Abstract
The mechanisms determining habitat use in animal populations have important implications for population dynamics, conservation, and management. Here, we investigated how an increase in annual numbers of brown bear females with cubs of the year (FCOY) in a growing, yet threatened population, could explain differences in the habitat characteristics around reproductive dens. Habitat characteristics around FCOY dens were compared between a low bear density period (1995–2005) and a period when the population was increasing (2006–2016). We also compared the distance to the nearest breeding area and to all other breeding areas observed during the same year. The results suggested that during the second period, breeding areas were closer to rivers, fruit trees, and anthropogenic sources of disturbance (trails, highways) than in 1995–2005. There were also shorter distances to the closest neighboring breeding area, while the mean distance among FCOY breeding areas increased as the population grew and expanded at the landscape level. These changes may reflect that the best den locations were increasingly occupied (i.e., ideal-despotic distribution), and may be further explained by the avoidance of conspecifics by FCOY in a critical time of the year, when newborn cubs are most vulnerable. We suggest that both density-dependent factors and human-related features of the landscape are crucial to understanding long-term dynamics in the habitat use of a threatened species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanisms determining habitat use in animal populations have important implications for population dynamics, conservation, and management (O’Neil et al. 2020). Habitat use of individuals in a population is expected to be functionally dependent on population density when individuals are distributed in a way that maximizes fitness and minimizes competition for resources. Density-dependence is a pivotal topic in ecological theory, historically studied in the context of population regulation (Gigliotti et al. 2020), particularly the effects of density on survival and reproduction (e.g., Hassell 1975; Fowler 1981; Murdoch 1994; Wolff 1997; Penteriani et al. 2006; Ferrer and Penteriani 2008), as well as in relation to recruitment, growth, body size, disease, and predation (e.g., Fowler 1987; Sinclair and Pech 1996; Stewart et al. 2005; Potapov et al. 2012).
Animals do not distribute randomly in space (Taylor et al. 1978). As such, substantial effort has been invested into understanding the role of habitat use in determining the spatial heterogeneity of population densities and the associated fitness consequences for individuals (Rosenzweig 1981; Morris 1987, 2003). When population density increases, the competition for space and resources is amplified (van Beest et al. 2014a), typically resulting in changes in the spatial behavior of individuals and populations at different scales. For instance, home range size (Kilpatrick et al. 2001; Dahle and Swenson 2003; Kjellander et al. 2004; Dahle et al. 2006), dispersal probability and distance (Ims and Andreassen 2005; Matthysen 2005), and fine-grained resource use of animals within habitats (van Beest et al. 2014b, a) may be altered with increasing density. According to ideal despotic distribution or ideal-dominant distribution (Evans and Fretwell 1973), the individuals that first occupy a given area may monopolize the most suitable habitats, resulting in a higher density of individuals occupying less suitable habitat as the population increases. While this concept has received much theoretical attention, there is little empirical evidence to explain patterns of habitat use when individuals compete for limited resources under the ideal-despotic scenario (O’Neil et al. 2020; Avgar et al. 2020).
Significant efforts have been invested in understanding how density-dependent phenomena influence coarse-grained parameters of spatial use and movements of large predators (Arditi et al. 2001; Edwards et al. 2002; Støen et al. 2006; Delgado et al. 2014; Sjödin et al. 2014; Šálek et al. 2015; Avgar et al. 2020; Clevenstine and Lowe 2021), but studies focused on density-dependent effects on fine-scale space use are still scarce (but see O’Neil et al. 2017; Gigliotti et al. 2020).
An increase in legislative protection (e.g., Bern Convention 1982, EU Habitats Directive 1992) and a global shift toward conservation-oriented management of large carnivores aided by legislation has led to the recovery of several populations across parts of North America and Europe (Bruskotter and Shelby 2010; Chapron et al. 2014; Boitani and Linnell 2015). However, as population densities of large predators increase, real or perceived conflicts with human activities (Fernández-Gil et al. 2016; Eklund et al. 2020; Zarzo-Arias et al. 2021) and threats to human lives (Penteriani et al. 2016) can increase, particularly if predators utilize areas closer to human settlements or activities. Human-carnivore conflict is one of the key threats to large carnivore populations and population recovery. Identifying the mechanisms underlying the variation in the spatial behavior of large carnivores is thus essential for providing adequate management recommendations that will both ensure the viability of carnivore populations and prevent and/or mitigate conflicts with humans.
Despite the general recovery of large carnivores, small, isolated populations are very vulnerable to stochastic events and the loss of key individuals (Linnell et al. 2005). The Cantabrian brown bear Ursus arctos inhabiting the North West Spain is an example of a vulnerable large carnivore population that has recently increased in size (Palomero et al. 2007; Pérez et al. 2014; Gonzalez et al. 2016). This population is isolated from other European bear populations and has been studied intensively for over 25 years through the monitoring of female bears with cubs of the year (FCOY, Palomero et al. 2007; Penteriani et al. 2018b). Monitoring FCOY allows for accurate and unduplicated counts, as they are easy to identify. Also, their smaller home range compared to solitary bears (Darling 1987; Benson and Chamberlain 2007), their tendency not to roam as far as solitary bears, and the fact that FCOY are more active during day time (Servheen 1983; Mattson 1997; Gardner et al. 2014; Penteriani et al. 2020b), make their annual counting an inexpensive and non-intrusive method to monitor trends of small populations (Ordiz et al. 2007; Penteriani et al. 2018b).
Beyond this population, brown bears are a model species to aid in the understanding of interactions between large carnivores and humans (e.g., Ordiz et al. 2012, 2013; Morales-González et al. 2020), as well as reproductive patterns in species typically considered as non-social and solitary (Støen et al. 2006; Ordiz et al. 2008). Although some population ranges are thought to be either stable or recently expanding (Chapron et al. 2014; Coogan et al. 2019), bears generally avoid humans spatially and temporally (Støen et al. 2015; Ordiz et al. 2017; Ladle et al. 2019; Penteriani et al. 2020b; Morales-González et al. 2020). However, as bear density increases in further, changes in their distribution and habitat use may potentially result in more intense use of sub-optimal areas, including those closer to human activity, which in turn could increase conflict and additional risks for individuals. A better understanding of the response of one of the most important, fitness-related components of bear populations—FCOY—to an increase in population density is crucial for the conservation of this species, especially if the response involves more frequent use of areas closer to humans for denning.
The aim of this study is to determine the differences, if any, in the spatial use of FCOY in response to an increase in population density of the western subpopulation of brown bears in the Cantabrian Mountain range. Female brown bears with newly born cubs use small areas after abandoning their winter dens, where they give birth to their offspring (e.g., Ordiz et al. 2007). Therefore, it is crucial to know the habitat characteristics of those small areas used, typically, for several weeks during the spring. In particular, we compared the landscape characteristics around reproductive dens and the spatial distribution of FCOY between two different periods in the evolution of the Cantabrian brown bear population from 1995 and 2016: a first period of slow increase of FCOY population (from 8 FCOY in 1995 to 12 in 2005) and a second period of faster increase of FCOY (from 15 FCOY in 2006 to 34 in 2016). We hypothesized that the observed increase in FCOY, which consequently increased population density, would result in (1) differing characteristics of used areas immediately around the reproductive dens between those two periods, in support of a potential ideal-despotic scenario (e.g., closer distance from FCOY breeding areas to sub-optimal habitat in closer proximity to human infrastructures); and (2) a decrease in the distance between FCOY, which may evidence distribution clumping around high quality areas.
Materials and methods
Study area and bear population
The focal bear population in NW Spain (hereafter, the Cantabrian population) is divided into two subpopulations, western and eastern, with little gene flow between them (Pérez et al. 2010; Gonzalez et al. 2016). The Cantabrian population was decimated during the twentieth century, and in the decade of 1990, it sustained only 70–5 bears (50–65 bears in the western subpopulation and 14–20 in the eastern one; Clevenger and Purroy 1991; Naves and Palomero 1993; Martínez Cano et al. 2016). In recent years the Cantabrian population has increased, in particular the western subpopulation (Pérez et al. 2014; Martínez Cano et al. 2016), which is the focus of the present study.
The western subpopulation (range around 7200 km2, Fig. 1) contains approximately 250 bears (Pérez et al. 2014) and inhabits the provinces of Asturias and León. The hilly and rugged study area has an altitude range from 100 to 2000 m a.s.l. and is characterized by an oceanic climate, with average annual temperatures of about 14 °C on the coast and 2–3 °C at the highest altitudes and high humidity. The tree layer is mainly dominated by oak (Quercus petraea, Quercus pyrenaica, and Quercus rotundifolia), beech (Fagus sylvatica), and chestnut (Castanea sativa) on north facing slopes, while drier, south facing slopes, are mainly dominated by oak (Q. petraea and Q. pyrenaica) and beech. Subalpine bushes (Juniperus communis, Vaccinium uliginosum, Vaccinium myrtillus, and Actostaphylos uva-ursi) dominate areas above the tree line, typically above 1700 m (Martínez Cano et al. 2016). Hundreds of years of human activities have transformed former forests into pastures and brushwood (Genista, Cytisus, Erica, and Calluna). Human population density is generally low in the area (< 15 inhabitants/km2) and the main economic activity is livestock farming, followed by mining, tourism, agriculture, and timber harvesting (Penteriani et al. 2020b). Areas surrounding this core bear range have experienced extensive urban and agriculture development, interspersed by an increasing network of transport infrastructure (Mateo-Sánchez et al. 2016). The closest bear population is in the Pyrenees, located almost 300 km east.
FCOY data
Since 1989, there has been a long-term monitoring program of the Cantabrian population primarily based on direct observations and annual counting of FCOY (Palomero et al. 2007; Gonzalez et al. 2016; Penteriani et al. 2018b, 2020b). The locations of brown bears were obtained from (a) direct bear observations georeferenced by personnel of the Principado de Asturias and Junta de Castilla y León, primarily the Patrullas Osos, that is, the Bear Patrols, as well as other guards of both regional governments, by the Asturian Foundation for the Conservation of Wildlife (FAPAS, Fondo para la Protección de los Animales Salvajes), the FOA (Fundación Oso de Asturias) and the Brown Bear Foundation (FOP, Fundación Oso Pardo); and (b) personal georeferenced observations of the authors. Direct observations were performed almost daily from den exit (the beginning of spring, i.e., late March—beginning of May; González-Bernardo et al. 2020) to the end of the mating period (June), generally from sunrise to sunset. Viewing points used by rangers and ourselves were evenly distributed over the entire bear range in the study area. Monitoring principally occurred during the three peaks of activity of females with cubs, i.e., around sunrise, between 12:00 and 15:00 h, and at sunset (González-Bernardo et al. 2020). Continuous monitoring of known breeding areas early in the season and good visibility (reproductive dens in the Cantabrian Mountains are generally located in rocky areas with sparse vegetation, where bear families are visible) minimized the delay in observing FCOYs after den exit (González-Bernardo et al. 2020). All observations involving animals were conducted with the appropriate permits and following ASM guidelines (Sikes 2016).
To test our hypotheses, data on FCOY were separated into two periods, based on the trend of the western bear subpopulation (Penteriani et al. 2018b): (1) the period from 1995 to 2005, which showed an increase of 4 FCOY in 10 years, representing the beginning of the slow bear population increase after its decline; and (2) the period from 2006 to 2016, with an increase of 19 FCOY in 10 years (Fig. 2), i.e., the time of the marked increase of the western bear subpopulation (Penteriani et al. 2020a).
Population of observed brown bear females with cubs (FCOY) in the western sector of the Cantabrian Mountains (north-western Spain) from 1995 to 2016. Hollow circles indicate a first period of stable FCOY population (from 8 FCOY in 1995 to 12 in 2005) and solid dots indicate a second period of faster increase of FCOY (from 15 FCOY in 2006 to 34 in 2016)
Landscape characteristics around FCOY reproductive dens
The average den exit date of FCOYs in the Cantabrian Mountains is 28 April ± 11.9 days, with only 19% of FCOYs exiting the den during the first half of April, while the remaining 32% and 49% of FCOYs leave the den during the second half of April and the first half of May, respectively (González-Bernardo et al. 2020). Thus, to describe the habitat surrounding reproductive dens (which exact position is frequently unknown), we only considered the first observation of each FCOY in April and until mid-May (n = 116 locations). Those observations were recorded immediately after den exit, i.e., when FCOY are very close to the den (Authors’ unpublished data; Gardner et al. 2014). FCOYs are individually identified and monitored based on the number of cubs and color markings (e.g., Ordiz et al. 2007), granting that the first observation of each FCOY (i.e., the observations that we used for further analyses) actually corresponded to a uniquely differentiated FCOY.
To characterize the landscape around each FCOY location, we downloaded the DEM (20 m), BTN200, and CORINE Land Cover layers (www.centrodedescargas.cnig.es) and we calculated (1) altitude; (2) slope orientation; (3) terrain ruggedness; and (4) minimum Euclidean distances to plantation (conifer) trees, crops, fruit trees, pastures, forests, shrublands, rocky areas, rivers, paved roads, trails and towns, which are landscapes features affecting bear habitat use (e.g., Nellemann et al. 2007; Ordiz et al. 2014). We classified the above variables into three categories (Table 1): landscape structure, landscape composition, and human footprint (i.e., landscape variables associated with humans).
Following Zuur et al. (2010), data were examined for outliers in the response and explanatory variables, collinearity between explanatory variables, and the nature of relationships between the response and explanatory variables. Four of the original 116 observations were removed from the final dataset, one due to missing data and three deemed as outliers (i.e., values with a high standard deviation and/or identified by the Grubb’s Test for Outliers, Grubbs 1969). We assessed collinearity among explanatory variables using Pearson correlation and Variation Inflation Index (VIF), leading to the removal of both the distance to buildings and the distance to towns (R > 0.7, VIF > 3).
To explore potential differences between the first and the second period in the landscape characteristics around FCOY reproductive dens, we used a Bernoulli generalized linear model (GLM) based on an exponential function (and logit link function) for each FCOY location with the period as the response variable (0: 1996 to 2005; 1: 2006 to 2016) and each of the variables defined in Table 1 as covariates.
Variations in FCOY proximity
We investigated any differences in the closest and mean proximity between neighboring FCOY within the same year between the two time periods (Ordiz et al. 2008). We removed two of the 112 locations as no other FCOY were observed in the same year. We used a Mann–Whitney U test to assess differences in the proximity between neighboring FCOY between the two periods.
All the statistical analyses were carried out in R 4.0.2 (R Development Core Team 2020) using arm (version 1.12.2), lme4 (version 1.1.28), lawstat (version 3.4), outliers (version 0.14), and pROC (version 1.18.0). The extraction of environmental variables and GIS analyses were conducted in both QGIS 3.12.3 (QGIS.org 2020) and ArcGIS 10.7.1 (ESRI 2019).
Results
FCOY den landscape
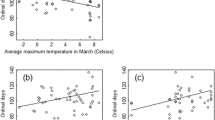
The Bernoulli GLM showed that FCOY reproductive dens were located significantly closer to rivers, trails, highways, and fruit trees during the period 2006–2016 than during the period 1995–2005 (Table 2, Figs. 3 and 4). Although lower altitudes are associated with these landscape features, the actual altitude covariate was marginally significant (P = 0.09). The overall model showed a pseudo R2 = 0.34 and its performance on correctly classifying the observations as belonging to the first or second period is considered acceptable (area under the receiver operating characteristic curve, AUC = 0.78, DeLong et al. 1988; Mandrekar 2010).
Mean fitted probability (solid line) of the distance from brown bear females with cubs reproductive dens to rivers, highways, trails and fruit trees during the two study periods. Data were modeled using a Bernoulli GLM. Black dots are observed data and shaded area is the 95% confidence intervals of the model
Variation in FCOY proximity
The mean closest distance to another FCOY in 1995–2005 was 11.7 km (median = 14 km), while it was 7.4 km in 2006–2016 (median 6.6 km), i.e., there was a significant decrease in the closest distance to a neighboring FCOY observed during the same year during the second period (Fig. 5), compared to the first one (W = 1454, N = 110, P < 0.01). On the contrary, the mean distance from a FCOY to all FCOY observed during the same year was 22.8 km in 2006–2016 (median = 22.5 km), while it was 20.0 km in 1995–2005 (median = 17.8 km), i.e., there was a significant increase in the distance among FCOY observed during the same year during the second period (Fig. 5), compared to the previous one (W = 746, N = 110, P < 0.05).
Discussion
Many studies have focused on modeling and understanding large carnivore habitat use. However, the effect of increasing densities on individuals’ spatial behavior is poorly understood. Our study highlights clear differences in brown bear habitat use of breeding areas between periods of different population densities. During the period of higher density, FCOYs selected areas closer to rivers, to anthropogenic sources of disturbance (trails, highways), and with higher densities of fruit trees, all typically associated with lower altitudes. There were shorter distances to the closest, neighboring breeding area in response to increasing bear numbers, while the mean distance among all (not only the closest) FCOY breeding areas increased in the second study period. In the latter period, the population not only increased locally (higher density), but also expanded at the landscape level (Zarzo-Arias et al. 2019).
A potential explanation for our results is a density-dependent mechanism driving habitat use of breeding areas by the most important segment of a brown bear population (adult, reproductive females) in the increasing, yet threatened population of the Cantabrian Mountains. FCOY habitat use patterns seem consistent with the ideal-despotic scenario, indicating preemptive use of breeding areas by FCOY. Preemptive behavior typically results in areas of higher quality being held earlier by more experienced or dominant individuals, allowing them to achieve higher fitness than those forced to occupy lower quality territories (Pulliam and Danielson 1991). Habitat use patterns following the ideal pre-emptive or ideal-dominant distributions are likely for recolonizing large carnivores in North America and Europe (Carter and Linnell 2016), and may include the potential for source–sink structured populations (O’Neil et al. 2020). Indeed, source-sink systems have the potential to determine that excess individuals emigrate from source areas during their lifetime and gather in sink areas, where their reproductive performances and survival are lower than in areas of higher quality (Timus et al. 2017). However, source–sink population dynamics are generally not considered in the conservation and management of brown bears and other large carnivores (Penteriani et al. 2018b; Morales-González et al. 2020; O’Neil et al. 2020), despite the fact that human-caused mortality for brown bears typically increases with closer proximity to human infrastructure (Steyaert et al. 2016b).
Preference for high-altitude, rugged terrain is likely a behavioral adaptation aimed at reducing FCOY movements, disturbance, and infanticide risk by conspecifics (the less FCOY move, the lower chances of meeting other bears), and favor increased survival in human-modified landscapes (Penteriani et al. 2020b). Restricted habitat availability due to density-dependent constraints and human encroachment in the Cantabrian human-modified landscape, together with the presence of male bears searching for reproductive opportunities, may promote encounters among bears during the mating season. This can, in turn, lead to a higher prevalence of infanticide events. Our finding that FCOY used areas closer to settlements and human activity in the second period of study, when the bear population has increased more rapidly, can also reflect that FCOY use areas closer to humans to segregate spatially from adult male bears, which are thought to avoid such areas (Nellemann et al. 2007). Although the lack of simultaneous positions of FCOY and adult bears does not allow to test this human-shield hypothesis, habitat selection is shown to be important for offspring survival of Scandinavian brown bears by helping to avoid infanticide by conspecifics (Steyaert et al. 2016a, b).
Litter size and cub mortality in the Cantabrian brown bear population have remained stable over the years (Penteriani et al. 2018b, 2020a), potentially linked to different key factors. First, the availability of main food resources in our study area might be considered relatively homogeneous over the breeding areas occupied by FCOY. As a result, even at times of high FCOY density, there likely is not high competition for food resources. Second, similar to other carnivores, common optimal litter sizes of Cantabrian brown bears do not vary according to environmental conditions (Gaillard et al. 2014; Gigliotti et al. 2020).
It is crucial to monitor long-term dynamics of spatial distribution and landscape use of threatened populations inhabiting human-modified landscapes. In such settings, key components of populations, such as FCOY, are challenged both by intra-specific and human-related factors. Namely, by an increasing density of conspecifics in areas characterized by heavy human encroachment. Improving our knowledge on bear habitat use in human-modified landscapes is essential to inform both managers and the general public. For example, managers and public should understand that seasonal, spring observations of FCOY nearby human settlements do not necessarily denote habituation to people (Ordiz et al. 2019), but just spatial avoidance of conspecifics in a scenario of sexual conflict (Steyaert et al. 2016a).
Indeed, our study has revealed that conservation and management of small and endangered populations must be based on long-term series of data and a careful consideration of the biology and population dynamics of the target species. The identification of differences in habitat use as a response to population density, such as those shown in our study, can be useful for predicting future densities and potential expansions of large carnivore populations (O’Neil et al. 2017, 2020). Furthermore, our study builds upon earlier research suggesting that population regulation mechanisms (e.g., reproductive suppression) that are typically attributed to group-living mammals also play a role in the population dynamics and space use of so-called solitary species, such as brown bears (Ordiz et al. 2008).
From a conservation-oriented perspective, our results inform the management of threatened species that are partially recovering former ranges in human-modified landscapes. In our case, an intraspecific factor (population density) interacts with sub-optimal habitat availability found closer to human settlements and activities. These interacting factors should be taken into account jointly in order to infer future population dynamics. This is crucial for landscape management and designations of critical habitat, i.e., to avoid displacement of FCOY at a critical period of the year, and for promoting awareness of potential wildlife-human conflicts.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Code availability
The R code used during the current study is available from the corresponding author on reasonable request.
References
Arditi R, Tyutyunov Y, Morgulis A et al (2001) Directed movement of predators and the emergence of density-dependence in predator–prey models. Theor Popul Biol 59:207–221. https://doi.org/10.1006/tpbi.2001.1513
Avgar T, Betini GS, Fryxell JM (2020) Habitat selection patterns are density dependent under the ideal free distribution. J Anim Ecol 89:2777–2787. https://doi.org/10.1111/1365-2656.13352
Benson JF, Chamberlain MJ (2007) Space use and habitat selection by female Louisiana black bears in the Tensas River Basin of Louisiana. J Wildl Manage 71:117–126. https://doi.org/10.2193/2005-580
Boitani L, Linnell JDC (2015) Bringing large mammals back: large carnivores in Europe. Rewilding European Landscapes. Springer International Publishing, Cham, pp 67–84
Bruskotter JT, Shelby LB (2010) Human dimensions of large carnivore conservation and management: introduction to the special issue. Hum Dimens Wildl 15:311–314. https://doi.org/10.1080/10871209.2010.508068
Carter NH, Linnell JDC (2016) Co-Adaptation is key to coexisting with large carnivores. Trends Ecol Evol 31:575–578. https://doi.org/10.1016/j.tree.2016.05.006
Chapron G, Kaczensky P, Linnell JDC et al (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346:1517–1519. https://doi.org/10.1126/science.1257553
Clevenger AP, Purroy FJ (1991) Demografia del oso pardo (Ursus arctos) en la Cordillera Cantábrica. Ecologia 5:243–256
Clevenstine AJ, Lowe CG (2021) Aggregation site fidelity and movement patterns of the protected marine predator giant sea bass (Stereolepis gigas). Environ Biol Fishes 104:401–417. https://doi.org/10.1007/s10641-021-01077-9
Coogan SCP, Coops NC, Janz DM et al (2019) Towards grizzly bear population recovery in a modern landscape. J Appl Ecol 56:93–99. https://doi.org/10.1111/1365-2664.13259
Dahle B, Swenson JE (2003) Home ranges in adult Scandinavian brown bears (Ursus arctos): effect of mass, sex, reproductive category, population density and habitat type. J Zool 260:S0952836903003753. https://doi.org/10.1017/S0952836903003753
Dahle B, Støen O-G, Swenson JE (2006) Factors incluencing home-range size in subadult brown bears. J Mammal 87:859–865. https://doi.org/10.1644/05-MAMM-A-352R1.1
Darling LM (1987) Habitat use by grizzly bear family groups in interior Alaska. Bears Their Biol Manag 7:169. https://doi.org/10.2307/3872623
del Delgado M, M, Penteriani V, Morales JM, et al (2014) A statistical framework for inferring the influence of conspecifics on movement behaviour. Methods Ecol Evol 5:183–189. https://doi.org/10.1111/2041-210X.12154
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837. https://doi.org/10.2307/2531595
Edwards GP, DE Preu N, Crealy IV, Shakeshaft BJ (2002) Habitat selection by feral cats and dingoes in a semi-arid woodland environment in central Australia. Austral Ecol 27:26–31. https://doi.org/10.1046/j.1442-9993.2002.01156.x
Eklund A, Johansson M, Flykt A et al (2020) Believed effect—a prerequisite but not a guarantee for acceptance of carnivore management interventions. Biol Conserv 241:108251. https://doi.org/10.1016/j.biocon.2019.108251
Evans PR, Fretwell SD (1973) Populations in a seasonal environment. J Anim Ecol 42:464. https://doi.org/10.2307/3303
Fernández-Gil A, Naves J, Ordiz A et al (2016) Conflict misleads large carnivore management and conservation: brown bears and wolves in spain. PLoS ONE 11:e0151541. https://doi.org/10.1371/journal.pone.0151541
Ferrer M, Penteriani V (2008) Non-independence of demographic parameters: positive density-dependent fecundity in eagles. J Appl Ecol 45:1453–1459. https://doi.org/10.1111/j.1365-2664.2008.01497.x
Fowler CW (1981) Density dependence as related to life history strategy. Ecology 62:602–610. https://doi.org/10.2307/1937727
Fowler CW (1987) A review of density dependence in populations of large mammals. In: Genoways HH (ed) Current Mammalogy. Springer, US, Boston, MA, pp 401–441
Gaillard J-M, Nilsen EB, Odden J et al (2014) One size fits all: Eurasian lynx females share a common optimal litter size. J Anim Ecol 83:107–115. https://doi.org/10.1111/1365-2656.12110
Gardner CL, Pamperin NJ, Benson JF (2014) Movement patterns and space use of maternal grizzly bears influence cub survival in Interior Alaska. Ursus 25:121–138. https://doi.org/10.2192/URSUS-D-14-00015.1
Gigliotti LC, Slotow R, Hunter LTB et al (2020) Context dependency of top-down, bottom-up and density-dependent influences on cheetah demography. J Anim Ecol 89:449–459. https://doi.org/10.1111/1365-2656.13099
Gonzalez EG, Blanco JC, Ballesteros F et al (2016) Genetic and demographic recovery of an isolated population of brown bear Ursus arctos L., 1758. PeerJ 4:e1928. https://doi.org/10.7717/peerj.1928
González-Bernardo E, Bombieri G, Mar Delgado M Del, Penteriani V (2020) The role of spring temperatures in the den exit of female brown bears with cubs in southwestern Europe. Ursus 2020:.https://doi.org/10.2192/URSUS-D-19-00015.1
Grubbs FE (1969) Procedures for detecting outlying observations in samples. Technometrics 11:1. https://doi.org/10.2307/1266761
Hassell MP (1975) Density-dependence in single-species populations. J Anim Ecol 44:283–295
Ims RA, Andreassen HP (2005) Density-dependent dispersal and spatial population dynamics. Proc R Soc B Biol Sci 272:913–918. https://doi.org/10.1098/rspb.2004.3025
Kilpatrick HJ, Spohr SM, Lima KK (2001) Effects of population reduction on home ranges of female white-tailed deer at high densities. Can J Zool 79:949–954. https://doi.org/10.1139/z01-057
Kjellander P, Hewison AJM, Liberg O et al (2004) Experimental evidence for density-dependence of home-range size in roe deer ( Capreolus capreolus L.): a comparison of two long-term studies. Oecologia 139:478–485. https://doi.org/10.1007/s00442-004-1529-z
Ladle A, Avgar T, Wheatley M et al (2019) Grizzly bear response to spatio-temporal variability in human recreational activity. J Appl Ecol 56:375–386. https://doi.org/10.1111/1365-2664.13277
Linnell JD, Promberger C, Boitani L, et al (2005) The linkage between conservation strategies for large carnivores and biodiversity: the view from the ‘“half-full”’ forests of Europe. In: Large carnivores and the conservation of biodiversity. pp 381–398
Mandrekar JN (2010) Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 5:1315–1316. https://doi.org/10.1097/JTO.0b013e3181ec173d
Martínez Cano I, Taboada FG, Naves J et al (2016) Decline and recovery of a large carnivore: environmental change and long-term trends in an endangered brown bear population. Proc R Soc B Biol Sci 283:20161832. https://doi.org/10.1098/rspb.2016.1832
Mateo-Sánchez MC, Gastón A, Ciudad C et al (2016) Seasonal and temporal changes in species use of the landscape: how do they impact the inferences from multi-scale habitat modeling? Landsc Ecol 31:1261–1276. https://doi.org/10.1007/s10980-015-0324-z
Matthysen E (2005) Density-dependent dispersal in birds and mammals. Ecography (cop) 28:403–416. https://doi.org/10.1111/j.0906-7590.2005.04073.x
Mattson DJ (1997) Sustainable grizzly bear mortality calculated from counts of females with cubs-of-the-year: an evaluation. Biol Conserv 81:103–111. https://doi.org/10.1016/S0006-3207(96)00149-8
Morales-González A, Ruiz-Villar H, Ordiz A, Penteriani V (2020) Large carnivores living alongside humans: brown bears in human-modified landscapes. Glob Ecol Conserv 22:e00937. https://doi.org/10.1016/j.gecco.2020.e00937
Morris DW (1987) Tests of density-dependent habitat selection in a patchy environment. Ecol Monogr 57:269–281. https://doi.org/10.2307/2937087
Morris DW (2003) Toward an ecological synthesis: a case for habitat selection. Oecologia 136:1–13. https://doi.org/10.1007/s00442-003-1241-4
Murdoch WW (1994) Population regulation in theory and practice. Ecology 75:271–287. https://doi.org/10.2307/1939533
Naves J, Palomero G (1993) Distribución del oso pardo en la Cordillera Cantábrica. El oso pardo. Icona, Madrid, pp 35–46
Nellemann C, Støen OG, Kindberg J et al (2007) Terrain use by an expanding brown bear population in relation to age, recreational resorts and human settlements. Biol Conserv 138:157–165. https://doi.org/10.1016/j.biocon.2007.04.011
O’Neil ST, Bump JK, Beyer DE (2017) Spatially varying density dependence drives a shifting mosaic of survival in a recovering apex predator ( Canis lupus ). Ecol Evol 7:9518–9530. https://doi.org/10.1002/ece3.3463
O’Neil ST, Vucetich JA, Beyer DE et al (2020) Territoriality drives preemptive habitat selection in recovering wolves: implications for carnivore conservation. J Anim Ecol 89:1433–1447. https://doi.org/10.1111/1365-2656.13199
Ordiz A, Rodríguez C, Naves J et al (2007) Distance-based criteria to identify minimum number of brown bear females with cubs in Europe. Ursus 18:158–167. https://doi.org/10.2192/1537-6176(2007)18[158:DCTIMN]2.0.CO;2
Ordiz A, Støen OG, Swenson JE et al (2008) Distance-dependent effect of the nearest neighbor: spatiotemporal patterns in brown bear reproduction. Ecology 89:3327–3335. https://doi.org/10.1890/07-1921.1
Ordiz A, Støen O-G, Sæbø S et al (2012) Do bears know they are being hunted? Biol Conserv 152:21–28. https://doi.org/10.1016/j.biocon.2012.04.006
Ordiz A, Støen O-G, Saebø S et al (2013) Lasting behavioural responses of brown bears to experimental encounters with humans. J Appl Ecol 50:306–314. https://doi.org/10.1111/1365-2664.12047
Ordiz A, Kindberg J, Sæbø S et al (2014) Brown bear circadian behavior reveals human environmental encroachment. Biol Conserv 173:1–9. https://doi.org/10.1016/j.biocon.2014.03.006
Ordiz A, Støen O-G, Delibes M, Swenson JE (2017) Staying cool or staying safe in a human-dominated landscape: which is more relevant for brown bears? Oecologia 185:191–194. https://doi.org/10.1007/s00442-017-3948-7
Ordiz A, Moen GK, Sæbø S et al (2019) Habituation, sensitization, or consistent behavioral responses? Brown bear responses after repeated approaches by humans on foot. Biol Conserv 232:228–237. https://doi.org/10.1016/j.biocon.2019.01.016
Palomero G, Ballesteros F, Nores C et al (2007) Trends in number and distribution of brown bear females with cubs-of-the-year in the Cantabrian Mountains, Spain. Ursus 18:145–157. https://doi.org/10.2192/1537-6176(2007)18[145:TINADO]2.0.CO;2
Penteriani V, Otalora F, Ferrer M (2006) Floater dynamics can explain positive patterns of density-dependent fecundity in animal populations. Am Nat 168:697–703. https://doi.org/10.1086/507995
Penteriani V, del Delgado M, M, Pinchera F, et al (2016) Human behaviour can trigger large carnivore attacks in developed countries. Sci Rep 6:20552. https://doi.org/10.1038/srep20552
Penteriani V, Delgado MDM, Krofel M et al (2018) Evolutionary and ecological traps for brown bears Ursus arctos in human-modified landscapes. Mamm Rev 48:180–193. https://doi.org/10.1111/mam.12123
Penteriani V, Zarzo-Arias A, Bombieri G et al (2018) Density and reproductive characteristics of female brown bears in the Cantabrian Mountains, NW Spain. Eur Zool J 85:312–320. https://doi.org/10.1080/24750263.2018.1499826
Penteriani V, Zarzo-Arias A, Del Mar DM et al (2020) Female brown bears use areas with infanticide risk in a spatially confined population. Ursus 2020:1–9. https://doi.org/10.2192/URSUS-D-18-00019R4
Penteriani V, Karamanlidis AA, Ordiz A, et al (2020a) Bears in human-modified landscapes: the case studies of the cantabrian, apennine, and Pindos Mountains. In: Bears of the World. Cambridge University Press, pp 260–272
Pérez T, Naves J, Vázquez JF et al (2010) Evidence for improved connectivity between Cantabrian brown bear subpopulations. Ursus 21:104–108. https://doi.org/10.2192/09SC018.1
Pérez T, Naves J, Vázquez JF et al (2014) Estimating the population size of the endangered Cantabrian brown bear through genetic sampling. Wildlife Biol 20:300–309. https://doi.org/10.2981/wlb.00069
Potapov A, Merrill E, Lewis MA (2012) Wildlife disease elimination and density dependence. Proc R Soc B Biol Sci 279:3139–3145. https://doi.org/10.1098/rspb.2012.0520
Pulliam HR, Danielson BJ (1991) Sources, sinks, and habitat selection: a landscape perspective on population dynamics. Am Nat 137:S50–S66. https://doi.org/10.1086/285139
Rosenzweig ML (1981) A theory of habitat selection. Ecology 62:327–335. https://doi.org/10.2307/1936707
Šálek M, Drahníková L, Tkadlec E (2015) Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mamm Rev 45:1–14. https://doi.org/10.1111/mam.12027
Servheen C (1983) Grizzly Bear food habits, movements, and habitat selection in the mission mountains. Montana J Wildl Manage 47:1026. https://doi.org/10.2307/3808161
Sikes RS, the Animal Care and Use Committee of the American Society of Mammalogists (2016) 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688. https://doi.org/10.1093/jmammal/gyw078
Sinclair ARE, Pech RP (1996) Density dependence, stochasticity, compensation and predator regulation. Oikos 75:164. https://doi.org/10.2307/3546240
Sjödin H, Brännström Å, Söderquist M, Englund G (2014) Population-level consequences of heterospecific density-dependent movements in predator–prey systems. J Theor Biol 342:93–106. https://doi.org/10.1016/j.jtbi.2013.09.019
Stewart KM, Bowyer RT, Dick BL et al (2005) Density-dependent effects on physical condition and reproduction in North American elk: an experimental test. Oecologia 143:85–93. https://doi.org/10.1007/s00442-004-1785-y
Steyaert SMJG, Zedrosser A, Elfström M et al (2016) Ecological implications from spatial patterns in human-caused brown bear mortality. Wildlife Biol 22:144–152. https://doi.org/10.2981/wlb.00165
Steyaert SMJG, Leclerc M, Pelletier F, et al (2016a) Human shields mediate sexual conflict in a top predator. Proc R Soc B Biol Sci 283:. https://doi.org/10.1098/rspb.2016.0906
Støen O-G, Zedrosser A, Sæbø S, Swenson JE (2006) Inversely density-dependent natal dispersal in brown bears Ursus arctos. Oecologia 148:356–364. https://doi.org/10.1007/s00442-006-0384-5
Støen O-G, Ordiz A, Evans AL et al (2015) Physiological evidence for a human-induced landscape of fear in brown bears (Ursus arctos). Physiol Behav 152:244–248. https://doi.org/10.1016/j.physbeh.2015.09.030
Taylor LR, Woiwod IP, Perry JN (1978) The density-dependence of spatial behaviour and the rarity of randomness. J Anim Ecol 47:383. https://doi.org/10.2307/3790
Timus N, Czekes Z, Rákosy L, Nowicki P (2017) Conservation implications of source-sink dynamics within populations of endangered Maculinea butterflies. J Insect Conserv 21:369–378. https://doi.org/10.1007/s10841-016-9906-6
van Beest FM, McLoughlin PD, Vander Wal E, Brook RK (2014) Density-dependent habitat selection and partitioning between two sympatric ungulates. Oecologia 175:1155–1165. https://doi.org/10.1007/s00442-014-2978-7
van Beest FM, Uzal A, Vander Wal E et al (2014) Increasing density leads to generalization in both coarse-grained habitat selection and fine-grained resource selection in a large mammal. J Anim Ecol 83:147–156. https://doi.org/10.1111/1365-2656.12115
Wolff JO (1997) Population regulation in mammals: an evolutionary perspective. J Anim Ecol 66:1. https://doi.org/10.2307/5959
Zarzo-Arias A, Penteriani V, Del Mar DM et al (2019) Identifying potential areas of expansion for the endangered brown bear (Ursus arctos) population in the cantabrian mountains (NW Spain). PLoS ONE 14:1–16. https://doi.org/10.1371/journal.pone.0209972
Zarzo-Arias A, Delgado MM, Palazón S et al (2021) Seasonality, local resources and environmental factors influence patterns of brown bear damages: implications for management. J Zool 313:1–17. https://doi.org/10.1111/jzo.12839
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
The local administrations of Junta de Castilla y León and Principado de Asturias provided us with the long-term database on brown bear observations and the authorizations needed to work in the brown bear restricted areas of the Cantabrian Mountains. We would like to thank David Cubero Bausela, Mercedes García Dominguez, and María Angeles Osorio Polo of the Junta de Castilla y Léon, and Teresa Sánchez Corominas, Víctor Vázquez, and Pedro García-Rovés of the Principado de Asturias. We are grateful to two anonymous reviewers and Dr Esther Kettel for providing helpful comments that have improved the manuscript.
Funding
V.P. and A.O. were financially supported by the I + D + I Project PID2020-114181 GB-I00 financed by the Spanish Ministry of Science and Innovation, the Agencia Estatal de Investigación (AEI), and the Fondo Europeo de Desarrollo Regional (FEDER, EU). AZA was financially supported by a Margarita Salas contract financed by the European Union-NextGenerationEU, Ministerio de Universidades y Plan de Recuperación, Transformación y Resiliencia, through the call of the Universidad de Oviedo (Asturias).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All observations involving animals were conducted with the appropriate permits and following ASM guidelines (Sikes 2016).
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Jan M. Wójcik
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uzal, A., Martinez-Artero, J., Ordiz, A. et al. Habitat characteristics around dens in female brown bears with cubs are density dependent. Mamm Res 67, 445–455 (2022). https://doi.org/10.1007/s13364-022-00640-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-022-00640-8