Abstract
Here in the present article, the state of art for nanotechnology-enabled nanogel theranostics and the upcoming concepts in nanogel-based therapeutics are summarized. The benefits, innovation, and prospects of nanogel technology are also briefly presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology has advanced tremendously for therapeutic delivery and diagnostic application in recent years. Several nanocarriers have been tested for translation to clinics (Marrache et al. 2013). These nanocarriers include liposomes, dendrimers, polymeric nanocarriers, hydrogels, and nanogels. This review will highlight the intelligent nanocarriers, “nanogels,” used for theranostics (therapy and diagnostics) applications. Nanogels are nanomaterials in the nanometer range (10 to 100 nm) and formed via a three-dimensional network of crosslinked polymer chains or materials from gel-like structures. Nanogels have advanced properties as compared to other existing nanocarriers. The limitation associated with different nanocarriers, such as liposomes, is their stability over time and polydispersibility. Polylactic-co-glycolic acid (PLGA) and chitosan nanoparticles are accompanied by drug burst release. The inorganic particles showed toxicity issues and immune responses in clinical trials (Zhang et al. 2022). Keeping these limitations in mind, nanogels have conducted improved features. According to the standard nomenclature, IUPAC nanogel is a gel nanoparticle of any shape with a diameter of 1–100 nm (Gold et al. 1997; Kar et al. 2017). These are formed by physical or chemical cross-linked polymers. The research has expanded over the years, and nanogels have gained attention in the material science (Asadian-Birjand et al. 2012; Topuz and Uyar 2022; Vashist et al. 2017) for the theranostics application. Recently, the theranostics application of nanocarriers has been utilized to treat various diseases where the diagnostic and therapeutic are integrated with a single platform (Wang et al. 2018a). Funkhouser first defined the term “theranostics” in 2002. Since then, a lot of progress has been made in developing nanocarriers in a hybrid format to have a combinatory effect. Various nanofillers like carbon dots (Zhao et al. 2022), metal nanoparticles (Lux et al. 2015), SPIONs (Mauro et al. 2019), carbon-based materials (Wang et al. 2018b), and quantum dots (Yang et al. 2014) have been used to develop nanogels for theranostics.
The current strategy of developing bio-polymeric theranostic agents has a pronounced conviction of incorporating inherent features of non-toxicity, biodegradability, biocompatibility, and high sensitivity. Literature reveals that the nano-range is preferred for various anti-cancer therapies (Cho et al. 2020a; Pei et al. 2018) and other biomedical applications (Kaewruethai et al. 2021; Vashist et al. 2018). The focus is on developing nanogels that are easy to synthesize with low-cost materials and result in biocompatibility and biodegradability and give no toxicity to the cellular microenvironment with intrinsic photoluminescence and increased photostability. A study demonstrated nanogels based on biodegradable photoluminescent polymer templates that are derived from compatible monomers like citric acid, maleic acid, L-cysteine, and polyethylene glycol (Gyawali et al. 2018). These nanogels possess strong potential for theranostics medicine and can be used for real-time fluorescence-based imaging. They are also deployed for the cell labeling (Gyawali et al. 2018). Nanogels are better candidates selected for enhancing tumor accumulation. A recent study showed the development of fucoidan-based theranostic nanogel, which has the unique feature of recovering its photoactivity in response to intracellular redox potential when internalized into cancer cells. This study gives an excellent platform for selecting near-IR imaging and improved photothermal therapy of tumors (Cho et al. 2020b).
Auto-fluorescent nanogels for theranostics
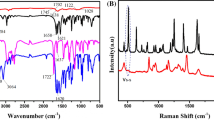
Researchers across the globe have put sincere efforts forward to develop efficient nanogel- based delivery systems to deliver hydrophobic drugs, cancer immunotherapy (Ma et al. 2022), oligonucleotides, and other bioactives across the BBB (Vinogradov et al. 2004). Recent research work on magneto-electric nanoparticles (MENP) from our group suggested that MENP-mediated drug delivery has the potential to improve the outcomes of anti-retroviral (ARV) therapy significantly and can transport neuron-resuscitating agents to cross BBB without altering the blood–brain barrier integrity (Kaushik et al. 2019, Kolishetti et al. 2022). Despite the tremendous success of the combined antiretroviral therapy (cART) in reducing the mortality rate in HIV-infected individuals, viral persistence remains a daunting challenge posing a significant barrier to HIV cure. The most critical challenge in existing therapies is that HIV infection requires lifelong adherence to daily dosing of ART, which may result in drug resistance. Besides this constant threat of the emergence of drug-resistant viral variants and overall cost compounded with adverse effects associated with ART necessitates the development of novel therapies. These therapies are aimed at targeting the integrated provirus within the host cellular DNA to eliminate the HIV viral reservoirs (Kaushik et al. 2019; Osborne et al. 2020). To address these challenges, our research group has developed and patented a very simple and stable bio-polymeric micro/nanogel system, which can be used for in vitro and in vivo theranostics applications for various diseases (Fig. 1). Owing to their novel characteristics, biopolymers, such as chitosan and hydroxyethyl cellulose (HEC), have gained huge importance for developing nanotechnology-based therapeutics and imaging tools. Bio-polymeric auto-fluorescent hydrogels in both micro- and nanoscales were developed using completely natural polymers chitosan, HEC, and sustainable resource polyol exhibiting complete biocompatibility (in the concentration range 10–100 µg/ml) tested over a wide range of host cells like astrocytes, PBMCs, and microglia (Vashist et al. 2020).
Moreover, their intrinsic fluorescence over a wide dynamic range of emission wavelengths (450–750 nm) and (710–810 nm) micro/nanogel particles (Fig. 2) obviates the requirement of an external fluorescent dye/reagent for their use in in vivo imaging-based theranostic applications (Vashist et al. 2020, 2019). The developed nanogel particles were detected with a 405-nm excitation laser and emission at 505–560 nm, demonstrating the maximum fluorescence intensity with 50 μg/ml of nanogel concentration. Our preliminary animal studies in Balb/c mice injected subcutaneously with 20 mg/kg of micro/nanogel particles revealed in vivo fluorescence of micro/nanogel particles using Ami HT-Spectral Instruments-based imaging (unpublished data not shown) underpinning them as a novel tool for in vivo imaging. Interestingly, the developed micro/nanogels significantly inhibited both viral transcription and viral release disrupting the HIV life cycle in T-cell enriched PBMCs, astrocytes, and macrophages, suggesting an inherent anti-HIV activity of synthesized micro/nanogels. Hence, the developed auto-fluorescent nanogels are anticipated to act as an anti-viral agent for HIV treatment and are proposed to be tested as a prophylactic agent against HIV in high-risk groups (Nair et al. 2022).

Copyright permission from reference Vashist et al. (2020) under Creative Commons Attribution (“CC BY”) license
Fluorescence properties of the nanogels at various concentrations using flow cytometer. A The mean intensity values of the single-particle population for each channel at an excitation wavelength of 405 nm. B Images acquired for the presence of fluorescence for and aggregated and single particle in each channel at an excitation wavelength of 405 nm.
Nanogel-based theranostics for CNS diseases and delivery across blood–brain barrier
Nanogels hold great potential as a drug delivery system for CNS diseases. The characteristic features of nanogels which make them unique are their high stability in physiological solutions, high drug encapsulation efficiency, biodegradability, biocompatibility, core–shell structures, and high permeability and stimuli responsiveness. These properties make them better nanocarriers than other existing nanocarriers and can thus cross the blood–brain barrier to a greater extent. Nanogels are a promising carrier for achieving effective therapeutic doses across the BBB. Nanogels have been deployed for managing various brain diseases like Alzheimer’s, Parkinson’s, multiple sclerosis, brain tumor, and neuroAIDS. The presence of tough BBB and blood-cerebrospinal fluid barrier (BCSFB) makes brain drug delivery very challenging. In this regard, nanogels can be engineered so that they can be transmigrated across BBB in a non-invasive manner (Cuggino et al. 2019). It is well known that therapeutics > 500 Da cannot cross the BBB. The therapeutic has to transmigrate across the BBB to treat brain diseases effectively. In one exciting study, fluorescently labeled poly(N-isopropylmethacrylamide) (p(NIPMAM)) nanogels were tested for their stiffness and their transport. It was observed that the more densely cross-linked nanogels were uptaken by brain endothelial cells. On the contrary, the less densely cross-linked nanogels showed the highest transcytosis property (Ribovski et al. 2021). Figure 3 demonstrates that nanogels with different stiffnesses tend to interact with the apical region of polarized brain endothelial cells (Ribovski et al. 2021). Another essential aspect in these years is nasal delivery of the nanogels for enhanced brain drug delivery. Hydrogen bond-enhanced nanogel for delivery of albiflorin to treat Parkinson’s disease (PD) was reported. Glycyrrhizic acid was utilized as a hydrogen bond crosslinker, preventing the drug’s premature release. These studies demonstrate the potential of nanogels for selective brain distribution and accumulation and are promising carriers for treating PD (Chen et al. 2022). Oxytocin-loaded angiopep-2-modified chitosan nanogels have been developed to address early intervention for Alzheimer's disease (AD). These nanogels inhibit the innate inflammatory response. Oxytocin was released and explicitly bound to upregulated oxytocin receptor and thus inhibiting the microglial activation and reducing the inflammatory cytokine levels (Ye et al. 2022). Numerous other recent studies have been reported showing the potential of nanogel-based theranostics for CNS diseases and delivery across blood–brain barrier (Arjun et al. 2022; Vdovchenko 2022; Vinogradov et al. 2004).

Copyright from reference Ribovski et al. 2021 Elsevier under Creative Commons CC-BY license
Low nanogel stiffness favors nanogel transcytosis across an in vitro blood–brain barrier.
Future prospects and conclusion
The ideal nanocarrier can be sustained in the human body for a longer circulation time to deliver the therapeutics with minimum or no side effects. The observed non-target effects can be reduced by passive targeting (Kitayama et al. 2022) or antibody-mediated active targeting of diseased tissue or organs (Li and Qi 2021). Tagging the nanocarrier with ligands and cell-penetrating peptides may facilitate their intracellular delivery (Khalil and Harashima 2023; Liu et al. 2022). Thus, nanogels are tunable and can be functionalized to make them transmigrate across physiological barriers (Zhao et al. 2021) such as gut barrier (Lee et al. 2020), blood–brain barrier (Ribovski et al. 2021), corneal epithelial barrier (Lin et al. 2021), and skin barrier (Cuggino et al. 2019). Smart nanogel may be designed with a capacity to circulate for longer intervals with sustained release, and droplet-based microfluidic development of nanogels for controlled drug delivery can be used to achieve efficiency. Using fluorescent probes and dyes in nanocarriers for imaging purposes bears severe limitations, such as experimental artifacts, toxicity, rapid clearance, photobleaching, and low specificity. In this regard, our nanogel technology has organic biopolymers with the least toxicity and more accuracy with detectable sensitivity. There is a wide range of applications for nanogels ranging from diagnostics to therapeutics. The developed nanogels can be deployed to deliver various biologics, nucleic acids, drugs, and RNA/DNA for the treatment of brain tumors and other carcinomas in the human body, in addition to HIV therapeutics. The existing imaging technologies, such as CT, MRI, PET, and FMT, have their strength and limitations (Lim et al. 2015). The advancements in nanomaterials used for theranostics include nanofillers like CNT, graphene, superparamagnetic nanoparticles, and Au nanoparticles that enhance MRI contrast. Thus, nanogels enable superior imaging with improved diagnostic efficacy. The other important parameter for nanogel-based therapeutics is to optimize the PK and PD properties of drugs loaded inside the nanogel. Thus, clinical translation is still a big challenge. Yet, the advancement in nanogel technology and research team collaborations from multidisciplinary areas may prove to be instrumental in developing a new class of nanogels for clinical use.
Data availability
The datasets generated for this review are available on request to the corresponding authors.
References
Arjun P, Freeman JL, Kannan RR (2022) Neurospecific fabrication and toxicity assessment of a PNIPAM nanogel encapsulated with trans-tephrostachin for blood-brain-barrier permeability in zebrafish model. Heliyon 8:e10237
Asadian-Birjand M, Sousa-Herves A, Steinhilber D, Cuggino JC, Calderon M (2012) Functional nanogels for biomedical applications. Curr Med Chem 19:5029–5043
Chen Y-B, Qiao T, Wang Y-Q, Cui Y-L, Wang Q-S (2022) Hydrogen bond-enhanced nanogel delivery system for potential intranasal therapy of Parkinson’s disease. Mater Des 219:110741
Cho MH, Li Y, Lo P-C, Lee H, Choi Y (2020a) Fucoidan-based theranostic nanogel for enhancing imaging and photodynamic therapy of cancer. Nano-Micro Letters 12:47
Cho MH, Li Y, Lo P-C, Lee H, Choi Y (2020b) Fucoidan-based theranostic nanogel for enhancing imaging and photodynamic therapy of cancer. Nano-Micro Letters 12:1–15
Cuggino JC, Blanco ERO, Gugliotta LM, Igarzabal CIA, Calderon M (2019) Crossing biological barriers with nanogels to improve drug delivery performance. J Control Release 307:221–246
Gold V, Loening KL, McNaught AD, Shemi P (1997) IUPAC compendium of chemical terminology, 2nd edn. Blackwell Science, Oxford
Gyawali D, Kim JP, Yang J (2018) Highly photostable nanogels for fluorescence-based theranostics. Bioactive Materials 3:39–47
Kaewruethai T, Laomeephol C, Pan Y, Luckanagul JA (2021) Multifunctional polymeric nanogels for biomedical applications. Gels 7:228
Kar M, Fechner L, Nagel G, Glitscher E, Rimondino GN, Calderón M (2017) Responsive nanogels for anti-cancer Therapy. Nanogels Biomed Appl 30:210
Kaushik A, Yndart A, Atluri V, Tiwari S, Tomitaka A, Gupta P, Jayant RD, Alvarez-Carbonell D, Khalili K, Nair M (2019) Magnetically guided non-invasive CRISPR-Cas9/gRNA delivery across blood-brain barrier to eradicate latent HIV-1 infection. Sci Rep 9:3928
Khalil IA, Harashima H (2023) Lipid‐based nanoparticles using cell‐penetrating peptides. Cell‐Penetrating Peptides: Design, Development and Applications 315–328
Kitayama Y, Yamada T, Kiguchi K, Yoshida A, Hayashi S, Akasaka H, Igarashi K, Nishimura Y, Matsumoto Y, Sasaki R (2022) In vivo stealthified molecularly imprinted polymer nanogels incorporated with gold nanoparticles for radiation therapy. J Mater Chem B 10:6784–6791
Kolishetti N, Vashist A, Arias AY, Atluri V, Dhar S, Nair M (2022) Recent advances, status, and opportunities of magneto-electric nanocarriers for biomedical applications. Mol Aspects Med 83:101046. https://doi.org/10.1016/j.mam.2021.101046
Lee Y, Sugihara K, Gillilland MG III, Jon S, Kamada N, Moon JJ (2020) Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater 19:118–126
Li L, Qi Z (2021) Recent advances in cell‐mediated drug delivery systems for nanomedicine and imaging. Fund Drug Deliv 263–284
Lim E-K, Kim T, Paik S, Haam S, Huh Y-M, Lee K (2015) Nanomaterials for theranostics: recent advances and future challenges. Chem Rev 115:327–394
Lin H-Y, Wang S-W, Mao J-Y, Chang H-T, Harroun SG, Lin H-J, Huang C-C, Lai J-Y (2021) Carbonized nanogels for simultaneous antibacterial and antioxidant treatment of bacterial keratitis. Chem Eng J 411:128469
Liu Y, Zhao Z, Li M (2022) Overcoming the cellular barriers and beyond: recent progress on cell penetrating peptide modified nanomedicine in combating physiological and pathological barriers. Asian J Pharma Sci 17(4):523–543
Lux J, White AG, Chan M, Anderson CJ, Almutairi A (2015) Nanogels from metal-chelating crosslinkers as versatile platforms applied to copper-64 PET imaging of tumors and metastases. Theranostics 5:277–288
Ma X, Li S-J, Liu Y, Zhang T, Xue P, Kang Y, Sun Z-J, Xu Z (2022). Bioengineered nanogels for cancer immunotherapy. Chem Soc Rev
Marrache S, Kumar Pathak R, Darley L, K, H Choi J, Zaver D, Kolishetti N, Dhar S (2013) Nanocarriers for tracking and treating diseases. Curr Med Chem 20:3500–3514
Mauro N, Scialabba C, Puleio R, Varvarà P, Licciardi M, Cavallaro G, Giammona G (2019) SPIONs embedded in polyamino acid nanogels to synergistically treat tumor microenvironment and breast cancer cells. Int J Pharm 555:207–219
Nair M, Raymond A, Vashist A (2022) Biotherapy for viral infections using biopolymer based micro/nanogels. US Patent App 17/615,940
Osborne O, Peyravian N, Nair M, Daunert S, Toborek M (2020) The paradox of HIV blood–brain barrier penetrance and antiretroviral drug delivery deficiencies. Trends Neurosci 43:695–708
Pei M, Jia X, Zhao X, Li J, Liu P (2018) Alginate-based cancer-associated, stimuli-driven and turn-on theranostic prodrug nanogel for cancer detection and treatment. Carbohyd Polym 183:131–139
Ribovski L, de Jong E, Mergel O, Zu G, Keskin D, van Rijn P, Zuhorn IS (2021) Low nanogel stiffness favors nanogel transcytosis across an in vitro blood–brain barrier. Nanomed Nanotechnol Biol Med 34:102377
Topuz F, Uyar T (2022) Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: drug delivery and beyond. Carbo Polym 120033
Vashist A, Atluri V, Raymond A, Kaushik A, Parira T, Huang Z, Durygin A, Tomitaka A, Nikkhah-Moshaie R, Vashist A, Agudelo M, Chand HS, Saytashev I, Ramella-Roman JC, Nair M (2020) Development of multifunctional biopolymeric auto-fluorescent micro- and nanogels as a platform for biomedical applications. Front Bioeng Biotechnol 8
Vashist A, Kaushik A, Madhavan NAIR, Florida International University FIU (2019) Micro/nano magnetic hydrogels with autofluorescence for therapeutic and diagnostic applications. U.S. Patent 10,344,100
Vashist A, Kaushik A, Vashist A, Bala J, Nikkhah-Moshaie R, Sagar V, Nair M (2018) Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov Today 23(7):1436–1443
Vashist A, Kaushik AK, Ahmad S, Nair M (2017) Nanogels for biomedical applications. Royal Society of Chemistry
Vdovchenko A (2022) Thermoresponsive poly-N-isopropylacrylamide nanogels for brain drug delivery
Vinogradov SV, Batrakova EV, Kabanov AV (2004) Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem 15:50–60
Wang F, Xiao J, Chen S, Sun H, Yang B, Jiang J, Zhou X, Du J (2018a) Polymer vesicles: modular platforms for cancer theranostics. Adv Mater 30:1705674
Wang H, Chen Q, Zhou S (2018b) Carbon-based hybrid nanogels: a synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem Soc Rev 47:4198–4232
Yang J, Yao MH, Wen L, Song JT, Zhang MZ, Zhao YD, Liu B (2014) Multifunctional quantum dot-polypeptide hybrid nanogel for targeted imaging and drug delivery. Nanoscale 6:11282–11292
Ye C, Cheng M, Ma L, Zhang T, Sun Z, Yu C, Wang J, Dou Y (2022) Oxytocin nanogels inhibit innate inflammatory response for early intervention in Alzheimer’s disease. ACS Appl Mater Interfaces 14:21822–21835
Zhang Y, Zou Z, Liu S, Miao S, Liu H (2022) Nanogels as novel nanocarrier systems for efficient delivery of CNS therapeutics. Front Bioeng Biotechnol 10
Zhao C, Sun S, Li S, Am Lv, Chen Q, Jiang K, Jiang Z, Li Z, Wu A, Lin H (2022) Programmed stimuli-responsive carbon dot-nanogel hybrids for imaging-guided enhanced tumor phototherapy. ACS Appl Mater Interfaces 14:10142–10153
Zhao Q, Zhang S, Wu F, Li D, Zhang X, Chen W, Xing B (2021) Rational design of nanogels for overcoming the biological barriers in various administration routes. Angew Chem Int Ed 60:14760–14778
Acknowledgements
Madhavan Nair thanks the support from the National Institutes of Health grants DA052271, DA042706, DA040537, DA037838, DA034547, and Florida Department of Health grant 8AZ04 and Institute of Neuroimmune Pharmacology, Herbert Wertheim College of Medicine, FIorida International University, Miami, FL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
US patent for “Micro/Nano Magnetic Hydrogels with Autofluorescence for Therapeutic and Diagnostic Applications” (US 10,344,100) 2019 with Arti Vashist, Ajeet K Kaushik, Madhavan P Nair as inventors. US non-provisional patent application for “Biotherapy for Viral infections using Biopolymer based Micro/Nanogels.” (US 17/615,940) 2022, with Madhavan P Nair, Arti Vashist, Andrea Raymond as inventors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vashist, A., Raymond, A.D., Chapagain, P. et al. Multi-functional auto-fluorescent nanogels for theranostics. J. Neurovirol. 29, 252–257 (2023). https://doi.org/10.1007/s13365-023-01138-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-023-01138-y