Abstract
The leopard cat (Prionailurus bengalensis) is the most common wild felid in Southeast Asia, yet little is known about the factors that affect their population density and occupancy in natural habitats. Although leopard cats are highly adaptable and reportedly can attain high densities in human-modified habitats, it is not clear which natural habitat is optimal for the species. Also, this felid has been preyed upon by large carnivores in Southeast Asia, yet the intra-guild effects of large carnivore presence on leopard cats are almost unknown. To shed light on these fundamental questions, we used data from camera trap surveys for felids to determine the leopard cat densities in three different forest types within Cambodia: continuous evergreen, mosaic dominated by evergreen (hereafter evergreen mosaic), and mosaic dominated by open dry deciduous forests (hereafter DDF mosaic). We also conducted occupancy analyses to evaluate the interactions of the leopard cats with three large carnivores: leopards (Panthera pardus), dholes (Cuon alpinus), and domestic dogs (Canis familiaris). The estimated density (individuals/100 km2 ± SE) was highest in the continuous evergreen (27.83 ± 7.68), followed by evergreen mosaic (22.06 ± 5.35) and DDF mosaic (13.53 ± 3.23). Densities in all three forest types were relatively high compared to previous studies. Domestic dogs were detected on all 3 sites, and leopards and dholes had sufficient records on only one site each. The occupancy probability of leopard cats was not affected by the presence or absence of any large carnivore, indicating that large carnivores and leopard cats occurred independently of each other. Our findings support the claim that leopard cats are habitat generalists, but we show that evergreen forest is the optimum natural habitat for this species in the region. The DDF mosaic appears to sustain lower densities of leopard cats, probably due to the harsh dry season and wildfires that led to reduced prey base, although this generalist felid was still able to occupy DDF in relatively moderate numbers. Overall, the adaptability of leopard cats to various forest types, and lack of negative interaction with large carnivores, helps to explain why this species is the most common and widespread felid in Southeast Asia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Southeast Asia is rich in biodiversity and has a high concentration of endemic fauna and flora species that comprise 18% of the global endemic plant and animal species (Myers et al. 2000; Sodhi et al. 2010). The biodiversity of Southeast Asia has dramatically declined as a result of human-related activities, including habitat destruction, over-hunting, pollution, and climate change (Sodhi et al. 2004; Sodhi and Brook 2006; Koh and Sodhi 2010). Habitat loss and deforestation in Southeast Asia are among the highest in the world (Sodhi et al. 2010), and forest cover continues to decline (Kim et al. 2015; Miettinen et al. 2011), even inside protected areas (Heino et al. 2015).
Wild felids are among the most threatened groups of terrestrial mammalian carnivores, with 25 of the 38 known species listed as globally threatened (Macdonald et al. 2010; Sunquist and Sunquist 2017). At least nine species of wild cats occur in mainland Southeast Asia, making it one of the most felid-diverse regions in the world (Burnham et al. 2012; Macdonald et al. 2012). The leopard cat (Prionailurus bengalensis) is the smallest felid (3–5 kg; Francis 2019) in Southeast Asia, and it is a generalist that occupies a broad range of habitats in both protected and non-protected areas (Ross et al. 2015; Sunquist and Sunquist 2017). Leopard cats that occur on Indonesian and Philippine islands recently have been classified as a different species, the Sunda leopard cat (P. javanensis; Kitchener et al. 2017), although it is similar in size and presumably has a similar ecology to mainland leopard cats. The diet of both species of leopard cats consists mostly of small (< 500 g) mammals, mainly Muridae, but also Sciuridae, and Tupaiidae (Rabinowitz 1990; Grassman 2000; Kamler et al. 2020a), and they occasionally feed on small carnivores (i.e., Mustelidae), lizards, birds, insects, amphibians, and plants (Rajaratnam et al. 2007; Xiong et al. 2016; Sunquist and Sunquist 2017). Because leopard cats have not declined dramatically across their range despite human-caused habitat changes, they are classified as Least Concern by the IUCN (Ross et al. 2015). In fact, densities of this small felid might be higher in human-modified landscape compared to natural landscapes. For example, densities of both leopard cat species were 2–21 individuals/100 km2 across protected areas (Table 1). However, they can attain unusually high densities (89 individuals/100 km2) in human-modified habitats, such as palm tree plantations (Chua et al. 2016), and they can prefer palm plantations over nearby natural forest, reportedly because of the greater abundance of small murid species in palm plantations (Rajaratnam et al. 2007). Higher abundances of leopard cats in human-dominated areas also have been reported in India (Srivathsa et al. 2015), and high tolerance for degraded habitat was confirmed for the Sunda leopard cat in Borneo (Wearn et al. 2013). However, little is known about the factors that affect leopard cat density in natural habitats, and it is not clear which natural habitat is optimal for this species.
Southeast Asia is dominated by evergreen and semi-evergreen forests, although open dry deciduous forests (DDF) cover about 15–20% of Southeast Asia (Wohlfart et al. 2014). Previous studies gave conflicting results about the effects of these forest types on leopard cat abundance. In northeastern Thailand, leopard cats were found to be most abundant in evergreen forests, moderately abundant in degraded forests, and almost non-existent in DDF (Petersen et al. 2019). However, in eastern Cambodia, leopard cats were found to be habitat generalists that regularly used DDF (Rostro-García et al. 2021). Evergreen forests would seemingly be better habitat for leopard cats because this habitat has a higher number and biomass of small rodents compared to DDF (Walker and Rabinowitz 1992; Petersen et al. 2019; Rostro-García et al. 2021). In contrast to evergreen forests, DDF forests in Southeast Asia typically have annual dry season fires which burn most of the grassy understory (Baker and Bunyavejchewin 2009; McShea et al. 2011; Pin et al. 2018) which can significantly decrease the seasonal biomass of small mammals (Walker and Rabinowitz 1992). However, there might be some benefits of DDF compared to evergreen forests for leopard cats, such as reduced numbers of predators such as leopards, which were previously shown to consume this small felid in eastern Cambodia (Rostro-García et al. 2018). Comparing leopard cat densities between evergreen forests and DDF would help determine which forest type is optimal for this species in Southeast Asia.
Leopard cats are preyed upon by larger carnivores, including leopards (Panthera pardus; Rostro-García et al. 2018) and dholes (Cuon alpinus; Kamler et al. 2020b), but little is known about the negative impacts of large carnivores on this small felid. Previous research gave conflicting results because some studies found high spatial overlap between both leopard cat species and large felids (Sunarto et al. 2015; Kyaw et al. 2021), whereas another study found that leopard cats avoided large felids (Vitekere et al. 2020); no studies have investigated the interactions of dholes and leopard cats. Also, domestic dogs (Canis familiaris) are sometimes abundant within protected areas of Southeast Asia, and they can have severe negative impacts on wildlife (Hughes and Macdonald 2013; Doherty et al. 2017; Gompper 2021). Therefore, domestic dogs probably also prey on leopard cats and they might have negative impacts on their populations. Understanding the relationships between leopard cats and large carnivores might help explain differences in their densities in both natural and human-modified habitats.
We used camera trap data to determine leopard cat densities in three sites in Cambodia that contained different forest types: continuous evergreen, evergreen mosaic, and DDF mosaic. We also conducted occupancy analyses to evaluate the interaction of leopard cats with three large carnivores: leopards, dholes, and domestic dogs. Based on previous studies, we predicted that the density of this small felid would be highest in continuous evergreen and lowest in DDF mosaic, owing to presumed differences in small rodent abundance (Walker and Rabinowitz 1992; Petersen et al. 2019; Rostro-García et al. 2021). We also predicted that all three large carnivores would have a negative impact on leopard cat occupancy because of their potential predation on this species (Rostro-García et al. 2018; Kamler et al. 2020b). Our study will help determine the effects of forest types and large carnivores on the density and occupancy of leopard cats in relatively natural habitats.
Study areas
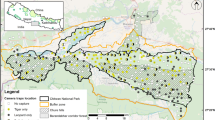
Camera trap surveys were conducted in the core zones of three protected areas in Cambodia: Central Cardamom National Park (CCNP, 11° 56′ N, 103° 29′ E), Phnom Prich Wildlife Sanctuary (PPWS, 12° 46ʹ N, 106° 52ʹ E), and Srepok Wildlife Sanctuary (SWS, 12° 50′ N, 107° 50′ E; Fig. 1). The CCNP (4013 km2) is dominated by evergreen and semi-evergreen forests in hilly terrain that forms part of the Cardamom Rainforest Landscape, situated in southwestern Cambodia; elevation ranges from 20 to 1540 m. The PPWS (2225 km2) consists of large patches of evergreen and semi-evergreen forests in hilly terrain and ridge lines, interspersed with DDF habitat on flat terrain; elevation ranges from 80 to 640 m. The SWS (3729 km2) is dominated by DDF habitat with small patches of evergreen and semi-evergreen forests in hilly terrain; elevation ranges from 100 to 400 m. Both PPWS and SWS are part of the Cambodia’s Eastern Plains Landscape that forms the largest extant of lowland dry forest in Southeast Asia. Camera-trapping grids in all study sites were located in natural forests, without villages, agricultural fields, plantations, or cattle grazing, and these sites are considered potential areas for tiger reintroduction in Cambodia (Gray et al. 2020).
Methods
Camera-trapping
All camera trap surveys were conducted during the dry season (December to May). In CCNP, from December 2013 to March 2014 cameras were placed in 81 locations within continuous evergreen forests in hilly terrain; this site was classified as continuous evergreen forest (Fig. 1; Table 2). In PPWS, from December 2012 to March 2013 camera traps were placed in 77 locations within evergreen and semi-evergreen forests in hilly terrain that were surrounded by DDF habitat; this site was classified as evergreen mosaic (Fig. 1; Table 2). In SWS, from December 2015 to February 2016 cameras were placed in 46 locations primarily within DDF habitat (87% of locations) in relatively flat terrain; this site was classified as DDF mosaic (Fig. 1; Table 2). Camera traps were placed along dirt roads, animal trails, abandoned logging roads, dry riverbeds, and ridge lines in the core zones of all sites. In all sites, paired camera traps were placed on opposite sides of the trail, and fastened to trees approx. 30–50 cm above the ground, and approx. 2–3 m from the center of the trails. In CCNP and PPWS, the focal animal of the camera trap survey was clouded leopards (Neofelis nebulosa), and the mean spacing between camera traps was 479 m and 725 m, respectively. In SWS, the focal animal of the camera trap survey was leopard, and the mean spacing between camera traps was 2516 m (Rostro-García et al. 2018).
Density estimation
Leopard cats were independently identified by three of the authors based on unique body-markings, and any discrepancies were jointly reviewed to reach a final agreement on identification (Rostro-García et al. 2018).We separated the pictures into left and right flanks and discarded those pictures that could not be identified. We also identified the sex of individuals when there were clear photographs of the rear end; an individual was defined as a male if its scrotum was visible, or as female if no scrotum was visible or if it was accompanied by young or appeared to be pregnant (Webb et al. 2020 ). On each site, we included in the analysis adult individuals in which both flanks were identified (Fig. S1 ), as well as those in which only one side was identified (we used the side that had the most individuals) to get a minimum number of individuals per site. We used 1-day occasions as a sampling period yielding a total of 78–94 occasions per study site (Table 2 ) and constructed a capture history that consisted of all identified mature individuals, camera trap station number, occasion ID, and sex (Royle et al. 2014 ).
Densities were estimated using spatially explicit capture-recapture (SCR) models under the Bayesian framework (Royle et al. 2014; Meredith 2020a). The R packages secr (Efford 2020), rgdal (Bivand et al. 2016), raster (Bivand et al. 2016), and makeJAGSmask (Meredith 2020b) were used for importing and formatting capture histories and creating the state space. We ran two models: (1) a spatial model with elevation as a covariate assuming that their densities would vary across the elevational gradient, and (2) capture probability (p) and scale parameter (σ) as a function of sex (Sollmann et al. 2011; Webb et al. 2020). The capture probability and the movement scale parameter for both sexes were estimated for each site. The elevation covariate was standardized by subtracting the values by its mean, and dividing by the standard deviation.
An effective survey area (state space) was created using QGIS 3.14 (QGIS Delopment Team 2020) by setting a buffer (4 times the movement scale parameter σ) around each camera-trap grid (Efford 2004). Unsuitable habitats, such as permanent rivers, were excluded from the effective survey area (Royle et al. 2014; Webb et al. 2020). We set data augmentation to 5 times the number of total identified individuals (Efford and Fewster 2013; Royle et al. 2014). We report posterior mean density with standard deviations and the 95% posterior highest density intervals (Penjor et al. 2018).
We fitted the model using a Bayesian approach implemented with JAGS (Plummer 2003) via program R (R Core Team 2020) by using R package jagsUI (Kellner et al. 2018). We ran three chains of Marko Chain Monte Carlo (MCMC) of 100,000 iterations each, discarded 10,000 during initial burn-in and 10,000 during adaptation per chain, and thinned by 10, which yielded 27,000 total posterior samples. The model convergence was assessed based on the Gelman-Rubin statistic (Rhat): the potential scale reduction factor and MCMC diagnostic trace plots (Gelman et al. 2013; Penjor et al. 2018). To assess model fit, we calculated Freeman-Tukey discrepancy between real and simulated data and calculated Bayesian p value where values between 0.05 and 0.95 indicate adequate fit.
Occupancy modeling
We conducted single-season two-species occupancy analyses (Waddle et al. 2010) to investigate the interaction between the leopard cat and three large carnivores: dholes, leopards, and domestic dogs (Table 3), which we considered dominant. To avoid zero inflation in the data (i.e., too many non-detections) and increase detection frequency, multiple days were pooled (Bischof et al. 2014; Penjor et al. 2019). We pooled the detection/non-detection data into 7-day occasions yielding a total of 12–14 sampling occasions per study site.
The hierarchical single-season two-species occupancy analysis allowed us to estimate the occupancy and detection probability of both dominant (i.e., large carnivores) and subordinate species (i.e., leopard cats) simultaneously (Waddle et al. 2010). We adopted the previous code (Meredith 2020a, c) to model one-way interaction between a dominant species and a subordinate species, where occupancy of subordinate species is affected by the presence/absence of the dominant species, but not vice versa. We fitted the model using a Bayesian approach implemented with JAGS (Plummer 2003) via program R (R Core Team 2020) using R packages jagsUI (Kellner et al. 2018) and wiqid (Meredith 2020c). We used uninformative uniform priors for all the parameters (i.e., dbeta[1, 1]). We ran three chains of Marko Chain Monte Carlo (MCMC) with 500,000 iterations, discarded 10,000 during initial burn-in and 10,000 during adaptation per chain, and thinned by 10, which yielded 147,000 total posterior samples. The model convergence was based on the Gelman-Rubin statistic for each parameter, where models were successfully converged with the Rhat value < 1.1 (Gelman et al. 2013; Bischof et al. 2014; Penjor et al. 2019). We report posterior means with standard deviations and 95% highest density credible intervals (Penjor et al. 2018, 2019). For each parameter, n.eff was a crude measure of effective sample size. We checked if 0 falls in the parameter’s 95% Bayesian Credible Interval (CI), and considered that it has a strong support if the 95% BIC did not overlap 0. For each model, the species interaction factor (SIF) was calculated between the leopard cats and the dominant species (SIF < 1 suggests species avoidance, SIF > 1 suggests species co-occur more frequently, and SIF = 1 suggests species occur independently; MacKenzie et al. 2004).
Results
Leopard cat density
We identified a total of 66 individual leopard cats from 15,492 trap days across all three sites (Table 2). Photos from all sites could be identified to an individual, except for 1 photo from CCNP, 1 photo from SWS, and 2 photos from PPWS that were discarded because of blurriness. The estimated population sizes (N ± SD) in the effective area were 45.36 ± 12.51 in CCNP, 56.93 ± 13.81 in PPWS, and 102.01 ± 24.37 in SWS. The model with capture probability and the movement scale parameter as a function of sex covariate estimated the density (no. individuals/100 km2 ± SD) as 27.82 ± 7.67 in CCNP, 22.06 ± 5.35 in PPWS, and 13 ± 3.23 in SWS (Fig. S2). We also estimated the expected number of individuals/100 km2 at each activity center within the study areas (Fig. S3). In SWS and PPWS, the capture probability of males was lower compared to that of females (Table 4). Overall, the capture probability was highest for females in SWS, and highest for males in CCNP (Table 4). In SWS and PPWS, the movement scale parameters (sigma) for males were 1–2 times higher than for females (Table 3). In contrast, in CCNP the movement scale parameter (sigma) for females was higher than for males (Table 4). The sex ratio of females to males in the population within the effective area was 1.2:1 in CCNP, 1.9:1 in PPWS, and 1.7:1 in SWS (Table 4; Fig. S2).
The Bayesian p values suggested that models including elevation as a spatial covariate and sex as a covariate fit our data better than the null model (Fig. S4). The models indicated adequate fit with p values ranging from 0.30 to 0.50 (Fig. S4). The SCR spatial covariate model tested the effect of elevation on leopard cat density in each study site and showed that elevation did not have a significant effect on density in any site because all the credible intervals overlapped zero (Table S1).
Occupancy modeling
Domestic dogs were detected in sufficient numbers for analysis in all sites (Table 3). However, leopards were only recorded in sufficient numbers for analysis in SWS, whereas dholes were only recorded in sufficient numbers for analysis in CCNP (Table 3).
The estimated occupancy probability of dholes was 0.58 ± 0.11 (mean ± SD) in CCNP (Table 5; Fig. S5). The occupancy of leopard cats was higher for the sites where dholes were present (0.74 ± 0.13) compared to the sites where dholes were absent (0.16 ± 0.12; Table 5), and SIF for the two species was 1.50. The detection probability of both species was relatively low (< 0.1; Table 5; Fig. S5).
The estimated occupancy probability of leopards was 0.46 ± 0.14 in SWS (Table 5; Fig. S6). The occupancy of leopard cats was similar for the sites where leopards were present (0.84 ± 11) compared to the sites where leopards were absent (0.74 ± 0.15; Table 5), and the SIF for the two species was 1.06. The detection probabilities of both species were similar (Table 5; Fig. S6).
The estimated occupancy probability of domestic dogs was 0.17 ± 0.05 in CCNP, 0.70 ± 0.18 in PPWS, and 0.79 ± 0.06 in SWS (Figs. S7, S8, and S9). In CCNP, the occupancy of leopard cats was about twice as high for the sites where dogs were present (0.78 ± 0.14) compared to the sites where dogs were absent (0.41 ± 0.09; Table 5), and the SIF was 1.67. In PPWS, the probability of occupancy of leopard cats was similar for the sites where dogs were present (0.65 ± 0.16) compared to the sites where dogs were absent (0.63 ± 0.24; Table 5), and the SIF was 0.98. Similarly, in SWS the probability of occupancy of leopard cats was similar for the sites where dogs were present (0.83 ± 0.09) compared to the sites where leopards were absent (0.72 ± 0.16; Table 5), and SIF was 1.03. In CCNP, the detection probabilities of domestic dogs and leopard cats were similar (Table 5; Fig. S7). In PPWS, the detection probability of leopard cats was about twice as high as domestic dogs (Table 5; Fig. S8), whereas in SWS the detection probability of domestic dogs was about twice as high as leopard cats (Table 5; Fig. S9).
Discussion
The leopard cat density was highest in CCNP and lowest in SWS, which supported our prediction that evergreen forests support higher densities of leopard cats compared to DDF. However, our results should be viewed with caution because the 95% credible intervals of the densities overlapped among all three sites. Nonetheless, our results were similar to Peterson et al. (2019), who found that leopard cat density in northeastern Thailand was higher in the evergreen and semi-evergreen forest compared to DDF, likely because the latter is a suboptimal habitat for this species. Similarly, Rabinowitz (1990) found that leopard cats used DDF less often than other habitats in western Thailand, owing to lower numbers of their preferred prey. Our results also suggest DDF is a suboptimal habitat for leopard cats, and we speculate that this was because of the harsh conditions within the DDF during the dry season, and the effects this has on the prey availability. Frequent annual dry season fires, both natural and human-caused (e.g., to enhance regrowth in the rainy season), occur in DDF after the dipterocarp trees loss their leaves, burning most of the grassy understory (McShea et al. 2011). The DDF is well adapted to dry season fires, which seem to have occurred in this habitat since the late Pleistocene (McShea et al. 2011), in contrast to evergreen forests which typically do not experience dry season fires. Previous research showed that evergreen forests and nearby DDF forests in Southeast Asia have similar biomass of small rodents during the rainy season, but after the dry season fires the biomass of small rodents becomes 5 times higher in evergreen forests compared to DDF (Walker and Rabinowitz 1992). Overall, the biomass of small rodents decreases about 76% in DDF forests from the rainy season until after the dry season fires (Walker and Rabinowitz 1992). Because leopard cats feed mostly on small rodents < 500 g (Kamler et al. 2020a; Rostro-García et al. 2021), the higher prey availability throughout the year in evergreen forests likely supports higher densities of this small felid compared to DDF.
Although leopard cats can attain unusually high densities in human-modified habitats, owing to superabundant small rodent numbers (Chua et al. 2016), their densities in natural habitats typically range from 2 to 18 individuals/100 km2 (Table 1). The only previous study to report a density > 18 individuals/km2 was Petersen et al. (2019), who found a density of 21.2 individuals/100 km2 in semi-evergreen forests in northeastern Thailand. Therefore, our study found two of the highest densities of leopard cats ever reported in natural habitat. When compared to previous studies, continuous evergreen or large patches of evergreen forests appear to be an optimal natural habitat for leopard cats in South and Southeast Asia (Table 1), probably due to relatively high numbers of small rodents in these forests. Although we found their density in DDF mosaic to be half of that found in a continuous evergreen forest, the density in DDF mosaic was still moderate compared to that reported in previous studies across various habitats (Table 1). We conclude that DDF appears to be a suboptimal habitat for leopard cats compared to evergreen forests; however, DDF can still sustain a modest population of this species, which is similar to that reported by Rostro-García et al. (2021).
The elevation did not significantly affect the density of leopard cats in any of the study sites, probably because there was not much variation in elevation across the study sites. Leopard cat densities might be affected by other factors not included in our study, such as local small rodent abundance, microhabitats, and possibly anthropogenic disturbances, including roads. We recommend that future studies investigate other factors that might influence densities of leopard cats in natural habitat, to gain a more complete understanding of the factors that influence their densities.
In SWS and PPWS, the capture probability of male leopard cats was lower than that of females, which likely corresponds to differences in movement patterns between the sexes. Males moved twice as far as females from their activity center, which likely resulted in low capture probability of males compared to females, especially if camera spacing was far relative to leopard cat home range sizes. Larger home ranges and greater distanced traveled by males compared to females are common in solitary felid species (Kamler and Gipson 2000; Goodrich et al. 2010; Simcharoen et al. 2014; Sarkar et al. 2016), and result from males establishing home ranges to encompass several female home ranges, whereas females establish their home ranges based on food resources and cover for their young (Sandell 1989). However, in CCNP males appeared to have higher capture probability, and had shorter distance movements compared to females. This result might have been because of the higher density and similar sex ratio in CCNP; thus, males might have had smaller home ranges because they did not need to travel as far to encompass several female home ranges.
The two-species occupancy analyses suggested that domestic dogs did not have a negative impact on leopard cat presence on any of the sites, which did not support our prediction. Nonetheless, leopard cats likely avoided domestic dogs temporally, because the former are almost strictly nocturnal (Lynam et al. 2013; Gray et al. 2014; Kamler et al. 2020a; Rostro-García et al. 2021) whereas domestic dogs are mostly diurnal in accordance with human activity (Kamler et al. 2012; Bianchi et al. 2020). We observed that domestic dogs were brought into all three sites by local people for the purposes of illegally hunting wildlife, including red muntjac (Muntiacus vaginalis), wild pig (Sus scrofa), and reptiles. Thus, domestic dogs likely negatively impact numerous other species inside the protected areas, especially in SWS where dogs were detected at high frequencies. Domestic dogs pose a threat to nearly 200 globally threatened species worldwide, and they have contributed to the extinctions of 11 vertebrates via depredations, disease transmission, competition, and hybridization (Doherty et al. 2017). Given the high rates of dog detections on our study sites, we recommend further research on domestic dogs and their impacts on wildlife within protected areas in Cambodia (Hughes and Macdonald 2013; Hughes et al. 2017). We also recommend that the management of all three protected areas makes a greater effort to enforce the laws and prevent domestic dogs and humans from illegally entering the core zones because such efforts are likely to have a positive impact on the overall biodiversity in the protected areas.
The occupancy probability of leopard cats was similar when leopards were present or absent in SWS, which did not support our prediction. This suggests that leopard cats were able to coexist with leopards in SWS. Although a previous study in SWS found that leopards consumed leopard cats, the latter only accounted for < 1% of the biomass consumed by leopards, and overall the leopard density was extremely low (1 leopard/100 km2; Rostro-García et al. 2018). This suggests that leopards rarely prey on leopard cats, at least not enough for this small felid to spatially avoid leopards, and that the low leopard density may have affected their interaction with leopard cats. Regardless, leopard and leopard cats have vastly different diets (Rostro-García et al. 2018, 2021; Kamler et al. 2020a); thus, they do not compete for the same food resources. Both felids were nocturnal in SWS (Rostro-García et al. 2018); thus; leopard cats did not appear to avoid leopards temporally. We conclude that due to large differences in body size and diets, leopard cats do not spatially or temporally avoid leopards, despite the occasional predation on this small felid by leopards, at least in areas were leopard densities are low such as SWS.
In CCNP, the occupancy probability of leopard cats was similar when dholes were present or absent, which did not support our prediction. This indicates that leopard cats were not spatially avoiding dholes, despite that dholes sometimes prey upon this small felid (Kamler et al. 2020b). Similar to the leopard, the diet of dholes contained < 1% biomass consumed of leopard cats, indicating they are rarely preyed upon by dholes. Also, dholes and leopard cats have vastly different diets (Kamler et al. 2020a, b), indicating that they do not compete for the same food resources. However, dholes are diurnal in Southeast Asia (Kamler et al. 2012), so leopard cats might avoid dholes temporally. We conclude that due to large differences in body size and diets, leopard cats do not spatially avoid dholes, despite the occasional predation on this small felid by dholes, although leopard cats might avoid dholes temporally.
Overall, the adaptability of leopard cats to various forest types, and lack of strong negative impacts caused by large carnivores, helps to explain why this species is the most common and widespread felid in Southeast Asia. Nonetheless, our camera-trapping surveys focused on larger felid species had different spacing between camera stations, and occurred in different habitats, all of which could have affected our results. However, we feel that potential differences in leopard cat detectability due to different methodologies were minimal given the relatively large number of detections at each site. Also, home ranges of leopard cats in Thailand averaged 12 km2 for 14 males and 14 km2 for 6 females (Grassman et al. 2005); thus, even the relatively large camera spacing of about 2.5 km in SWS theoretically would have missed few, if any, leopard cats. Another caveat is that our investigation was limited to broad scale analysis of habitat and occupancy. Thus, leopard cats might have avoided large carnivores using mechanisms at finer scales. For example, time-to-encounter analysis might have detected leopard cat avoidance of large carnivores. Additionally, leopard cats might have avoided large carnivores at the level of the home range, feeding site, or resting site (Rostro-Garía et al. 2015). Furthermore, in our study sites the small- and medium-sized felids and canids, such as clouded leopards, Asian golden cats (Catopuma temminckii), jungle cats (Felis chaus), marbled cats (Pardofelis marmorata), and golden jackals (Canis aureus), were absent or occurred in such low numbers that they could not be included in the analyses. Leopard cats might compete more with small and meso-felids, which are known to feed more on small rodents compared to large carnivores (Kamler et al. 2020a). Nonetheless, previous studies showed that the occupancy of leopard cats was not affected by the presence of Asian golden cats (Kamler et al. 2020a) or jungle cats (Rostro-García et al. 2021), indicating leopard cats might not spatially avoid meso-felids. Furthermore, leopard cats were not found in the scats of Asian golden cats (Kamler et al. 2020a), jungle cats (Rostro-García et al. 2021), and jackals (Kamler et al. 2021), indicating this small felid might not be preyed upon frequently by these mesocarnivores. Regardless, we recommend that future studies examine in more detail if mesocarnivores affect the density or occupancy of leopard cats. Additionally, we recommend that future researches consider multi-scale analyses when investigating the interactions of leopard cats and both large and mesocarnivores. Only by studying leopard cats within different carnivore communities can we gain a more complete understanding of coexistence mechanisms, and the important factors that affect the density and occupancy of Southeast Asia’s smallest felid.
Data availability
Data are available upon request.
References
Baker PJ, Bunyavejchewin S (2009) Fire behavior and fire effects across the forest landscape of continental Southeast Asia. In: Cochrane MA (ed) Tropical fire ecology. Springer, Chichester, pp 311–334
Bashir T, Bhattacharya T, Poudyal K, Sathyakumar S, Qureshi Q (2013) Estimating leopard cat Prionailurus bengalensis densities using photographic captures and recaptures. Wildl Biol 19:462–472
Bianchi RdC, Olifiers N, Riski LL, Gouvea JA, Cesário CS, Fornitano L, Zanirato GL, Yan de Oliveira M, de Morais KD, Ribeiro RL, D’Andrea PS (2020) Dog activity in protected areas: behavioral effects on mesocarnivores and the impacts of a top predator. Eur J Wildl Res 66:36
Bischof R, Ali H, Kabir M, Hameed S, Nawaz MA (2014) Being the underdog: an elusive small carnivore uses space with prey and time without enemies. J Zool 293:40–48
Bivand R, Keitt T, Rowlingson B, Pebesma E, Sumner M, Hijmans R, Rouault E, Warmerdam F, Ooms J, Rundel C (2016) Rgdal: bindings for the geospatial data abstraction library. R package
Burnham D, Hinks AE, Macdonald DW (2012) Life and dinner under the shared umbrella: patterns in felid and primate communities. Folia Primatol 83:148–170
Chua MA, Sivasothi N, Meier R (2016) Population density, spatiotemporal use and diet of the leopard cat (Prionailurus bengalensis) in a human-modified succession forest landscape of Singapore. Mammal Res 61:99–108
Doherty TS, Dickman CR, Glen AS, Newsome TM, Nimmo DG, Ritchie EG, Vanak AT, Wirsing AJ (2017) The global impacts of domestic dogs on threatened vertebrates. Biol Cons 210:56–59
Efford M (2004) Density estimation in live-trapping studies. Oikos 106:598–610
Efford M (2020) secr: spatially explicit capture-recapture models. 4.3.1
Efford MG, Fewster RM (2013) Estimating population size by spatially explicit capture-recapture. Oikos 122:918–928
Francis C (2019) Field guide to the mammals of South-east Asia, 2nd edn. Bloomsbury Publishing, New York
Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB (2013) Bayesian data analysis. CRC Press, New York
Gompper ME (2021) Adding nuance to our understanding of dog–wildlife interactions and the need for management. Integr Comp Biol 61:93–102
Goodrich JM, Miquelle DG, Smirnov EN, Kerley LL, Quigley HB, Hornocker MG (2010) Spatial structure of Amur (Siberian) tigers (Panthera tigris altaica) on Sikhote-Alin Biosphere Zapovednik, Russia. J Mammal 91:737–748
Grassman LI (2000) Movements and diet of the leopard cat Prionailurus bengalensis in a seasonal evergreen forest in south-central Thailand. Acta Theriol 45:421–426
Grassman LI, Tewes ME, Silvy NJ, Kreetiyutanout K (2005) Spatial organization and diet of the leopard cat (Prionailurus bengalensis) in north-central Thailand. J Zool 266:45–54
Gray T, Channa P, Chanrattanak P, Sovanna P (2014) The status of jungle cat and sympatric small cats in Cambodia’s Eastern Plains. Cat News 8:19–23
Gray TN, Grainger MJ, Grosu R (2020) Conservation decision-making under uncertainty: identifying when to reintroduce tiger Panthera tigris to Cambodia. Conserv Sci Pract 2:e187
Heino M, Kummu M, Makkonen M, Mulligan M, Verburg PH, Jalava M, Räsänen TA (2015) Forest loss in protected areas and intact forest landscapes: a global analysis. PLoS ONE 10:e0138918
Hughes J, Macdonald DW (2013) A review of the interactions between free-roaming domestic dogs and wildlife. Biol Conserv 157:341–351
Hughes J, MacDonald DW, Boitani L (2017) Roaming free in the rural idyll: dogs and their connections with wildlife. In: Serpell J (ed) The domestic dog: its evolution, behaviour and interactions with people. Cambridge University Press, Cambridge, pp 369–384
Kamler JF, Gipson PS (2000) Home range, habitat selection, and survival of bobcats, Lynx rufus, in a prairie ecosystem in Kansas. Can Field-Naturalist 114:388–394
Kamler JF, Johnson A, Vongkhamheng C, Bousa A (2012) The diet, prey selection, and activity of dholes (Cuon alpinus) in northern Laos. J Mammal 93:627–633
Kamler JF, Inthapanya X, Rasphone A, Bousa A, Vongkhamheng C, Johnson A, Macdonald DW (2020a) Diet, prey selection, and activity of Asian golden cats and leopard cats in northern Laos. J Mammal 101:1267–1278
Kamler JF, Thatdokkham K, Rostro‐García S, Bousa A, Caragiulo A, Crouthers R, In V, Pay C, Pin C, Prum S, Vongkhamheng C (2020b) Diet and prey selection of dholes in evergreen and deciduous forests of Southeast Asia. J Wildl Manag 84:1396–1405
Kamler JF, Minge C, Rostro-García S, Gharajehdaghipour T, Crouthers R, In V, Pay C, Pin C, Sovanna P, Macdonald DW (2021) Home range, habitat selection, density, and diet of golden jackals in the Eastern Plains Landscape, Cambodia. J Mammal 102:636–650
Kellner K, Kellner MK, SystemRequirements J (2018) Package ‘jagsUI’ A wrapper around ‘rjags’ to streamline ‘JAGS’ analyses
Kim DH, Sexton JO, Townshend JR (2015) Accelerated deforestation in the humid tropics from the 1990s to the 2000s. Geophys Res Lett 42:3495–3501
Kitchener AC, Breitenmoser-Würsten C, Eizirik E, Gentry A, Werdelin L, Wilting A, Yamaguchi N, Abramov AV, Christiansen P, Driscoll C, Duckworth JW (2017) A revised taxonomy of the Felidae. The final report of the Cat Classification Task Force of the IUCN/SSC Cat Specialist Group. Cat News S11:1–80
Koh LP, Sodhi NS (2010) Conserving Southeast Asia’s imperiled biodiversity: scientific, management, and policy challenges. Biodivers Conserv 19:913–917
Kyaw PP, Macdonald DW, Penjor U, Htun S, Naing H, Burnham D, Kaszta Ż, Cushman SA (2021) Investigating carnivore guild structures: spatial and temporal relationships amongst threatened felids in Myanmar. ISPRS Int J Geo Inf 10:808
Lynam AJ, Jenks KE, Tantipisanuh N, Chutipong W, Ngoprasert D, Gale GA, Steinmetz R, Sukmasuang R, Bhumpakphan N, Grassman Jr LI, Cutter P (2013) Terrestrial activity patterns of wild cats from camera-trapping. Raffles Bull Zool 61:407–415
Macdonald DW, Loveridge AJ, Nowell K (2010) Dramatis personae: an introduction to the wild felids. In: Macdonad DW, Loveridge AJ (eds) Biology and conservation of wild felids. Oxford University Press, Oxford, pp 3–58
Macdonald DW, Burnham D, Hinks AE, Wrangham R (2012) A problem shared is a problem reduced: seeking efficiency in the conservation of felids and primates. Folia Primatol 83:171–215
MacKenzie DI, Bailey LL, Nichols JD (2004) Investigating species co-occurrence patterns when species are detected imperfectly. J Anim Ecol 73:546–555
McShea WJ, Davies SJ, Bhumpakphan N (2011) The ecology and conservation of seasonally dry forests in Asia. Smithsonian Institution Scholarly Press, Washington, D.C.
Meredith M (2020a) Bayes with JAGS – a tutorial for wildlife researchers. https://bcss.org.my/tut/bayes-with-jags-a-tutorial-for-wildlife-researchers/occupancy-modelling/two-species-occupancy-models/. Accessed Sept 2020
Meredith M (2020b) makeJAGSmask. Habitat matrices for use with SECR analysis in JAGS or BUGS. https://mmeredith.net/R/. Accessed Sept 2020
Meredith M (2020c) wiqid: quick and dirty estimates for wildlife populations. R package, version 0.3.0. https://CRAN.R-project.org/package=wiqid . Accessed Sept 2020
Miettinen J, Shi C, Liew SC (2011) Deforestation rates in insular Southeast Asia between 2000 and 2010. Glob Change Biol 17:2261–2270
Mohamed A, Sollmann R, Bernard H, Ambu LN, Lagan P, Mannan S, Hofer H, Wilting A (2013) Density and habitat use of the leopard cat (Prionailurus bengalensis) in three commercial forest reserves in Sabah, Malaysian Borneo. J Mammal 94:82–89
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853
Penjor U, Macdonald DW, Wangchuk S, Tandin T, Tan CKW (2018) Identifying important conservation areas for the clouded leopard Neofelis nebulosa in a mountainous landscape: inference from spatial modeling techniques. Ecol Evol 8:4278–4291
Penjor U, Tan CKW, Wangdi S, Macdonald DW (2019) Understanding the environmental and anthropogenic correlates of tiger presence in a montane conservation landscape. Biol Conserv 238:108196
Petersen WJ, Savini T, Steinmetz R, Ngoprasert D (2019) Estimating leopard cat Prionailurus bengalensis Kerr, 1792 (Carnivora: Felidae) density in a degraded tropical forest fragment in northeastern Thailand. J Threat Taxa 11:13448–13458
Pin C, Ngoprasert D, Gray TN, Savini T, Crouthers R, Gale GA (2018) Utilization of waterholes by globally threatened species in deciduous dipterocarp forest of the Eastern Plains Landscape of Cambodia. Oryx 54:572–582
Plummer M (2003) JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. DSC 2003 Working Papers, Vienna, Austria
QGIS Delopment Team (2020) QGIS geographic information system. Open Source Geospatial Foundation Project
R Core Team (2020) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed Sept 2020
Rabinowitz A (1990) Notes on the behavior and movements of leopard cats, Felis bengalensis, in a dry tropical forest mosaic in Thailand. Biotropica:397–403
Rajaratnam R, Sunquist M, Rajaratnam L, Ambu L (2007) Diet and habitat selection of the leopard cat (Prionailurus bengalensis borneoensis) in an agricultural landscape in Sabah, Malaysian Borneo. J Trop Ecol 23:209–217
Rasphone A, Kamler JF, Tobler M, Macdonald DW (2021) Density trends of wild felids in northern Laos. Biodivers Conserv 30:1881–1897
Ross J, Brodie J, Cheyne S, Hearn A, Izawa M, Loken B, Lynam A, McCarthy J, Mukherjee S, Phan C, Rasphone A, Wilting A (2015) Prionailurus bengalensis. The IUCN Red List of Threatened Species 2015: e.T18146A50661611. https://doi.org/10.2305/IUCN.UK.2015-4.RLTS.T18146A50661611.en. Downloaded on 12 June 2020
Rostro-García S, Kamler JF, Hunter LTB (2015) To kill, stay or flee: the effects of lions and landscape factors on habitat and kill site selection of cheetahs in South Africa. PLoS ONE 10:e0117743
Rostro-García S et al (2018) An adaptable but threatened big cat: density, diet and prey selection of the Indochinese leopard (Panthera pardus delacouri) in eastern Cambodia. R Soc Open Sci 5:171187
Rostro‐García S, Kamler JF, Minge C, Caragiulo A, Crouthers R, Groenenberg M, Gray TN, In V, Pin C, Sovanna P, Kéry M (2021) Small cats in big trouble? Diet, activity, habitat use, and occupancy of jungle cats and leopard cats in threatened dry deciduous forests, Cambodia. Ecol Evol 11:4205–4217
Royle JA, Chandler RB, Sollmann R, Gardner B (2014) Spatial capture-recapture. Academic Press, Boston
Sandell M (1989) The mating tactics and spacing patterns of solitary carnivores. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution, vol 1. Cornell University Press, Ithaca, pp 164–182
Sarkar MS, Ramesh K, Johnson JA, Sen S, Nigam P, Gupta SK, Murthy RS, Saha GK (2016) Movement and home range characteristics of reintroduced tiger (Panthera tigris) population in Panna Tiger Reserve, central India. Eur J Wildl Res 62:537–547
Simcharoen A, Savini T, Gale GA, Simcharoen S, Duangchantrasiri S, Pakpien S, Smith JL (2014) Female tiger Panthera tigris home range size and prey abundance: important metrics for management. Oryx 48:370–377
Sodhi NS, Brook BW (2006) Southeast Asian biodiversity in crisis. Cambridge University Press, Cambridge
Sodhi NS, Koh LP, Brook BW, Ng PK (2004) Southeast Asian biodiversity: an impending disaster. Trends Ecol Evol 19:654–660
Sodhi NS, Posa MRC, Lee TM, Bickford D, Koh LP, Brook BW (2010) The state and conservation of Southeast Asian biodiversity. Biodivers Conserv 19:317–328
Sollmann R, Furtado MM, Gardner B, Hofer H, Jácomo AT, Tôrres NM, Silveira L (2011) Improving density estimates for elusive carnivores: accounting for sex-specific detection and movements using spatial capture–recapture models for jaguars in central Brazil. Biol Conserv 144:1017–1024
Srivathsa A, Parameshwaran R, Sharma S, Karanth KU (2015) Estimating population sizes of leopard cats in the Western Ghats using camera surveys. J Mammal 96:742–750
Sunarto S, Kelly MJ, Parakkasi K, Hutajulu MB (2015) Cat coexistence in central Sumatra: ecological characteristics, spatial and temporal overlap, and implications for management. J Zool 296:104–115
Sunquist M, Sunquist F (2017) Wild cats of the world. University of Chicago Press, Chicago
Vitekere K, Wang J, Karanja H, Consolée KT, Jiang G, Hua Y (2020) Dynamic in species estimates of carnivores (leopard cat, red fox, and north Chinese leopard): a multi-year assessment of occupancy and coexistence in the Tieqiaoshan Nature Reserve, Shanxi Province, China. Animals 10:1333
Waddle JH, Dorazio RM, Walls SC, Rice KG, Beauchamp J, Schuman MJ, Mazzotti FJ (2010) A new parameterization for estimating co-occurrence of interacting species. Ecol Appl 20:1467–1475
Walker S, Rabinowitz A (1992) The small-mammal community of a dry-tropical forest in central Thailand. J Trop Ecol:57–71
Wearn OR, Rowcliffe JM, Carbone C, Bernard H, Ewers RM (2013) Assessing the status of wild felids in a highly-disturbed commercial forest reserve in Borneo and the implications for camera trap survey design. PLoS One 8:e77598
Webb EL, Choo YR, Kudavidanage EP, Amarasinghe TR, Bandara UG, Wanninayaka WA, Ravindrakumar P, Nimalrathna TS, Liang SH, Chua MA (2020) Leopard activity patterns in a small montane protected area highlight the need for integrated, collaborative landscape conservation. Glob Ecol Conserv 23:e01182
Wohlfart C, Wegmann M, Leimgruber P (2014) Mapping threatened dry deciduous dipterocarp forest in South-east Asia for conservation management. Trop Conserv Sci 7:597–613
Xiong M, Shao X, Long Y, Bu H, Zhang D, Wang D, Li S, Wang R, Yao M (2016) Molecular analysis of vertebrates and plants in scats of leopard cats (Prionailurus bengalensis) in southwest China. J Mammal 97:1054–1064
Acknowledgements
These research projects were conducted under the permission granted by the General Department of Administration for Nature Conservation and Protection, Ministry of Environment (MoE), and Forestry Administration, Ministry of Agriculture, Forestry and Fishery (MAFF). We thank the field teams who helped collect the data, including MoE rangers, WWF Cambodia staff, and local porters. We also thank WWF Cambodia for coordination, logistical, and fieldwork support in SWS and PPWS, and Mike Meredith for providing statistical advice.
Funding
The camera trap studies in PPWS and CCNP were funded by the Robertson Foundation through Wildlife Conservation Research Unit (WildCRU), Department of Zoology, University of Oxford, with support provided by WWF Cambodia, FFI Cambodia, and the Ministry of Environment. The camera trap studies in SWS were funded by Panthera with support provided by WWF Cambodia and the Forestry Administration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Our research was carried out following the legal standards of Cambodia’s Natural Protected Area law, the Ministry of Environment, and Forestry Law, Ministry of Agriculture, Forestry and Fishery, and the guidelines provided by the University of Oxford, Biomedical Sciences, Animal Welfare and Ethical Review Body (AWERB).
Consent for publication
The manuscript has been approved by all co-authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Krzysztof Schmidt
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pin, C., Phan, C., Kamler, J.F. et al. Density and occupancy of leopard cats across different forest types in Cambodia. Mamm Res 67, 287–298 (2022). https://doi.org/10.1007/s13364-022-00634-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-022-00634-6