Abstract
Despite the high diversity of bats in neotropics, traditional methods such as mist nets, harp traps and roost detection have limitations in capturing that diversity in a landscape, with most detected species restricted to those that forage in the undergrowth or enclosed spaces. Therefore, acoustic records become a tool that complements and enhances the efforts to get more complete bat inventories while avoiding alterations in usual foraging activities and disruption in their life cycles. This study describes the acoustic parameters (spectral and temporal variables) of the echolocation pulses of insectivorous bats to characterise different species of bats in Southwest Colombia acoustically. We recorded echolocation calls between December 2017 and May 2020 in the Andean and Pacific regions of the Department of Nariño. We analysed 81 sequences of echolocation calls from eight bat species belonging to three families: Vespertilionidae, Molossidae and Emballonuridae. We perform recordings on free-flying bats with identity corroboration by capture for recording in flight rooms and examination in the hand. Myotis riparius and Lasiurus blossevillii were recorded for the first time in the Nariño Department. M. albescens, M. keaysi, M. riparius and L. blossevillii (Vespertilionidae) had pulses of frequency modulated (FM) with a quasi-constant frequency (QCF) ending; Molossus molossus, Tadarida brasiliensis and Promops centralis (Molossidae) had pulses with constant frequency (CF) and QCF; and Saccopteryx bilineata (Emballonuridae) had pulses with QCF. This study contributes to the efforts to facilitate the identification of insectivorous bats of the Neotropics using the acoustic monitoring approaches, represents a reference to compare the acoustic studies in Southwestern Colombia and contributes to increasing our knowledge of the bat diversity in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insectivorous bats are among the most diverse mammals in the Neotropical region (Solari and Martínez-Arias 2014). This group shows a broad variation in their diets, morphology and ecological adaptations (Baker et al. 2012). Additionally, its echolocation system is based on sounds produced by the larynx (Davies et al. 2013). These adaptations allow insectivorous bats to cover a range of habitats from the lowlands to high mountain forests (Patterson et al. 2003; Meyer and Kalko 2008). However, there is an overall lack of natural history information on Neotropical insectivorous bats as well as difficulties in their appropriate taxonomic identification due to acoustic similarities among species with phenotypic plasticity (Obrist 1995), hybridisation processes and cryptic groups (Walters et al. 2013) or with species showing a large amount of intra and interspecific variation (Kalko and Schnitzler 1993; Jung et al. 2014).
The specialised echolocation system allows bats to move, orient and obtain food in different habitats (Schnitzler and Kalko 2001). Ecological studies have traditionally employed conventional capture methods such as mist nets or harp traps (Kunz 1988; O’Farrell and Miller 1997). The feeding habits of bats may bias the records with traditional methodologies. For instance, phyllostomid bats make better use of lower vegetation strata, increasing the capture rates in mist nets (Simmons and Voss 1998). Meanwhile, since some insectivorous species use the upper strata of the vegetation to catch their prey in flight, the rates of mist net captures are reduced in the understory. However, these insectivorous bats are efficiently recorded using ultrasonic detectors (Kalko et al. 2008). Therefore, the analysis of echolocation calls by the measuring of its acoustic spectro-temporal parameters has supported the insectivorous bat species identification in several studies (Fullard et al. 1994; Rautenbach et al. 1996; McCracken et al. 1997; O’Farrell and Gannon 1999; Ochoa et al. 2000; Russo and Jones 2002; Gillam and McCracken 2007). More specifically, these identifications can be carried out by analysing of the echolocation pulses in the search phase (set of pulses emitted by the bat when it moves or looks for food). It is characterised by presenting parameters with stereotypical values, which means that variables such as initial and final frequencies, pulse duration and interpulse interval, among others, remain little variable. For this reason, the pulses emitted during the search phase are used to describe and identify different species (Fenton and Bell 1981).
More than 50% of the world’s bat families are represented in Colombia (Solari et al. 2013), which includes 72 genera and 205 species (Ramírez-Chaves et al. 2016). The Department of Nariño is an area of high bat species richness (Ramírez-Chaves and Noguera-Urbano 2010), with 23 species of insectivorous bats reported (Calderón-Leytón et al. 2020). To date, field studies in Nariño have focused on the analysis of plant-bat interactions (Cabrera-Pantoja 2007), species inventories (Ramírez-Chaves and Noguera-Urbano 2010), activity patterns and species abundance (Martínez 2007). Our study provides the first description of insectivorous bat echolocation calls for this region and contributes to building a bat sound library of the region, which may serve as a reference to identify, validate and compare recordings from different species and localities.
Materials and methods
Study area
This study was conducted in eight localities of the Nariño Department in Colombia through an altitudinal gradient of 8 to 2668 m a.s.l. covering the Pacific region in the municipalities of Tumaco (Maragrícola farm), Barbacoas (Rio Ñambí Natural Reserve and Altaquer settlement) and Ricaurte (Pilispi settlement); and the Andean region in the municipalities of Puerres (Palos Verdes settlement), Pasto (Briceño settlement), El Peñol (San Antonio settlement) and Taminango (Remolino settlement) (Fig. 1; Table 1). Rainfall in the Pacific region is abundant throughout the year, varying from 2500 to about 7000 mm, with temperatures ranging from 24 to 28 °C. Meanwhile, the Andean region’s rainfall varies from 1000 to 6000 mm annually and temperatures from 6 to 24 °C (Solarte et al. 2007).
Map of the study area. The Department of Nariño representing the Pacific and the Andean regions and sampled localities (shown as black dots; see details in Table 1) in an elevational gradient. (A) Department of Nariño in southwest Colombia in South America
Capture methods and species identification
Fieldwork was carried out between December 2017 and May 2020. We captured bats using ten mist nets (8 or 12 m long by about 2 m high) placed in the understory, the canopy and where bats shelter. We sampled 3 days per locality between 18:00 and 06:00 h for a total sampling effort of 3925 net-h. To increase detectability, we include different habitat types (open areas near water bodies and pathways). The captured individuals were identified up to the species level using taxonomic keys and bibliographic reference materials (e.g., Gardner 2007; Díaz et al. 2016) and original descriptions of species groups. Specimens of each species were deposited in the Mammal Collection of the Museum of Vertebrate Zoology at the Universidad de Nariño (hereafter PSO-CZ): Myotis albescens (5), Myotis keaysi (1), Lasiurus blossevillii (1), Myotis riparius (2), Molossus molossus (10), Tadarida brasiliensis (2), Promops centralis (1) and Saccopteryx bilineata (3).
Recording echolocation pulses
In order to get a first approach to the bat diversity in the area, we identified all the captured individuals at the species level as follows (number of individuals per species in brackets): M. albescens (5), M. keaysi (1), L. blossevillii (1), M. riparius (5), M. molossus (10), T. brasiliensis (5) and S. bilineata (4). Later, each individual was placed inside a closed flight room (2 m long × 2 m wide × 2 m high). We set up a room with thin material (insect awning cloth) to avoid sound bouncing and large amounts of echo in recordings since more porous and shiny fabrics may reduce the reflection of several frequencies (Estrada-Villegas et al. 2012). Once the individual was quiet, it was left alone in the flight room, and its vocalisations were recorded continuously for around 30 min. This allowed to generate several files per individual when flying inside the flight room. Additionally, we get recordings of free-flying bats under natural conditions (Zamora et al. 2020) from 6:00 p.m. to 6:00 a.m. Individuals were recorded in real-time with the Echo Meter EM3 recorder (Wildlife Acoustics, Inc, Maynard, MA, USA), configured with a sampling frequency of 250 kHz in WAV format at a 16-bit resolution.
Finally, for the acoustic analysis and the descriptions of the calls, we only selected recordings of echolocation pulses emitted by individuals in free flight only in the study area selected. We used flight room recordings to determine distinguishable traits and patterns as a reference to confirm species identification of free-flying individuals, but they were not used in the analysis. For example, in the case of M. molossus, this species is characterised by exhibiting alternating and frequency-modulated (FM) pulses that can promote longer sounds to allow for changes in FM (Marín 2009). This property allowed the verification that each species emitted characteristic pulses that were distinguishable from other species.
To deal with pseudo-replication problems that would have arisen from including several pulses from the same individual in the analysis in a non-controlled manner, we choose sequences from individuals recorded in different sampling points in each type of vegetation, recorded on separate days and times, following Orozco-Lugo et al. (2013). We ensure no pseudo-replication in echolocation call sequences since the bat species recorded show differences in their activity patterns between individuals, demonstrating periods of bimodal activity, unimodal activity or some peaks in activity during the night (Rivero-Monteagudo and Mena 2023). Additionally, this activity can vary according to sex, reproductive condition, energy requirements and time of the year (O’Donnel 2002). We identify free-flying species through comparisons with publications and the library of echolocation calls of bats from Ecuador (Rivera-Parra and Burneo 2013).
Acoustic analyses
We selected a minimum of ten sequences for each species for the analyses. Each sequence represents a vocalisation emitted by an individual. We followed the call classification by Estrada-Villegas et al. (2012). We assumed a sequence to be a regular succession of calls, the latter being a discrete sound emission equivalent to a series of echolocation pulses. Therefore, we selected five pulses in the search phase of each sequence. These pulses were selected because they had the highest energy in decibels (generally the fundamental frequency, where the bat concentrates the greatest amount of energy during the emission of the echolocation pulses). Pulses were determined using a zero-crossing analysis in the Kaleidoscope Pro v.4.5.0 software (Wildlife Acoustics, Inc, Maynard, MA, USA). We generated spectrograms using a Hamming window, a 256 FFT window size and a 50% overlap.
We quantified nine structural features of echolocation pulses (Rivera-Parra 2011; Martinez-Medina et al. 2021): (1) Fstart (starting frequency of the selection being analysed for each of the five pulses), (2) Fend (the final frequency of the selection being analysed for each of the five pulses; it was selected from the point of the oscillogram where the amplitude increased or decreased in a monotonic way), (3) Fmin (minimum full spectrum frequency, which is an estimate of the minimum signal frequency), (4) Fmax (maximum full spectrum frequency, which is an estimate of the maximum signal frequency), (5) Fmean (average full spectrum frequency, which is the mean spectrum power frequency weighted by amplitude), (6) Fpeak (full spectrum peak frequency, which is the maximum spectrum frequency), (7) bandwidth (difference between Fmax and Fmin). These frequencies were measured in kilohertz (kHz) (Fig. 2). We measured variables 1 through 6 using the “spectral analysis” function in Kaleidoscope. In addition, we measured two time-related parameters (milliseconds, ms): (8) duration (time between the start point and the pulse endpoint) and (9) interval (difference between the initial time of the first and second pulses).
Spectrogram of echolocation calls from A S. bilineata and B spectrogram with power spectra of echolocation calls from L. blossevillii, showing the acoustic parameters measured and analysed in this study. The colour intensity corresponds to the signal’s amplitude at a given frequency and at a given point in time
Statistical analysis
We obtained descriptive statistics such as the mean and standard deviation for each spectral and temporal variable from free-flying bat recordings (five pulses of each of the ten sequences per species). Subsequently, we performed a discriminant function analysis (DFA) based on the different species of bats belonging to Vespertilionidae, Molossidae and Emballonuridae. This multivariate statistical technique, using Bayes’ theorem, allows us to analyse whether there are significant differences between groups of objects with respect to a set of variables measured on them. In the case of the prior probability, it indicates the probability that any observation belongs to a class, in this case, the eight recorded species of bats. The variables used were Fstart, Fend, Fmin, Fmax, Fmean, Fpeak, bandwidth, duration and interval. The standardised discriminant function coefficients were used to determine the contribution each variable made to the ability of discriminant function analysis to classify calls (Rivera-Parra and Burneo 2013; Borcard et al. 2014).
Results
We obtained a total of 81 sequences of echolocation calls coming from 81 free-flying individuals from the family Vespertilionidae (Myotis albescens (10), M. keaysi (10), M. riparius (10) and Lasiurus blossevillii (10)), Molossidae (Molossus molossus (10), Tadarida brasiliensis (10) and Promops centralis (11)) and Emballonuridae (Saccopteryx bilineata (10)). For the flight room recordings, we obtained a total of 80 sequences coming from 31 individuals from all species except P. centralis since it was not recorded in the flight room considering the evidence of the flight room consequences on high variation in their pulses and recommendations to avoid using these methods in species adapted to open areas (see Mora et al. 2004; Martinez-Medina et al. 2021; Table 2). However, the identity of this species was determined by the detection of its shelter and the manual identification under the skin and skull of each species recorded in this study.
Vespertilionidae
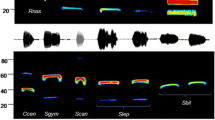
The species belonging to this family presented FM echolocation pulses. The species L. blossevillii recorded in San Antonio (El Peñol) (Fstart 63.77 ± 4.77, Fend 37.45 ± 1.05, duration 4.52 ± 0.31 and interval 52.4 ± 2.83) presented the lowest frequency range in its pulses. In contrast, M. keaysi (Fstart 109.05 ± 2.35, Fend 57.35 ± 0.66, duration 3.48 ± 0.33 and interval 55.15 ± 9.47) of the subfamily Myotinae, (Río Ñambí Natural Reserve, Barbacoas) presented the highest Fstart followed by M. albescens (Fstart 103.29 ± 6.44, Fend 38.05 ± 2.56, duration 2.51 ± 0.28 and interval 72.1 ± 4.99) recorded in Palos Verdes (Puerres) and M. riparius (Fstart 99.94 ± 5.97, Fend 46.93 ± 0.61, duration 4.46 ± 0.27 and interval 65.75 ± 1.89) (Ricaurte) which had the lowest Fstart and Fend values of this subfamily (Table 2; Fig. 3).
Molossidae
We identified echolocation calls of three species (Fig. 4). M. molossus (Fstart 32.51 ± 0.69, Fend 24.35 ± 0.92, duration 9.90 ± 2.04 and interval 234.8 ± 1.17) recorded in El Remolino (Taminango) exhibited pulses of FM. Additionally, this species presented alternated pulses (i.e., low-frequency pulses followed by high-frequency pulses). T. brasiliensis (Fstart 30.3 ± 1.96, Fend 22.24 ± 1.64, duration 15.04 ± 2.05 and interval 319.5 ± 8.17) recorded in Pilispi (Ricaurte) presented constant frequency (CF) pulses with the lowest slope that is exhibited at the end of the pulse. P. centralis (Fstart 19.04 ± 1.31, Fend 28.07 ± 1.13, duration 7.34 ± 1.52 and interval 261.1 ± 8.08) (Briceño, Pasto) presented low quasi-constant frequency (QCF) calls. The start frequency was lower than the end frequency, with the highest energy in the first harmonic (Table 2).
Emballonuridae
We recorded Saccopteryx bilineata (Fstart 31.21 ± 0.21, Fend 43.45 ± 0.24, duration 8.11 ± 0.0004 and interval 128.1 ± 0.003) at the Maragrícola farm (Tumaco). We also detected its refuges and captured and collected three specimens deposited in the PSO-CZ. Echolocation calls were narrowband and multi-harmonic pulses with QCF (Fig. 5). Fmax was present in the second harmonic (Table 2). None of the pulses of the ten sequences showed alternation, even when feeding buzzes were recorded.
Differences between temporal and spectral variables
Our results show that the level of accuracy of the DFA performed with four species of the Vespertilionidae family, three species of the Molossidae family and one Emballonurid species was high (93.25%, P < 0.05). The first discriminant function accounted for 68.67% of the variation in the data, while the second function accounted for 24.58% of the variation (Fig. 6; Table S.1). Temporal and spectral features (Fstart, Fmax and bandwidth) were the most important features to differentiate the eight insectivorous bat species recorded in our study (Table S.2).
Discussion
In this study, we characterised the echolocation calls of eight species of insectivorous bats of the Vespertilionidae, Molossidae and Emballonuridae families from Southwest Colombia. M. riparius and L. blossevillii are recorded for the first time in the Department of Nariño. Even though both species have been recorded in other locations in Colombia, we described in detail the spectro-temporal parameters of their pulses. Considering the results derived from the statistical analyses of the spectral and temporal variables in this study, it is crucial to highlight the challenge of making direct comparisons with existing literature. This difficulty arises from the fact that, up to this point, not all authors have comprehensively examined spectral and temporal variables. Instead, a partial focus has been common, with some emphasising specific variables such as Fstart, Fend, Fmax, duration and interval in their analyses.
Vespertilionidae
It should be noted that although the general structure of the echolocation pulses in the search phase recorded in free-flight is like that reported in the literature, it is necessary to understand that the spectral and temporal parameters may or may not be like others found in works carried out in other countries. This may be due to the following: (1) the correlation between foraging areas and the structure of the acoustic signal (Neuweiler 1984; 1989); (2) the relationship between the type of habitat, the search strategy and the type of food (Kalko 1995); or (3) the body size of the coexisting species (Aldridge and Rautenbach 1987; Norberg and Rayner 1987). In general, the structure of the echolocation pulses for the Vespertilionidae family could be distinguished by the frequency modulated (FM) with an ending quasi-constant frequency (QCF), similar to what was recorded by O’Farrell and Miller (1999). According to Hase et al. (2018), the final variations in the pulses contain information on each individual’s identity, allowing individuals to discriminate their pulses from both conspecific and heterospecific pulses.
The measurements of the spectral and temporal variables for the species M. albescens were different from those reported by other studies, indicating that this species has terminal frequencies ranging from 43 to 46 kHz (Surlykke and Kalko 2008; Estrada-Villegas et al. 2012; Arias Aguilar 2017). This variation of pulses within populations of the same species may be because they are located in different geographic regions, as well as the type of habitat and environmental conditions (Heller and Helversen 1989; Barclay and Brigham 2004; Ratcliffe et al. 2004, Jung et al. 2007). In previous studies, Myotis keaysi was reported to have short pulses (approximately 2.5 ms; Rydell et al. 2002). Contrastingly, we found that this species’ pulses presented a duration between 3.15 and 3.8 ms. The difference in duration results from the challenges the bat faces in its natural environment. If these bats forage in open areas, they not only use longer pulses than individuals that fly in the understory but also have longer intervals between pulses, presumably to ensure that all echoes from the previous call are received before the next call is issued (Jones and Holderied 2007). Moreover, it is important to consider that variations in duration may be attributed to differences among the following: (1) individual bats; (2) recording conditions, including factors such as the distance between the bat and the microphone; and (3) adaptive adjustments associated with foraging habitats, such as proximity to obstacles and levels of acoustic noise (e.g., Kalko and Schnitzler 1993; Obrist 1995).
However, there were similarities in the Fstart and the Fend and the structure of the echolocation pulses. These similarities in the spectral variables may be explained by the fact that this species usually emits its most typical pulses when it flies in open areas (Rydell et al. 2002).
Regarding the echolocation pulses of M. riparius, we describe the presence of harmonics (not previously reported) and lower Fmax values in contrast with those reported by Fenton et al. (1999; Fmax values of 121.9 ± 3.1). These differences can be attributed mainly to the methodology used by the authors since they recorded this species in a permanent greenhouse made of mesh. Although this is a good option for recording vocalisations, it may not represent of the bat’s vocalisations in their natural habitat. Our records of the L. blossevillii calls showed lower Fmax and Fmin values than those reported by other studies (see Kraker-Castañeda et al. 2013). The differences in these frequencies correspond to variations in the emission of echolocation signals due to the structural complexity of the areas where they fly (e.g., whether individuals forage at the understory or canopy). Kraker-Castañeda et al. (2013) recorded L. blossevillii in grassland and forest while we recorded the species over a body of water. M. riparius and L. blossevillii are new records for the Department of Nariño (Ricaurte and El Peñol, respectively). Although the echolocation pulses emitted by these species have already been described to differing degrees of detail (Fenton et al. 1999; Kraker-Castañeda et al. 2013), more studies are needed to help clarify taxonomic difficulties associated with the similarity of their vocalisations.
In Colombia, M. riparius has been recorded in Valle del Cauca and Boyacá departments (Gardner 2007), covering an altitudinal range of 50–150 and 100–1100 m, respectively (Moratelli et al. 2013). This species prefers wooded areas and forages along streams, rivers, trails and open areas (Simmons and Voss 1998). However, we recorded this species at 1492 m a.s.l. in an urbanised area. On the other hand, L. blossevillii is reported in humid and dry forests of the departments of Cundinamarca and Cauca (Gardner 2007) from 200 to 2600 m a.s.l. (Gonzalez et al. 2016). We recorded the species at 1395 m a.s.l. in San Antonio (El Peñol), but recent explorations throughout the Andean region in the Department of Nariño indicate that this species is also distributed in other localities such as the municipalities of Sotomayor, Guaitarilla and Samaniego (Arévalo-Cortés et al. unpublished data).
Molossidae
The family Molossidae exhibits excellent plasticity in the structure of its echolocation pulses in the search phase, which implies frequency changes and frequency alternations (Mora et al. 2004; Gillam and McCracken 2007). According to Jung et al. (2014), the species M. molossus can present two pulses, a low frequency pulse followed by a higher frequency pulse, which is in line with our results (Fig. 4). Alternating their frequencies will allow a bat to distinguish between echoes from different pulses emitted. Pairing may allow the bat to process the echoes of the first pulse while emitting the second. This pattern could also present an advantage in prey capture (Jung et al. 2014).
In several studies carried out on the species T. brasiliensis, values of Fstart (Jung et al. 2014; Krauel et al. 2018), Fmax (Ratcliffe et al. 2004; Briones-Salas et al. 2013) and duration (Gillam and Montero 2016) are similar to those we report here, supporting the idea of the differences between the variables for this species not related to habitat structure (Ratcliffe et al. 2004). Even though Molossidae species exhibit high plasticity in their echolocation calls, the spectral and temporal variables of P. centralis presented distinctive pulses, as has been reported in other studies (Jung et al. 2014; Froidevaux et al. 2020). Regardless that P. centralis may present a flexible adjustment of the echolocation system to face sensory challenges in the environment, the identity of each echolocation call was preserved.
Emballonuridae
The family Emballonuridae includes species of insectivorous bats that generally feed in open areas, on water surfaces or at the edges of vegetation (Jung et al. 2007). This foraging pattern suggests that the echolocation pulses of the species belonging to this family cover a small bandwidth and generally contain a QCF with certain species adding an FM component (Kalko and Schnitzler 1998; Jung et al. 2007; MacSwiney et al. 2008). In the case of S. bilineata echolocation pulses, the second harmonic contains higher energy than the first one.
S. bilineata also presented slightly lower frequencies, which differs from the results obtained by Biscardi et al. (2004; Fmax values of 43.8 ± 0.3 kHz; Fminvalues 39.2 ± 2.1 kHz). Ratcliffe et al. (2011) reported that this species uses two different pulse sequences, a first pulse at 45 kHz followed by a second pulse at 48 kHz (Barclay 1983; Jung et al. 2007). This information differs from our findings. Although we recorded the echolocation calls of this species through methods such as flight rooms and free flight, we did not observe the vocal behaviour of different sequences of pulses since the frequencies were maintained uniformly over time in a sequence and did not show doubling alternation in the pulses (Fig. 5). It should be noted that we corroborate the identification of this species with the collected individuals (Table 3) and its external and cranial morphology.
According to Aldridge and Rautenbach (1987), the fact that this species does not present two different pulse sequences may be due to the correlation between the foraging areas and the structure of the acoustic signal. For instance, environmental selection pressures such as local microclimate and vegetation structure can influence the spectro-temporal structure of echolocation calls. Ratcliffe et al. (2011) found that S. bilineata produces sequences of pulses at a single frequency when it is close to its perching site while it emits pulses of alternating frequency at the feeding site. However, we found the species to emit pulses at the same frequency, regardless of whether prey was detected or captured, suggesting that this species emits pulses of the same frequency when it is in open areas as we recorded it in the Maragrícola farm, Tumaco, which contains open areas with artificial water bodies.
Differences between temporal and spectral variables
The results of the DFA are consistent with the results of Rivera-Parra (2011), in which the species are separated by the variables Fstart, Fmax and bandwidth (Table S.2). While these differences represent diverse foraging strategies, habitat preferences and phylogenetic relations between the families, they are consistent with an adaptation of signal design to foraging habitat (Surlykke and Kalko 2008). For instance, low frequencies, short bandwidths, long durations and extended intervals commonly occur in bats that forage in open spaces (Siemers et al. 2001). Meanwhile, high frequencies, long bandwidths, short durations and narrow intervals widely occur in bats that forage in cluttered spaces (Jones and Holderied 2007).
It is essential to note that the sampling areas in this study exhibit diverse natural and environmental conditions. Consequently, the analysis of certain spectral and temporal variables of echolocation pulses (Fmax, Fmean and duration) in low-lying areas tends to yield higher values. This is attributed to the predominantly open nature of these spaces, where both the inherent echoes and background noise are diminished. As a result, there is an enhanced specificity in the detection and capture of prey (Schnitzler and Kalko 2001; Estrada-Villegas et al. 2012). In contrast, Schnitzler and Kalko (2001) and Estrada-Villegas et al. (2012) assert that species often emit pulses at lower frequencies with longer wavelength ranges in areas characterised by obstacles. This adaptation is employed to facilitate the detection of sounds from the environment with greater amplitude, particularly in environments where capturing prey involves dealing with various obstacles.
Although surveys have been carried out in Southwestern Colombia, studies on the chiropteran fauna still need to be included in this region. Traditional methods such as mist nets or harp traps have limitations in the diversity estimation of insectivorous bats due to their low detection of species that forage in open areas and canopy. For this reason, it is necessary to complement existing survey methods with bioacoustics tools and analyse echolocation calls as a basis for the taxonomic identification of insectivorous bats (Pech-Canche et al. 2010; Martinez-Medina et al. 2021). Additionally, we suggest identifying bat roosts with the help of local communities to obtain more complete species inventories.
To date, only seven insectivorous species have been reported in the Department of Nariño: Eptesicus brasiliensis (Barbacoas), E. andinus (Barbacoas), M. albescens (Barbacoas and Tumaco), M. keaysi (Barbacoas and Ricaurte), M. nigricans (Barbacoas and Tumaco), M. oxyotus (Mallama) and Rhogeessa io (Leiva and Taminango) (Ramírez-Chaves and Noguera-Urbano 2010). Until 2018, only 29 specimens corresponding to the Vespertilionidae family had been deposited in the PSO-CZ museum (Calderón-Leytón 2017; Calderón-Leytón et al. 2020). With this work, we contribute new information about the diversity of bats of the family Vespertilionidae that inhabit the ecosystems in the southwestern region of Colombia. Here, we report the first observations in Nariño of the species M. riparius (Ricaurte) and L. blossevillii (El Peñol) supported with audio recordings and skin vouchers with contributions to the acoustic libraries (regional and national) and an increase of 42 the number of Vespertilionidae specimens for the PSO-CZ. The lack of systematic sampling may explain why these species were not previously recorded in the region.
The information provided for the combination of several survey methodologies is essential to know the real biodiversity in our territories and understand their vulnerabilities. For instance, bats have been highly affected due inappropriate management by the local community to attend public health and agricultural issues, where the common solution is the indiscriminate extermination. This entails the loss of bat populations that fulfil functions for the maintenance of various processes that ensure the persistence of natural ecosystems; therefore, this project impacts and expands the conservation or research of wildlife because (i) it generates an expansion of the knowledge of the diversity of bats in the Andean region of the department of Nariño, (ii) proposes a baseline on the biological diversity of bats in the department, which may help compare with other biological groups and conducting vulnerability analysis due to climate change or the valuation of environmental services, (iii) is aware of local communities, considering the different ecosystem services bats provide and (iv) generates valuable knowledge for the conservation, management and manipulation of bats in Colombia.
Data availability
Acoustic files generated during this study were deposited in the regional library of sounds at the Universidad de Nariño -SONAR (see Table S3). A copy of these files is available in the acoustic public repository Xeno-Canto accessing to the following URL: https://xeno-canto.org/set/8577.
References
Aldridge HD, Rautenbach IL (1987) Morphology, echolocation and resource partitioning in insectivorous bats. J Anim Ecol 56:763. https://doi.org/10.2307/4947
Arias Aguilar AP (2017) Morcegos insetívoros aéreos neotropicais: Identificação acústica e padrões de estruturação de assembleias. Universidade Federal Do Rio Grande Do Sul. http://hdl.handle.net/10183/163714
Baker RJ, Bininda-Emonds O, Mantilla-Meluk H, Porter C, Van Den Bussche R (2012) Molecular time scale of diversification of feeding strategy and morphology in new world leaf-nosed bats (Phyllostomidae): a phylogenetic perspective. Evol Hist Bats Foss Mol Morphol 385–409. https://doi.org/10.1017/CBO9781139045599.012
Barclay RMR (1983) Echolocation calls of emballonurid bats from Panama. J Comp Physiol 151:515–520
Barclay RMR, Brigham RM (2004) Geographic variation in the echolocation calls of bats: A complication for identifying species by their calls. In: Bat echolocation research: tools, techniques, and analysis Brigham RM, Kalko EKV, Jones G, Parsons S, Limpens HJGA (eds) Bat Conservation International, Austin, Texas, pp 144–149
Biscardi S, Orprecio J, Fenton MB et al (2004) Data, sample sizes and statistics affect the recognition of species of bats by their echolocation calls. Acta Chiropterologica 6:347–363. https://doi.org/10.3161/001.006.0212
Borcard D, Gillet F, Legendre P (2014) Numerical ecology with R, second edition. Springer International Publishing AG. New York, NY. https://doi.org/10.1007/978-1-4419-7976-6
Briones-Salas M, Peralta-Pérez M, García-Luis M (2013) Acoustic characterization of new species of bats for the State of Oaxaca, Mexico. Therya 4:15–32. https://doi.org/10.12933/therya-13-106
Cabrera-Pantoja MJ (2007) Uso del recurso alimentario por murciélagos nectarívoros del género Anoura (Chiroptera: Phyllostomidae) en un bosque de niebla de la Reserva Natural La Planada-Nariño. Universidad de Nariño, Departamento de Biología, Pasto, Nariño, pp 80
Calderón-Leytón JJ, Arévalo-Cortés J, Cabrera-Ojeda C, Arcos O, Noguera-Urbano EA (2020) Colección Mastozoológica Universidad de Nariño (Colección Zoológica P.S.O-CZ 041). Mammal Notes 6:1–5
Calderón-Leytón J (2017) Colección de mamíferos voladores y no voladores de la Universidad de Nariño. https://ipt.biodiversidad.co/sib/resource.do?r=amiferos_unarino. Accessed 7 May 2021
Davies KTJ, Maryanto I, Rossiter SJ (2013) Evolutionary origins of ultrasonic hearing and laryngeal echolocation in bats inferred from morphological analyses of the inner ear. Front Zool 10:1–15. https://doi.org/10.1186/1742-9994-10-2
Díaz MM, Solari S, Aguirre LF et al (2016) Clave de Identificación de los Murcielagos de Sudamérica. Programa de Conservación de los Murciélagos de Argentina. https://ri.conicet.gov.ar/handle/11336/156765
Estrada-Villegas S, McGill BJ, Kalko EKV (2012) Determinants of species evenness in a neotropical bat ensemble. Oikos 121:927–941. https://doi.org/10.1111/j.1600-0706.2011.19837.x
Fenton MB, Bell GP (1981) Recognition of species of insectivorous bats by their echolocation calls. J Mammal 62:233–243. https://doi.org/10.2307/1380701
Fenton MB, Rydell J, Vonhof MJ, Eklöf J, Lancasteret WC (1999) Constant frequency and frequency-modulate components in the echolocation calls of three species of small bats (Emballonuridae, Thyropteridae, and Vespertilionidae). Can J Zool 77:1891–1900. https://doi.org/10.1139/z99-168
Froidevaux JSP, Roemer C, Lemarchand C et al (2020) Second capture of Promops centralis (Chiroptera) in French Guiana after 28 years of mist-netting and description of its echolocation and distress calls. Acta Amaz 50:327–334
Fullard JH, Simmons JA, Saillant PA (1994) Jamming bat echolocation: the dogbane tiger moth Cycnia tenera times its clicks to the terminal attack calls of the big brown bat Eptesicus fuscus. J Exp Biol 194:285–98
Gardner A (2007) Mammals of South America, Volume 1 marsupials, xenarthrans, shrews, and bats. Chicago. https://doi.org/10.1644/08-mamm-r-296.1
Gillam EH, McCracken GF (2007) Variability in the echolocation of Tadarida brasiliensis: effects of geography and local acoustic environment. Anim Behav 74:277–286. https://doi.org/10.1016/j.anbehav.2006.12.006
Gillam EH, Montero BK (2016) Influence of call structure on the jamming avoidance response of echolocating bats. J Mammal 97:14–22. https://doi.org/10.1093/jmammal/gyv147
Gonzalez E, Barquez R, Miller B (2016) Lasiurus blossevillii. In: IUCN red list threat. Species. https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T88151055A22120040.en.
Hase K, Kadoya Y, Maitani Y et al (2018) Bats enhance their call identities to solve the cocktail party problem. Commun Biol 1:1–8. https://doi.org/10.1038/s42003-018-0045-3
Heller KG, Helversen OV (1989) Resource partitioning of sonar frequency bands in rhinolophoid bats. Oecologia 80:178–186. https://doi.org/10.1007/BF00380148
Jones G, Holderied MW (2007) Bat echolocation calls : adaptation and convergent evolution bat echolocation calls : adaptation and convergent evolution. Proc R Soc b: Biol Sci 274:905–912. https://doi.org/10.1098/rspb.2006.0200
Jung K, Kalko EKV, Von Helversen O (2007) Echolocation calls in Central American emballonurid bats: signal design and call frequency alternation. J Zool 272:125–137. https://doi.org/10.1111/j.1469-7998.2006.00250.x
Jung K, Molinari J, Kalko EKV (2014) Driving factors for the evolution of species-specific echolocation call design in new world free-tailed bats (Molossidae). PLoS One 9:. https://doi.org/10.1371/journal.pone.0085279
Kalko E (1995) Insect pursuit, prey capture and echolocation in pipistrelle bats (Microchiroptera). Anim Behav 50:861–880. https://doi.org/10.1016/0003-3472(95)80090-5
Kalko EKV, Schnitzler H (1993) Plasticity in echolocation signals of European pipistrelle bats in search flight: implications for habitat use and prey detection, pp 415–428. https://doi.org/10.1007/BF00170257
Kalko EKV, Estrada-Villegas S, Schmidt M, Wegmann M, Chrisoph FJM (2008) Flying high—assessing the use of the aerosphere by bats. Integr Comp Biol 48:0–73. https://doi.org/10.1093/icb/icn030
Kalko E, Schnitzler H (1998) The roles of echolocation and olfaction in two Neotropical fruit-eating bats, Carollia perspicillata and C. castanea, feeding on Piper. Behav Ecol Sociobiol 42:397–409
Kraker-Castañeda C, Santos-Moreno A, García-García J (2013) Riqueza de especies y actividad relativa de murciélagos insectívoros aéreos en una selva tropical y pastizales en Oaxaca, México. Mastozool Neotrop 20:255–267
Krauel JJ, Ratcliffe JM, Westbrook JK, McCracken GF (2018) Brazilian free-tailed bats (Tadarida brasiliensis) adjust foraging behaviour in response to migratory moths. Can J Zool 96:513–520. https://doi.org/10.1139/cjz-2017-0284
Kunz TH (1988) Ecological and behavioral methods for the study of bats, Smithsonian. Washington, DC, London. https://doi.org/10.5860/choice.26-4489
MacSwiney GM, Clarke FM, Racey PA (2008) What you see is not what you get: the role of ultrasonic detectors in increasing inventory completeness in Neotropical bat assemblages. J Appl Ecol 45:1364–1371. https://doi.org/10.1111/j.1365-2664.2008.01531.x
Marín LA (2009) Caracterización preliminar de los sonidos de ecolocación de dos especies de murciélagos (mammalia : chiroptera) presentes en la Estación Biológica El Frío, Estado de Apure, Venezuela. Trabajo de grado. Pontificia Universidad Javeriana. http://hdl.handle.net/10554/8576
Martínez JM (2007) Composición y estructura de la comunidad de murciélagos en la Reserva Natural Río Ñambí- Nariño, Colombia. Universidad de Nariño, Pasto, Colombia, pp 72
Martinez-Medina D, Sánchez J, Zurc D et al (2021) Estándares para registrar señales de ecolocalización y construir bibliotecas de referencia de murciélagos en Colombia. Biota Colomb 22:36–56. https://doi.org/10.21068/c2021.v22n01a03
McCracken G, Hayes J, Cevallos J et al (1997) Observations on the distribution, ecology, and behaviour of bats on the Galapagos Islands. J Zool. https://doi.org/10.1111/j.1469-7998.1997.tb01974.x
Meyer CFJ, Kalko EKV (2008) Assemblage-level responses of phyllostomid bats to tropical forest fragmentation: Land-bridge islands as a model system. J Biogeogr 35:1711–1726. https://doi.org/10.1111/j.1365-2699.2008.01916.x
Mora EC, Macias S, Vater M et al (2004) Specializations for aerial hawking in the echolocation system of Molossus molossus ( Molossidae, Chiroptera ). Naturwissenschaften 94:380–383. https://doi.org/10.1007/s00359-004-0519-2
Moratelli R, Gardner AL, De Oliveira JA, Wilson DE (2013) Review of myotis (chiroptera, vespertilionidae) from Northern South America, including description of a new species. Am Museum Novit 1–36. https://doi.org/10.1206/3780.2
Neuweiler G (1984) Foraging, echolocation and audition in bats. Sci Nat 71:446–455. https://doi.org/10.1007/BF00455897
Neuweiler G (1989) Foraging ecology and audition in echolocating bats. Trends Ecol Evol 4:160–166. https://doi.org/10.1016/0169-5347(89)90120-1
Norberg U, Rayner J (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. R Soc London B 316:335–427. https://doi.org/10.1098/rstb.1987.0030
O’Donnel FJ (2002) Influence of sex and reproductive status on nocturnal activity of long-tailed bats (Chalinolobus tuberculatus). J Mammal 83:794–803
O’Farrell MJ, Gannon WL (1999) A comparison of acoustic versus capture techniques for the inventory of bats. Am Soc Mammal 80:24–30
O’Farrell MJ, Miller BW (1997) A new examination of echolocation calls of some neotropical bats (Emballonuridae and Mormoopidae). J Mammal 78:954–963. https://doi.org/10.2307/1382955
O’Farrell MJ, Miller BW (1999) Use of vocal signatures for the inventory of free-flying neotropical bats1. Biotropica 31:507–516. https://doi.org/10.1111/j.1744-7429.1999.tb00394.x
Obrist MK (1995) Flexible bat echolocation: the influence of individual, habitat and conspecifics on sonar signal design. Behav Ecol Sociobiol 36:207–219. https://doi.org/10.1007/BF00177798
Ochoa J, Farrell MJO, Miller BW (2000) Contribution of acoustic methods to the study of insectivorous batdiversity in protected areas from northern Venezuela. Acta Chiropterologica 2:171–183
Orozco-Lugo L, Guillén-Servent A, Valenzuela-Galván D, Arita HT (2013) Descripción de los pulsos de ecolocalización de once especies de murciélagos insectívoros aéreos de una selva baja caducifolia en Morelos, México. Therya 4:33–46. https://doi.org/10.12933/therya-13-103
Patterson BD, Willig MR, Stevens RD (2003) Trophic strategies, niche partitioning, and patterns of ecological organization. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, USA
Pech-Canche JM, MacSwiney GC, Estrella E (2010) Importancia de los detectores ultrasónicos para mejorar los inventarios de murciélagos Neotropicales. Therya 1:221–228. https://doi.org/10.12933/therya-10-17
Ramírez-Chaves H, Noguera-Urbano EA (2010) Lista preliminar de los mamíferos (Mammalia: Theria) del departamento de Nariño, Colombia. Biota Colomb 11:117–140
Ramírez-Chaves HE, Suárez-Castro AF, González-Maya JF (2016) Cambios recientes a la lista de mamiferos de Colombia. Mammal Notes 3:1–19
Ratcliffe JM, ter Hofstede HM, Avila-Flores R et al (2004) Conspecifics influence call design in the Brazilian free-tailed bat, Tadarida brasiliensis. Can J Zool 82:966–971. https://doi.org/10.1139/z04-074
Ratcliffe JM, Jakobsen L, Kalko EKV, Surlykke A (2011) Frequency alternation and an offbeat rhythm indicate foraging behavior in the echolocating bat, Saccopteryx bilineata. J Comp Physiol A Neuroethol Sensory, Neural, Behav Physiol 197:413–423. https://doi.org/10.1007/s00359-011-0630-0
Rautenbach IL, Whiting MJ, Fenton MB (1996) Bats in riverine forests and woodlands: a latitudinal transect in Southern Africa. Can J Zool 74:312–322. https://doi.org/10.1139/z96-039
Rivera-Parra P, Burneo SF (2013) Primera biblioteca de llamadas de ecolocalización de murciélagos del Ecuador. Therya 4:79–88. https://doi.org/10.12933/therya-13-104
Rivera-Parra P (2011) Caracterización de la fauna de quirópteros del Parque Nacional Yasuní en base a llamadas de ecolocación. Pontificia Universidad Catolica Del Ecuador. http://repositorio.puce.edu.ec/handle/22000/4534
Rivero-Monteagudo J, Mena JL (2023) Hourly activity patterns of the insectivorous bat assemblage in the urban – rural landscape of Lima, Peru. J Mammal 20:1–13. https://doi.org/10.1093/jmammal/gyad015
Russo D, Jones G (2002) Identification of twenty-two bat species (Mammalia: Chiroptera) from Italy by analysis of time-expanded recordings of echolocation calls. J Zool 258:91–103. https://doi.org/10.1017/S0952836902001231
Rydell J, Arita HT, Santos M, Granados J (2002) Acoustic identification of insectivorous bats ( order Chiroptera ) of Yucatan, Mexico. J Zool 257:27–36. https://doi.org/10.1017/S0952836902000626
Schnitzler H, Kalko E (2001) Echolocation by insect-eating bats. Bioscience 51:557–569
Sikes RS, Bryan JA (2016) 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688. https://doi.org/10.1093/jmammal/gyw078
Siemers BM, Kalko E, Schnitzler H-U (2001) Echolocation behavior and signal plasticity in the Neotropical bat Myotis nigricans (Schinz, 1821) (Vespertilionidae): a convergent case with European species of Pipistrellus? Behav Ecol Sociobiol 50:317–328. https://doi.org/10.1007/s002650100379
Simmons N, Voss R (1998) The mammals of Paracou, French Guiana: a Neotropical lowland rainforest fauna, Part 1 Bats. Bull Am Museum Nat Hist 237:1–215
Solari S, Martínez-Arias V (2014) Cambios recientes en la sistemática y taxonomía de murciélagos Neotropicales (Mammalia: Chiroptera). Therya 5:167–196
Solari S, Muñoz-Saba Y, Rodríguez-Mahecha JV, Defler TR, Ramírez-Chavez HE, Trujillo F (2013) Riqueza, endemismo y conservación de los mamíferos de Colombia. Mastozool Neotrop 20:301–365
Solarte MA, Narváez G, Rivas G, Bacca AE, Muñoz D, Calderón JJ, Torres C, Figueroa V, Rengifo J, Martínez P, Dávila MT, Cepeda B, Castillo G (2007) Proyecto Estado del arte de la información biofísica y socioeconómica de los páramos de Nariño. Tomo I. Grupo de investigación en Biología de Paramos y Ecosistemas Andinos, Universidad de Nariño - Corporación Autónoma Regional de Nariño CORPONARIÑO. Pasto, Nariño, Colombia, pp 60. https://corponarino.gov.co/expedientes/intervencion/biodiversidad/tomo01introducion.pdf
Surlykke A, Kalko EKV (2008) Echolocating bats cry out loud to detect their prey. PLoS One 3:1–10. https://doi.org/10.1371/journal.pone.0002036
Walters CL, Collen A, Lucas T et al (2013) Challenges of using bioacoustics to globally monitor bats. In: Adams RA, Pedersen SC (eds) Bat evolution, ecology, and conservation, pp 479–499. https://doi.org/10.1007/978-1-4614-7397-8
Zamora-Gutierrez V, Ortega J, Avila-Flores R et al (2020) The Sonozotz project: Assembling an echolocation call library for bats in a megadiverse country. Ecol Evol 00:1–16. https://doi.org/10.1002/ece3.6245
Acknowledgements
We thank the Department of Biology and the Evolutionary Ecology Research Group (GIEE) of the Universidad de Nariño, the Asociación GAICA and the Faculty of Biological and Agricultural Sciences, Poza Rica, Tuxpan at the Universidad Veracruzana. We thank JM Pech, the Group of Terrestrial Vertebrates in Veracruz, Mexico and E Torres, JL Guerra, M Villarreal and C Paz for field assistance. We thank D Spaan for the valuable comments that helped improve the manuscript. JA-C thanks the scholarship provided by Fundación CeiBA. RAF-G thanks the Dirección General de Asuntos del Personal Académico DGAPA-UNAM and the Consejo Nacional de Humanidades, Ciencia y Tecnología de México (CONAHCyT, SNII CVU No. 635378).
Funding
This study was supported by the office of the Vice-president for Research at the Universidad de Nariño (VIIS-UDENAR).
Author information
Authors and Affiliations
Contributions
JA-C and JT-F conceived the idea, collected the data and performed the analyses. JJC-L and RAF-G supported the design of the study and supervised the research. SAM-M and DZ support the validation. JA-C and RAF-G wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study followed the guidelines for the use of wild mammal species in research (Sikes and Bryan 2016) and was carried out under the permit for collecting wildlife species of biological diversity for non-commercial scientific research purposes provided by the regional environmental authority (CORPONARIÑO; Resolution No., 126—19/02/2015).
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Facundo Luna.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arévalo-Cortés, J., Tulcan-Flores, J., Zurc, D. et al. Description of the echolocation pulses of insectivorous bats with new records for Southwest Colombia. Mamm Res 69, 231–244 (2024). https://doi.org/10.1007/s13364-023-00734-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-023-00734-x