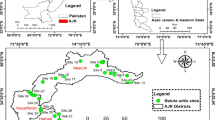
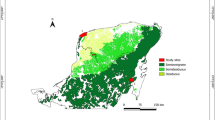
Abstract
A classic question in community ecology is how species coexist within a community. Studies have sought to understand how species occurrence vary according to habitat structure, space, food, predators, and competitors. Small mammals are widely used as a model system in community ecology, since they represent the most diverse group of mammals in the neotropical forests. Hence, we investigated whether microhabitat features, food resource (fruits), and presence of medium and large mammals can explain fine-spatial scale richness, abundances, and habitat use of small mammals in a forest in Brazil. Three species represented 83% of all captured individuals (Didelphis albiventris, Oligoryzomys nigripes, Akodon montensis). Species richness, abundance, and habitat use of small mammals were affected positively by the distance of bamboo (Chusquea sp.) thickets. The occurrence of predators (carnivores and omnivores) and potential competitors (large herbivores), however, did not affect richness, abundance, and habitat use of small mammals at small spatial scales. Our findings suggest that the bamboo patches can influence spatial distribution and shape small mammal communities in tropical forests.


Similar content being viewed by others
References
Akkawi P, Galetti M, Mendes CP, Villar N (2020) Dominance hierarchy on palm resource partitioning among Neotropical frugivorous mammals. J Mammal 101:697–709. https://doi.org/10.1093/jmammal/gyaa052
Amorim FW et al (2020) Good heavens what animal can pollinate it? A fungus-like holoparasitic plant potentially pollinated by opossums. Ecology 101:e03001. https://doi.org/10.1002/ecy.3001
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer. https://doi.org/10.1007/978-0-387-74075-1
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s Information Criterion. J Wildl Manag 74:1175–1178
Banks-Leite C et al (2014) Using ecological thresholds to evaluate the costs and benefits of set-asides in a biodiversity hotspot. Science 345:1041–1045. https://doi.org/10.1126/science.1255768
Barros CS, Püttker T, Pinotti BT, Pardini R (2015) Determinants of capture-recapture success: an evaluation of trapping methods to estimate population and community parameters for Atlantic forest small mammals. Zoologia (Curitiba) 32:334–344. https://doi.org/10.1590/s1984-46702015000500002
Barton K (2019) MuMIn: multi-model inference. R package version 1.43.6. https://CRAN.R-project.org/package=MuMIn. Accessed 10 Feb 2021
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-7. Retrived from http://CRAN.R-project.org/package=lme4. Accessed 10 Feb 2021
Ben-Moshe A, Dayan T, Simberloff D (2001) Convergence in morphological patterns and community organization between Old and New World rodent guilds. Am Nat 158:484–495
Bergallo HG, Magnusson WE (1999) Effects of climate and food availability on four rodent species in southeastern Brazil. J Mammal 80:472–486. https://doi.org/10.2307/1383294
Bergallo HdG, Magnusson WE (2004) Factors affecting the use of space by two rodent species in Brazilian Atlantic forest 68
Bovendorp RS (2013) História natural e ecologia de duas espécies de roedores simpátricas da tribo Oryzomyini (Cricetidae: Sigmodontinae) na Floresta Atlântica. Escola Superior de Agricultura “Luiz de Queiroz”. Universidade de São Paulo
Bovendorp RS, McCleery RA, Galetti M (2017a) Optimising sampling methods for small mammal communities in Neotropical rainforests. Mammal Rev 47:148–158. https://doi.org/10.1111/mam.12088
Bovendorp RS, Villar N, de Abreu-Junior EF, Bello C, Regolin AL, Percequillo AR, Galetti M (2017b) Atlantic small-mammal: a dataset of communities of rodents and marsupials of the Atlantic forests of South America. Ecology 98:2226. https://doi.org/10.1002/ecy.1893
Bovendorp RS, Brum FT, McCleery RA, Baiser B, Loyola R, Cianciaruso MV, Galetti M (2018) Defaunation and fragmentation erode small mammal diversity dimensions in tropical forests. Ecography 42:23–35. https://doi.org/10.1111/ecog.03504
Bovendorp RS, Heming NM, Percequillo AR (2020) Bottom-up effect: a rodent outbreak following the bamboo blooming in a Neotropical rainforest. Mamm Res. https://doi.org/10.1007/s13364-020-00505-y
Brooks M et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.3929/ethz-b-000240890
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. A practical information - theoretical approach. Spinger, New York
Cabral JC, Granzinolli MAM, Motta-Junior JC (2006) Dieta do quiriquiri, Falco sparverius (Aves: Falconiformes), na Estação Ecológica de Itirapina, SP. Rev Bras Ornitol 14:393–399
Campanello PI, Gatti MG, Ares A, Montti L, Goldstein G (2007) Tree regeneration and microclimate in a liana and bamboo-dominated semideciduous Atlantic Forest. For Ecol Manag 252:108–117
Carvalho FMV, Pinheiro PS, Fernandez FAS, Nessimian JL (1999) Diet of small mammals in Atlantic forest fragments in southeastern Brazil. Rev Bras Zoociencias 1:91–101
Chesson P, Kuang JJ (2008) The interaction between predation and competition. Nature 456:235–238
Dalmagro A, Vieira E (2005) Patterns of habitat utilization of small rodents in an area of Araucaria forest in Southern Brazil. Austral Ecol 30:353–362
Delciellos AC, Barros CS, Prevedello JA, Ferreira MS, Cerqueira R, Vieira MV (2018) Habitat fragmentation affects individual condition: evidence from small mammals of the Brazilian Atlantic Forest. J Mammal 99:936–945. https://doi.org/10.1093/jmammal/gyy078
DeMattia EA, Curran LM, Rathcke BJ (2004) Effects of small rodents and large mammals on Neotropical seeds. Ecology 85:2161–2170
Dirzo R, Mendoza E, Ortiz P (2007) Size-related differential seed predation in a heavily defaunated neotropical rain forest. Biotropica 39:355–362
Dueser RD, Hallett JG (1980) Competition and habitat selection in a forest-floor small mammal fauna vol 35. Oikos
Durigan G, Franco GADC, Saito M, Baitello JB (2000) Estrutura e diversidade do componente arbóreo da floresta na Estação Ecológica dos Caetetus, Gália, SP. Rev Bras Bot 23(371):383
Facure KG, Giaretta AA (1996) Food habits of carnivores in a coastal Atlantic forest of Brazil. Mammalia 60:499–502
Fonseca G, Kierulff M (1989) Biology and natural history of Brazilian Atlantic forest small mammals Bulletin of the Florida State Museum. Biological Sciences 34:99–152
Fonseca G, Robinson J (1990) Forest size and stucture: competitive and predator effects on small mammal communities. Biol Conserv 53:265–294
Galetti M, Bovendorp RS, Guevara R (2015a) Defaunation of large mammals leads to an increase in seed predation in the Atlantic forests. Glob Ecol Conserv 3:824–830. https://doi.org/10.1016/j.gecco.2015.04.008
Galetti M et al (2015b) Defaunation affects the populations and diets of rodents in Neotropical rainforests. Biol Conserv 190:2–7. https://doi.org/10.1016/j.biocon.2015.04.032
Galetti M, Rodarte RR, Neves CL, Moreira M, Costa-Pereira R (2016) Trophic niche differentiation in rodents and marsupials revealed by stable isotopes. PLoS One 11:e0152494. https://doi.org/10.1371/journal.pone.0152494
Galetti M et al (2017) Defaunation and biomass collapse of mammals in the largest Atlantic forest remnant. Anim Conserv 20:270–281. https://doi.org/10.1111/acv.12311
Galetti M et al (2021) Causes and consequences of large-scale defaunation in the Atlantic forest. In: The Atlantic forest, pp 297–324. https://doi.org/10.1007/978-3-030-55322-7_14
Graipel ME, Cherem JJ, Miller PRM, Glock L (2003) Trapping small mammals in the forest understory: a comparison of three methods. Mammalia 67:551–558
Grelle CEV (2003) Forest structure and vertical stratification of small mammals in a secondary Atlantic forest, southeastern Brazil. Stud Neotropical Fauna Environ 38:81–85
Henderson RW, Dixon JR, Soini P (1987) On the seasonal incidence of tropical snakes. Milwaukee Public Mus Contrib Biol Geol 17:1–15
HilleRisLambers J, Adler PB, Harpole W, Levine JM, Mayfield MM (2012) Rethinking community assembly through the lens of coexistence theory. Annu Rev Ecol Evol Syst 43:227–248
Honorato R, Crouzeilles R, Ferreira MS, Grelle CEV (2015) The effects of habitat availability and quality on small mammals abundance in the Brazilian Atlantic Forest. Natureza & Conservação 13:133–138. https://doi.org/10.1016/j.ncon.2015.11.010
Hutchinson GE (1957) Population studies - animal ecology and demography - concluding remarks. Cold Spring Harb Symp Quant Biol 22:415–427
Hutchinson GE, MacArthur RH (1959) A theoretical ecological model of size distributions among species of animals. Am Nat 93:117–125
Iob G, Vieira EM (2008) Seed predation of Araucaria angustifolia (Araucariaceae) in the Brazilian Araucaria Forest: influence of deposition site and comparative role of small and ‘large’ mammals. Plant Ecol 198:185–196. https://doi.org/10.1007/s11258-007-9394-6
Leite Y, Costa L, Stallings J (1996) Diet and vertical space use of three sympatric opossums in a Brazilian Atlantic forest reserve. J Trop Ecol 12:435–440
Lima DO, Azambuja BO, Camilotti VL, Caceres NC (2010) Small mammal community structure and microhabitat use in the austral boundary of the Atlantic forest. Brazil Zool 27:99–105
Lima RAF, Rother DC, Muler AE, Lepsch IF, Rodrigues RR (2012) Bamboo overabundance alters forest structure and dynamics in the Atlantic Forest hotspot. Biol Conserv 147:32–39. https://doi.org/10.1016/j.biocon.2012.01.015
Lopez L, Terborgh J (2007) Seed predation and seedling herbivory as factors in tree recruitment failure on predator-free forested islands. J Trop Ecol 23:129. https://doi.org/10.1017/s0266467406003828
Lüdecke D, Makowski D, Waggoner P, Patil I (2020) Package ‘performance’.
Macarthur R, Levins R (1964) Competition, habitat selection, and character displacement in a patchy environment. Proc Natl Acad Sci U S A 51:1207–1210. https://doi.org/10.1073/pnas.51.6.1207
Magioli M, Ferraz KMPM (2021) Deforestation leads to prey shrinkage for an apex predator in a biodiversity hotspot. Mamm Res 66:245–255. https://doi.org/10.1007/s13364-021-00556-9
Melo GL, Sponchiado J, Machado AF, Caceres NC (2011) Small-mammal community structure in a South American deciduous Atlantic forest. Community Ecol 12:58–66. https://doi.org/10.1556/ComEc.12.2011.1.8
Melo GL, Miotto B, Peres B, Cáceres NC (2013) Microhabitat of small mammals at ground and understorey levels in a deciduous, southern Atlantic Forest. An Acad Bras Cienc 85:727–736
Moura MC, Vieira MV, Cerqueira R (2009) Occasional intraguild predation structuring small mammal assemblages: the marsupial Didelphis aurita in the Atlantic Forest of Brazil. Austral Ecol 34:481–489. https://doi.org/10.1111/j.1442-9993.2009.01948.x
Naxara L, Pinotti BT, Pardini R (2009) Seasonal microhabitat selection by terrestrial rodents in an old-growth Atlantic Forest. J Mammal 90:404–415. https://doi.org/10.1644/08-mamm-a-100.1
Oksanen J et al (2016) Vegan: community ecology package. R package version 2.3-5. https://CRAN.R-project.org/package=vegan. Accessed 27 Jan 2021
Olifiers N, Gentile R, Fiszon JT (2005) Relation between small-mammal species composition and anthropic variables in the Brazilian Atlantic forest. Braz J Biol 65:495–501
Oliveira FFR, Nessim R, Costa LP, Leite YLR (2007) Small mammal ecology in an urban Atlantic forest fragment in southeastern Brazil. Lundiana 8:27–34. https://doi.org/10.35699/2675-5327.2007.23171
Paglia AP et al (2012) Lista anotada dos mamiferos do Brasil / Annotated checklist of Brazilian mammals. Occas Pap Conserv Int 6:1–76
Pardini R, de Souza SM, Braga-Neto R, Metzger JP (2005) The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol Conserv 124:253–266. https://doi.org/10.1016/j.biocon.2005.01.033
Patton JL, Pardiñas UFJ, D’Elía G (2015) Mammals of South America. Volume 2: rodents, vol 2. University of Chicago Press, Chicago
Pedó E, Freitas TRO, Hartz SM (2010) The influence of fire and livestock grazing on the assemblage of non-flying small mammals in grassland-Araucaria forest ecotones, southern Brazil. Zoologia (Curitiba Impr) 27:533–540
Pinotti BT, Naxara L, Pardini R (2011) Diet and food selection by small mammals in an old-growth Atlantic forest of south-eastern Brazil. Stud Neotropical Fauna Environ 46:1–9
Pinotti BT, Pagotto CP, Pardini R (2012) Habitat structure and food resources for wildlife across successional stages in a tropical forest. For Ecol Manag 283:119–127. https://doi.org/10.1016/j.foreco.2012.07.020
Pinotti BT, Pagotto CP, Pardini R (2015) Wildlife recovery during tropical forest succession: assessing ecological drivers of community change. Biotropica 47:765–774
Puttker T, Meyer-Lucht Y, Sommer S (2006) Movement distances of five rodent and two marsupial species in forest fragments of the coastal Atlantic rainforest, Brazil. Ecotropica 12:131–139
Püttker T, Pardini R, Meyer-Lucht Y, Sommer S (2008) Responses of five small mammal species to micro-scale variations in vegetation structure in secondary Atlantic Forest remnants. Brazil BMC Ecol 8:9
Püttker T, Bueno AA, dos Santos de Barros C, Sommer S, Pardini R (2013) Habitat specialization interacts with habitat amount to determine dispersal success of rodents in fragmented landscapes. J Mammal 94:714–726. https://doi.org/10.1644/12-mamm-a-119.1
Püttker T, Barros CS, Pinotti BT, Bueno AA, Pardini R (2019) Co-occurrence patterns of rodents at multiple spatial scales: competitive release of generalists following habitat loss? J Mammal 100:1229–1242
R Core Team (2019) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrived from http://www.R-project.org/. Accessed 15 Dec 2021
Rother DC, Rodrigues RR, Pizo MA (2016) Bamboo thickets alter the demographic structure of Euterpe edulis population: a keystone, threatened palm species of the Atlantic forest. Acta Oecol 70:96–102
Santos F et al (2019) Prey availability and temporal partitioning modulate felid coexistence in Neotropical forests. PLoS One 14:e0213671
Sikes RS, Animal C, Use Committee of the American Society of M (2016) 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688. https://doi.org/10.1093/jmammal/gyw078
Silva RB (2005) Ecologia do rato-da-taquara (Kannabateomys amblyonyx) no Parque Estadual de Itapuã, São Leopoldo, RS. Universidade do Vale do Rio dos Sinos
Tabanez MF et al (2005) Plano de manejo da Estação Ecológica dos Caetetus Instituto Florestal. Série Registros 29:1–104
Terborgh J et al (2001) Ecological meltdown in predator-free forest fragments. Science 294:1923–1926. https://doi.org/10.1126/science.1064397
Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the Neotropics. J Mammal 87:757–765. https://doi.org/10.1644/05-mamm-a-285r2.1
Veloso HP, Filho ALRR, Lima JCA (1991) Classificação da vegetação brasileira, adaptada a sistema universal. vol 1. IBGE
Vieira MF, de Carvalho-Okano RM (1996) Pollination biology of Mabea fistulifera (Euphorbiaceae) in southeastern Brazil Biotropica:61-68
Vieira EM, De Moraes DA (2003) Carnivory and insectivory in Neotropical marsupials Predators with pouches: the biology of carnivorous marsupials:271-284
Vieira EM, Monteiro Filho ELA (2003) Vertical stratfication of small mammals in the Atlantic rain forest of south-eastern Brazil. J Trop Ecol 19:501–507
Vieira E, Pizo M, Izar P (2003) Fruit and seed exploitation by small rodents of the Brazilian Atlantic forest. Mammalia 67:533–539
Vieira EM, Paise G, Machado PHD (2006) Feeding of small rodents on seeds and fruits: a comparative analysis of three species of rodents of theAraucaria forest, southern Brazil. Acta Theriol 51:311–318. https://doi.org/10.1007/bf03192683
Vieira EM, Ribeiro JF, Iob G (2011) Seed predation of Araucaria angustifolia (Araucariaceae) by small rodents in two areas with contrasting seed densities in the Brazilian Araucaria forest. J Nat Hist 45:843–854. https://doi.org/10.1080/00222933.2010.536265
Acknowledgements
We thank the Fundação Florestal (COTEC Proc. SMA no. 002.169/2017) for allowing us to work at ESEC. We also thank Sérgio Nazareth, Sean Keuroghlian, and other colleagues for their assistance with the fieldwork. We are deeply in debt with two anonymous reviewers who made their suggestions in the earlier versions to improve the quality of this manuscript.
Funding
This project was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo, Programa Biota (FAPESP, Proc 2014/01986-0). CLA received a Master’s fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Financial code 001, Proc PPG – Unesp - RC: 2017), MG a Senior Fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and RSB a Postdoctoral Fellowship from FAPESP (Proc 2013/25441-0).
Author information
Authors and Affiliations
Contributions
CLA, MCC, MG, and RSB contributed to the study conception and design. Data collection was performed by CLA and RSCA. Data analysis was performed by MCC and NMH. The first draft of the manuscript was written by CLA, NMH, and RSB, and MCC and MG commented and contributed on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Thales Renato Ochotorena de Freitas
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Sample grid design for capturing small mammals and medium and large mammals in the Caetetus Ecological Station, Brazil. Large Sherman trap (23 × 7.5 × 8.5 cm) are represented by dark rectangle, small Sherman trap (23 × 7.5 × 8.5 cm) are represented by light gray rectangle, and Tomahawk (42.0 × 12 × 15 cm) are represented by open rectangle. The open circles connected by continuous line represent the pitfall traps connected by plastic fence. The cameras trap is represented by the dark blue triangle for the period to December 2017 to January 2018 and dark green from January to February 2018 in the grid
Rights and permissions
About this article
Cite this article
André, C.L., Côrtes, M.C., Heming, N.M. et al. Bamboo shapes the fine-scale richness, abundance, and habitat use of small mammals in a forest fragment. Mamm Res 67, 199–218 (2022). https://doi.org/10.1007/s13364-021-00616-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-021-00616-0