood activity curve, covering the duration of the scan. Discrete plasma samples were divided by the corresponding whole blood samples to obtain the plasma-over-blood (POB) data.
An exponential approach to a constant POB model was fitted (Gunn et al. 2000), to generate the metabolite-corrected plasma input function. This POB value was then multiplied by the whole blood curve to generate a total plasma curve. A sigmoid model was used to fit the parent fraction data.
The resulting fitted parent fraction profile was multiplied by the total plasma curve and then smoothed post-peak using a tri-exponential fit to derive the required parent plasma input function. For each scan, a time delay correction was fitted and applied to the input function to account for any time delay between blood sample measurement and the tomographic measurements of the tissue data. Free fraction in plasma (fp) was measured through ultrafiltration (Amicon Ultra regenerated cellulose MWCO 30 kDa, Millex, Ireland) in triplicate using Tris buffer (0.1M, pH = 7.4) to determine and enable correction for non-specific binding.
[11C] PBR28 positron emission tomography-computed tomography (PET-CT) data processing
All brain [11C] PBR28 PET-CT data from the participants were analysed using the MIAKAT™ v4.3.17 pipeline via the same process. PET data were reconstructed using filtered back projection, corrected for attenuation and scatter (based on low-dose CT acquisition scan). Dynamic data were binned into 26 frames (durations: 8 × 15 s, 3 × 1 min, 5 × 2 min, 5 × 5 min and 5 × 10 min). Motion correction in the dynamic PET data was performed via frame-to-frame image registration of the non-attenuation corrected PET image to the participants’ structural T1-magnetic resonance image using SPM5 (Wellcome Trust Centre for Neuroimaging), with a mutual information cost function.
The CIC neuroanatomical atlas version 2.0 was nonlinearly deformed into the participant’s space, via structural T1-MRI data mapping, to obtain a personalised anatomical parcellation of regions of interest (ROIs). The following ROIs were chosen to assess levels of binding based on regions investigated in previously published TSPO PET studies in people with HIV (Boerwinkle et al. 2020; Coughlin et al. 2014; Garvey et al. 2014; Hammoud et al. 2005; Rubin et al. 2018; Vera et al. 2016; Wiley et al. 2006): whole brain, frontal lobe, occipital lobe, temporal lobe, parietal lobe, amygdala, hippocampus, posterior cingulate gyrus, anterior cingulate, basal ganglia, globus pallidus, striatum, caudate, putamen, thalamus, cerebellum, brainstem, midbrain, pons and medulla. Each ROI was then applied to the dynamic PET data to derive regional time-activity curves.
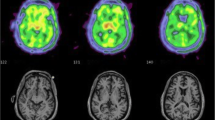
Model fitting and parameter estimation was performed using the MIAKAT™ pipeline, implemented in MATLAB™ R2019b (The MathWorks, Natick, MA, USA). A two-tissue compartment model using the metabolite-corrected plasma input function was applied to the dynamic PET data using a fixed blood volume correction of 5%. For each ROI evaluated, the main outcome measured was the total volume of distribution (VT) estimated from the rate constant as described previously (Gunn et al. 2000). The secondary outcome, distribution volume ratio (DVR), was calculated by normalising the VT at each ROI to cortical grey matter, a pseudo-reference region previously investigated in a [11C] PBR28PET study in people with HIV (Vera et al. 2016).
Statistical analysis
Participant demographics and clinical parameters were described using median (interquartile range) and total (percentage), as appropriate. Differences in variables between participants who started ART during acute HIV infection and chronic HIV infection were analysed using the Mann–Whitney U test and Fisher’s exact test, as appropriate. Difference in variables between participants who started ART during acute HIV infection, participants who started ART during chronic HIV infection and control participants were analysed using the Kruskall-Wallis test. Statistical analyses were conducted using SPSS version 25 (IBM Corp, Armonk, NY, US) and p-values < 0.05 were considered statistically significant throughout.
Differences in VT and DVR at the pre-selected ROIs between the participants who started ART during acute HIV infection, participants who started ART during chronic HIV infection and control participants were compared using the Kruskall-Wallis and Mann–Whitney U-tests, as appropriate.
Results
Participant characteristics
Participant demographics and clinical parameters are described in Table 1. Seventeen participants living with HIV (9 who initiated ART during acute HIV infection and 8 who initiated ART during chronic HIV infection) and 8 control participants completed [11C] PBR28 PET brain imaging and were included. All participants included in the study were male and high-affinity binders on TSPO genotypic testing. Apart from age and bodyweight, no additional demographic or clinical data were available for the control participants.
Overall, the control participants were younger (median (IQR) 21 years (20, 25)) compared with participants who initiated ART during acute HIV infection (median (IQR) 40 years (30, 46)), who were in turn, younger than the participants who initiated ART during chronic HIV infection (median (IQR) 44 years (41, 47)) (Table 1). Of the three groups, participants who initiated ART during acute HIV infection had the lowest median bodyweight (median (IQR) 74.7 kg (69.1, 74.9)).
Participants living with HIV were predominantly of white ethnicity (Table 1). Overall, participants who initiated ART during acute HIV infection versus chronic HIV infection had a shorter duration since HIV diagnosis (median (IQR) 3.3 years (2.6, 4.5) vs 15.0 years (3.0, 15.5), p = 0.04) and shorter duration on ART (median (IQR) 3.2 years (2.5, 4.4) vs 4.0 years (3.0, 13.5), p = 0.05) (Table 1).
Median pre-treatment HIV RNA load was numerically lower in participants who initiated ART during acute HIV infection compared with chronic HIV infection (median (IQR) 4.4 (3.2, 5.7) vs 5.3 (4.8, 5.4) log10 HIV RNA copies/mL, p = 0.61) and current CD4+ T-cell count was similar between the two HIV-positive groups (Table 1). Participants who initiated ART during acute HIV infection had higher nadir CD4+ T-cell count, higher current CD4+/CD8+ T-cell ratio and lower current CD8+ T-cell count compared to participants who initiated ART during chronic HIV infection (Table 1).
At the point when the brain PET-CT scan was performed, the majority of participants who initiated ART during acute HIV infection were on INSTI-based ART regimens (67%), whereas for the participants who initiated ART during chronic HIV infection, equal numbers of participants were on protease inhibitor (PI)-based and non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART regimens.
[11C] PBR28 total volume of distribution (VT) results
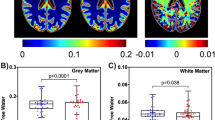
Table 2 describes the mean (standard deviation) [11C] PBR VT binding at the selected brain regions, stratified according to the study groups. Overall, no statistically significant differences in [11C] PBR28 (VT) binding between the three groups of participants at any of the selected regions of interest were observed (unadjusted p-value > 0.05 by the Kruskall-Wallis test for differences between the three groups at all 20 pre-selected ROIs) (Table 2).
Between the three groups, participants who initiated ART during chronic HIV infection demonstrated a trend towards higher mean [11C] PBR28 (VT) binding followed by control participants, with participants who initiated ART during acute HIV infection displaying lowest mean [11C] PBR28 (VT) binding at the majority of the ROIs: whole brain, frontal lobe, occipital lobe, temporal lobe, amygdala, hippocampus, posterior cingulate gyrus, anterior cingulate gyrus, caudate, thalamus, cerebellum, brainstem, midbrain and pons (Table 2 and Fig. 1).
[11C] PBR28 distribution volume ratio (DVR) results
Table 3 describes the differences in [11C] PBR28 DVR normalised to cortical grey matter at the 20 pre-selected anatomical brain ROIs between the three groups of participants. We observed no statistically significant differences in [11C] PBR28 DVR binding between the three groups of participants at any of the pre-selected regions of interest (unadjusted p-value > 0.05 for differences between the three groups at all 20 ROIs, using the Kruskall-Wallis test) (Table 3).
Mean [11C] PBR28 DVR results at the pre-selected ROIs were variable and showed no consistent pattern in trends towards differences between the three groups of participants (Table 3 and Fig. 2).
Conclusions
This study compares neuroinflammation using [11C] PBR28 binding as a proxy in people who initiated ART in acute HIV infection, people who initiated ART in chronic HIV infection and control participants. While no statistically significant differences in [11C] PBR28 binding were observed between the three groups, our analyses consistently demonstrated higher absolute mean [11C] PBR28 binding amongst people who initiated ART during chronic HIV infection compared with people who initiated ART during acute HIV infection and control participants at the majority of brain anatomical regions studied. Our findings should be interpreted with caution due to the small sample size of our pilot study, however this initial signal may herald a potential true signal that warrants further investigation. The trend towards lower absolute [11C] PBR28 binding in participants who initiated ART during acute infection compared with chronic infection corroborates the theory that early ART initiation soon after HIV acquisition may attenuate the neuroinflammatory responses widely reported in persons with HIV who initiated ART during chronic HIV infection (Ulfhammer et al. 2018; Yilmaz et al. 2008).
While some previous TSPO studies in people with HIV have identified statistically significant differences in TSPO binding at certain anatomical locations in people with HIV on ART compared with control participants, we did not observe this in our study. Reasons for the discrepant results between the various TSPO studies in people with HIV include the fact that the previous studies have used different TSPO radiotracers and different methodologies to quantify TSPO binding (Alagaratnam & Winston 2022). Quantification of TSPO binding remains a rapidly evolving field and our study utilised newer models and processes for quantification based on current gold-standard techniques that had not yet been determined at the time when the previous studies were conducted. In a previous study from our group, (Vera et al. 2016) estimated the continuous [11C] PBR28 plasma-to-blood ratio using a constant model in the plasma input function. However, we observed that an exponential-approaching-constant model provided a better fit for the data in the majority of the participants in our study. Brain [11C] PBR28 data for people who initiated ART during chronic HIV was provided from Vera et al.’s study (Vera et al. 2016), but the same control participants’ data were not available for us to use in our analysis, which may also explain the discrepancy seen between the results of our analyses. Additionally, brain [11C] PBR28 signals from grey matter were analysed, as at the time, it was considered the optimal strategy for identifying microglial activation. However, given that HIV disease affects both the grey and white matter throughout the brain, our analyses here included brain [11C] PBR28 signals from both grey and white matter. Furthermore, our study is limited by the sensitivity of [11C]PBR28; although [11C]PBR28 has greater sensitivity than previous TSPO ligands, it may not have sufficient sensitivity to determine differences in ligand binding between the three cohorts of participants in this study.
Whether to use absolute [11C] PBR28 binding (VT) or [11C] PBR28 VT binding normalised to a reference region (DVR) to report regional brain [11C] PBR28 binding remains hotly debated. Some studies have suggested a poor test–retest reproducibility with absolute TSPO VT binding due to intra-individual factors and normalisation to a reference or pseudo-reference region can control for unaccounted physiological factors such as stress responses, hormone-mediated changes in TSPO expression, blood cholesterol changes due to food intake and other genotypic factors that may affect TSPO radioligand uptake in the brain (Coughlin et al. 2014; Drugan 1996; Gavish et al. 1999; Jučaite et al. 2012). These factors may impact cerebral radioligand signal in TSPO PET imaging studies and can also cancel out non-binding effects on the TSPO radioligand signals. A study using [11C] DPA-713, a second-generation TSPO radioligand, demonstrated improved test–retest reproducibility when [11C] DPA-713 VT binding was normalised to a reference region (Coughlin et al. 2014). A major concern with normalising TSPO binding to a reference region is losing the ability to detect changes in the region used as the reference region, by effectively cancelling out the signal in the region of interest by normalising to another region also displaying a signal. Ideally, the reference region chosen should be unaffected by the disease under investigation. However, HIV disease generally affects the whole central nervous system, and a reliable disease-free brain TSPO reference region in people with HIV has not yet been identified. For this reason, we have chosen to use absolute [11C] PBR28 VT binding as our primary outcome, with [11C] PBR28 DVR binding normalised to cortical grey matter as the secondary outcome. Urgent consensus is required on the optimal methodology for determining [11C] PBR28 binding and the optimal reference region for TSPO studies. Until a gold-standard method of measuring TSPO radiotracer uptake is developed and accepted, interpreting and comparing results from TSPO PET neuroimaging studies will remain challenging.
Strengths of our study include the phenotypically well-described cohorts of participants living with HIV. Participants who initiated ART during acute HIV infection were strictly within 3 months of confirmed primary HIV infection. [11C] PBR28 binding quantification was performed using the gold-standard methodology using continuous plasma input function via arterial sampling.
On the converse, limitations include the small sample size due to the high cost of performing PET neuroimaging studies, and the control participants who were not demographically matched to the people living with HIV. Overall, participants who initiated ART during chronic HIV infection were older and had higher body weight upon enrolment into the study, which may reflect the normal ageing processes. While control participants were on average younger than both groups of HIV-positive participants, the control participants’ body weight were similar to that seen in participants who initiated ART during chronic HIV infection. To date, published findings on the effect of age on TSPO expression have been inconclusive with some studies demonstrating higher TSPO binding with increasing age and no effect in others (Cagnin et al. 2001; Paul et al. 2019; Rissanen et al. 2018; Schuitemaker et al. 2012; Tuisku et al. 2019). Body mass index has been shown to negatively correlate with brain [11C] PBR28 VT binding (Tuisku et al. 2019), however complete body mass index data was not available for our analysis. We are also limited by a lack of data on alcohol use, recreational drug use and smoking history which could be confounders to our findings.
Astrocytes and microglia play very different roles in the central nervous system but emerging data suggests that reactive astrocytes may also have a contributory role to TSPO expression signals (Lavisse et al. 2012) and it is becoming increasingly recognised that [11C] PBR28 binding can represent pro-, anti- and mixed inflammatory phenotypes (Owen et al. 2017). Thus, TSPO expression signals in this setting should be interpreted with caution and further studies are warranted to determine the phenotype (pro-, anti- and mixed inflammatory) of the signals identified and the cell type contributing to the [11C] PBR28 binding signals (microglia and astrocytes), by correlating brain TSPO binding results with neuropathological specimens with histochemistry and fluid biomarkers.
In summary, this study assessed the effect of early ART initiation soon after HIV acquisition on neuroinflammation using a novel molecular neuroimaging technique. We observed no significant differences in neuroinflammation, using [11C]PBR28 binding as a proxy between people who initiated ART during chronic HIV infection, people who initiated ART during primary HIV infection and control participants. A trend towards higher neuroinflammation in people who initiated ART during chronic infection compared with acute infection and control participants was observed, suggesting early ART initiation may reduce neuroinflammation.
Data availability
No datasets were generated or analysed during the current study.
References
Alagaratnam J, Winston A (2022) Molecular neuroimaging of inflammation in HIV. In Clin Exp Immunol 210(1). https://doi.org/10.1093/cei/uxab013
Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2005) Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. https://doi.org/10.1093/jnen/64.6.529
Banati RB, Egensperger R, Maassen A, Hager G, Kreutzberg GW, Graeber MB (2004) Mitochondria in activated microglia in vitro. J Neurocytol. https://doi.org/10.1007/s11068-004-0515-7
Boerwinkle AH, Strain JF, Burdo T, Doyle J, Christensen J, Su Y, Wisch JK, Cooley SA, Vaida F, Smith MD, Jafri H, Paul RH, Benzinger TLS, Ances BM (2020) Comparison of [11C]-PBR28 Binding Between Persons Living With HIV and HIV-Uninfected Individuals. J Acquired Immune Defic Syndr 85(2). https://doi.org/10.1097/QAI.0000000000002435
Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB (2001) In-vivo measurement of activated microglia in dementia. Lancet. https://doi.org/10.1016/S0140-6736(01)05625-2
Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, Roosa HV, Gage KL, Stathis M, Rais R, Rojas C, McGlothan JL, Watkins CC, Sacktor N, Guilarte TR, Zhou Y, Sawa A, Slusher BS, Caffo B, Pomper MG (2014) Regional brain distribution of translocator protein using [11C]DPA-713 PET in individuals infected with HIV. J NeuroVirol. https://doi.org/10.1007/s13365-014-0239-5
Drugan RC (1996) Peripheral benzodiazepine receptors: Molecular pharmacology to possible physiological significance in stress-induced hypertension. In Clinical Neuropharmacology. https://doi.org/10.1097/00002826-199619060-00002
Edén A, Marcotte TD, Heaton RK, Nilsson S, Zetterberg H, Fuchs D, Franklin D, Price RW, Grant I, Letendre SL, Gisslén M (2016) Increased Intrathecal Immune Activation in Virally Suppressed HIV-1 Infected Patients with Neurocognitive Impairment. PLoS ONE 11(6). https://doi.org/10.1371/journal.pone.0157160
Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, Winston A (2014) Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS 28(1):67–72. https://doi.org/10.1097/01.aids.0000432467.54003.f7
Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, Weizman A (1999) Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev
Gunn RN, Lammertsma AA, Grasby PM (2000) Quantitative analysis of [carbonyl-11C]WAY-100635 PET studies. Nucl Med Biol. https://doi.org/10.1016/S0969-8051(00)00115-3
Hammoud DA, Endres CJ, Chander AR, Guilarte TR, Wong DF, Sacktor NC, McArthur JC, Pomper MG (2005) Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J NeuroVirol 11(4). https://doi.org/10.1080/13550280500187351
Hellmuth J, Slike BM, Sacdalan C, Best J, Kroon E, Phanuphak N, Fletcher JLK, Prueksakaew P, Jagodzinski LL, Valcour V, Robb M, Ananworanich J, Allen IE, Krebs SJ, Spudich S (2019) Very Early Initiation of Antiretroviral Therapy During Acute HIV Infection Is Associated With Normalized Levels of Immune Activation Markers in Cerebrospinal Fluid but Not in Plasma. J Infect Dis. https://doi.org/10.1093/infdis/jiz030
Hogg RS, Heath KV, Yip B, Craib KJ, O’Shaughnessy MV, Schechter MT, Montaner JS (1998) Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA, J Am Med Assoc 279(6):450–454. https://doi.org/10.1001/jama.279.6.450
Jučaite A, Cselényi Z, Arvidsson A, Åhlberg G, Julin P, Varnäs K, Stenkrona P, Andersson J, Halldin C, Farde L (2012) Kinetic analysis and test-retest variability of the radioligand [11C](R)-PK11195 binding to TSPO in the human brain - a PET study in control subjects. EJNMMI Res. https://doi.org/10.1186/2191-219X-2-15
Lavisse S, Guillermier M, Hérard AS, Petit F, Delahaye M, Van Camp NV, Haim LB, Lebon V, Remy P, Dollé F, Delzescaux T, Bonvento G, Hantraye P, Escartin C (2012) Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci. https://doi.org/10.1523/JNEUROSCI.1487-12.2012
Liu GJ, Middleton RJ, Hatty CR, Kam WWY, Chan R, Pham T, Harrison-Brown M, Dodson E, Veale K, Banati RB (2014) The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. https://doi.org/10.1111/bpa.12196
May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, Hay P, Johnson M, Palfreeman A, Gilson R, Chadwick D, Martin F, Hill T, Walsh J, Post F, Fisher M, Ainsworth J, Jose S, Leen C, Sabin C (2014) Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS (London, England) 28(8):1193–1202. https://doi.org/10.1097/QAD.0000000000000243
Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurolog Sci 202(1–2). https://doi.org/10.1016/S0022-510X(02)00207-1
Nightingale S, Dreyer AJ, Saylor D, Gisslén M, Winston A, Joska JA (2021) Moving on From HAND: Why We Need New Criteria for Cognitive Impairment in Persons Living With Human Immunodeficiency Virus and a Proposed Way Forward. Clin Infect Dis 73(6). https://doi.org/10.1093/cid/ciab366
Oliveira MF, Chaillon A, Nakazawa M, Vargas M, Letendre SL, Strain MC, Ellis RJ, Morris S, Little SJ, Smith DM, Gianella S (2017) Early Antiretroviral Therapy Is Associated with Lower HIV DNA Molecular Diversity and Lower Inflammation in Cerebrospinal Fluid but Does Not Prevent the Establishment of Compartmentalized HIV DNA Populations. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1006112
Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R, Lewis YL, Libri V, Barletta J, Ramada-Magalhaes J, Kamalakaran A, Nutt DJ, Passchier J, Matthews PM, Gunn RN, Rabiner EA (2014) Determination of [11C]PBR28 binding potential in vivo: A first human TSPO blocking study. J Cereb Blood Flow Metab. https://doi.org/10.1038/jcbfm.2014.46
Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, Galloway D, Williams JB, Lehr J, Mandhair H, Peferoen LAN, Taylor PC, Amor S, Antel JP, Matthews PM, Moore CS (2017) Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J Cereb Blood Flow Metabol 37(8). https://doi.org/10.1177/0271678X17710182
Pace M, Frater J (2014) A cure for HIV: Is it in sight? Exp Rev Anti-Infect Ther 12(7):783–791. https://doi.org/10.1586/14787210.2014.910112
Paul S, Gallagher E, Liow JS, Mabins S, Henry K, Zoghbi SS, Gunn RN, Kreisl WC, Richards EM, Zanotti-Fregonara P, Morse CL, Hong J, Kowalski A, Pike VW, Innis RB, Fujita M (2019) Building a database for brain 18 kDa translocator protein imaged using [11C]PBR28 in healthy subjects. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X18771250
Rissanen E, Tuisku J, Vahlberg T, Sucksdorff M, Paavilainen T, Parkkola R, Rokka J, Gerhard A, Hinz R, Talbot PS, Rinne JO, Airas L (2018) Microglial activation, white matter tract damage, and disability in MS. Neurology - Neuroimmunology Neuroinflammation. https://doi.org/10.1212/nxi.0000000000000443
Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, Mcarthur JC, Collier AC, Evans SR, Ellis RJ (2007) The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS (london, England) 21(14):1915–1921. https://doi.org/10.1097/QAD.0b013e32828e4e27
Rubin LH, Sacktor N, Creighton J, Du Y, Endres CJ, Pomper MG, Coughlin JM (2018) Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 32(12). https://doi.org/10.1097/QAD.0000000000001858
Schouten J, Wit FW, Stolte IG, Kootstra NA, Van Der Valk M, Geerlings SE, Prins M, Reiss P (2014) Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between hiv-infected and uninfected individuals: The age H IV cohort study. Clin Infect Dis 59(12):1787–1797. https://doi.org/10.1093/cid/ciu701
Schuitemaker A, van der Doef TF, Boellaard R, van der Flier WM, Yaqub M, Windhorst AD, Barkhof F, Jonker C, Kloet RW, Lammertsma AA, Scheltens P, van Berckel BNM (2012) Microglial activation in healthy aging. Neurobiol Aging. https://doi.org/10.1016/j.neurobiolaging.2010.09.016
Simioni S, Cavassini M, Annoni J-M, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave J-P, Giacobini E, Hirschel B, Du Pasquier RA. (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS (london, England) 24(9):1243–1250. https://doi.org/10.1097/QAD.0b013e3283354a7b
Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA (1995) Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci 15(7 Pt 2):5263–5274. http://www.ncbi.nlm.nih.gov/pubmed/7623150
Tuisku J, Plavén-Sigray P, Gaiser EC, Airas L, Al-Abdulrasul H, Brück A, Carson RE, Chen MK, Cosgrove KP, Ekblad L, Esterlis I, Farde L, Forsberg A, Halldin C, Helin S, Kosek E, Lekander M, Lindgren N, Marjamäki P, Cervenka S (2019) Effects of age, BMI and sex on the glial cell marker TSPO — a multicentre [11C]PBR28 HRRT PET study. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-019-04403-7
Ulfhammer G, Eden A, Mellgren S, Fuchs D, Zetterberg H, Hagberg L, Nilsson S, Yilmaz A, Gisslen M (2018) Persistent central nervous system immune activation following more than 10 years of effective HIV antiretroviral treatment. AIDS. https://doi.org/10.1097/QAD.0000000000001950
Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, Rabiner EA, Kalk N, Bishop C, Gunn RN, Matthews PM, Winston A (2016) Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology 86(15):1425–1432. https://doi.org/10.1212/WNL.0000000000002485
Wiley CA, Lopresti BJ, Becker JT, Boada F, Lopez OL, Mellors J, Meltzer CC, Wisniewski SR, Mathis CA (2006) Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J NeuroVirol 12(4). https://doi.org/10.1080/13550280600873868
Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslén M (2008) Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. https://doi.org/10.1097/QAI.0b013e31815ace97
Acknowledgements
This project was funded by the NIHR Imperial Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. Infrastructure support for this research was provided by the NIHR Imperial Biomedical Research Centre (BRC). We would like to thank all participants and staff involved in the study.We would especially like to thank the following groups and individuals for their contributions (listed alphabetically): GSK plc for providing the healthy volunteer PET brain neuroimaging database. Imperial College Healthcare NHS Trust. Imperial College London, London, UK. Jim Myers. Invicro, a Konica Minolta Company. Eugenii A. Rabiner. Roger Gunn. Saleem Azeem
Funding
This project was funded by the NIHR Imperial Biomedical Research Centre (BRC), grant reference number: P57282. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. Infrastructure support for this research was provided by the NIHR Imperial Biomedical Research Centre (BRC). J Alagaratnam has received financial support to attend scientific conferences from MSD and Gilead Sciences. J Thornhill has received speaker fees by Gilead Sciences, and conference registration fees from ViiV healthcare. J Underwood is supported by the Medical Research Council [grant number MR/T023791/1]. P Edison was funded by the Medical Research Council and now by Higher Education Funding Council for England (HEFCE). He has also received grants from Alzheimer’s Research, UK, Alzheimer’s Drug Discovery Foundation, Alzheimer’s Society, UK, Alzheimer’s Association, US, Medical Research Council, UK, Novo Nordisk, Piramal Life Sciences and GE Healthcare. P Edison is a consultant to Roche, Pfizer, Cyodyn, Biohaven Pharmaceuticals and Novo Nordisk. He has received speaker fees from Novo Nordisk, Pfizer, Nordea, Piramal Life Science. He has received educational and research grants from GE Healthcare, Novo Nordisk, Piramal Life Science/Life Molecular Imaging, Avid Radiopharmaceuticals and Eli Lilly. He is an external consultant to Novo Nordisk and a member of their Scientific Advisory Board. S Fidler received funding and research grants to Imperial College London from the National Institutes of Health (NIH), Bill & Melinda Gates Foundation (BMGF) and Medical Research Council (MRC).A Winston has received honoraria or research grants on behalf of Imperial College London or been a consultant or investigator in clinical trials sponsored by Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, Roche and ViiV Healthcare.
Author information
Authors and Affiliations
Contributions
JA: drafting and revising the manuscript, study concept and design, analysis and interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, study supervision. JT: obtained funding, revising the manuscript, study concept or design, acquisition of data, study supervision, analysis or interpretation of data, accepts responsibility for conduct of research and final approval ZF: revising the manuscript, technical support for the analysis and interpretation of data JHV: revising the manuscript, study concept or design, analysis and interpretation of data JU: obtained funding, revising the manuscript, study concept or design, acquisition of data, study supervision, analysis or interpretation of data, accepts responsibility for conduct of research and final approval RH: revising the manuscript, acquisition of data, study supervision GS: technical support for the analysis and interpretation of data DO: revising the manuscript, study concept or design, analysis or interpretation of data PE: revising the manuscript, study concept or design, analysis or interpretation of data SF: obtained funding, revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, study supervision AW: conceptualised the idea for the study, obtained funding, drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, study supervision. All authors provided critical review of the draft manuscript for intellectual content and have seen and approved the final version.
Corresponding author
Ethics declarations
Ethical Approval
The studies included in this manuscript were approved by UK Research Ethics Committees (REC) (reference numbers 16/LO/0096 and 12/LO/1570). Permission was obtained from the UK Administration of Radioactive Substances Advisory Committee (ARSAC) (reference numbers: RPC 630/3764/34269 and 630/3764/ 29163) for the administration of [11C] PBR28.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alagaratnam, J., Thornhill, J.P., Fan, Z. et al. Differences in neuroinflammation in people who started antiretroviral treatment during primary versus chronic HIV infection: an 18kDa Translocator protein (TSPO) positron emission tomography (PET) study. J. Neurovirol. (2024). https://doi.org/10.1007/s13365-024-01200-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13365-024-01200-3