Abstract
A species’ potential distribution can be modelled adequately only if no factor other than habitat availability affects its occurrences. Space use by stone marten Martes foina is likely to be affected by interspecific competition with the strictly related pine marten Martes martes, the latter being able to outcompete the first species in forested habitats. Hence, to point out the environmental factors which determine the distribution and density of the stone marten, a relatively understudied mesocarnivore, we applied two non-invasive survey methods, camera-trapping and faecal-DNA based genetic analysis, in an Alpine area where the pine marten was deemed to be absent (Val Grande National Park N Italy). Camera trapping was conducted from October 2014 to November 2015, using up to 27 cameras. Marten scats were searched for between July and November 2015 and, to assess density, in spring 2017. Species identification was accomplished by a PCR-RFLP method, while 17 autosomal microsatellites were used for individual identification. The stone marten occurred in all available habitats (83% of trapping sites and 73.2% of scats); nonetheless, habitat suitability, as assessed using MaxEnt, depended on four major land cover variables—rocky grasslands, rocks and debris, beech forests and chestnut forests—, martens selecting forests and avoiding open rocky areas. Sixteen individuals were identified, of which 14 related to each other, possibly forming six different groups. Using capwire estimators, density was assessed as 0.95 (0.7–1.3) ind/km2. In the study area, the widespread stone marten selected forested areas, attaining density values like those reported for the pine marten in northern Europe and suggesting that patterns of habitat selection may depend on the relative abundance of the two competing martens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Affecting ecosystem function, structure and dynamics, mesocarnivores play important roles in natural communities and are considered sensitive indicators of environmental health and change in forested and aquatic habitats, particularly wherever large carnivores have been driven to extinction by human interference (Buskirk and Zielinski 2003; Roemer et al. 2009). Evaluating mesocarnivore distribution and abundance is thus essential for investigating trophic cascades and predator-prey density-dependent relationships and conservation-aimed management (Williams et al. 2002).
Nonetheless, with a few exceptions (red fox Vulpes vulpes, European badger Meles meles and Eurasian otter Lutra lutra), there is relatively little research on mesocarnivores (Brooke et al. 2014). Among the mustelid family, which largely contribute to the diversity of European mesocarnivores, the stone marten Martes foina has currently been understudied (Proulx et al. 2004), probably as an indirect consequence of its absence in the British Isles, which have long played a leader role in the investigation of mustelid ecology (e.g. Mcdonald 2002; O'Mahony et al. 2017; Mathews et al. 2018). Although the stone marten is widespread through much of continental Europe and central Asia—from Portugal in the west as far as north-western China in the east (Proulx et al. 2004)—, data on its spatial ecology are scarce (e.g. Abramov et al. 2006; Ruiz-Gonzalez et al. 2008), research having been focused on habitat selection (reviewed by Virgós et al. 2012). To the best of our knowledge, density data are reported by only three available studies, conducted in either rural (Switzerland 0.7–2 ind/km2, Lachat Feller 1993; Germany: 2 adult and 1.5 juvenile ind/km2, Herrmann 2004) or urban areas (4.7–5.8 ind/km2, Herr et al. 2009) by means of radiotracking. In northern Italy, stone marten density has been assessed in an agricultural area using camera-trapping and the Random Encounter Model proposed by Rowcliffe et al. (2008). Although population density was similar to that recorded in rural Switzerland (0.96 ind/km2; Ronchi 2016), the REM has been demonstrated to largely underestimate marten density (Balestrieri et al. 2016a).
Although being best adapted to warm climates (Proulx et al. 2004), the stone marten has been recorded from sea level up to 4200 m in Nepal (Oli 1994), while in Europe it occurs up to 2400 m a.s.l. on the Alps (Genovesi and De Marinis 2003). As other Martes species, the stone marten prefers forested habitats (Virgós et al. 2012); in southern Europe, it is often associated to mosaics of forest and field patches (Sacchi and Meriggi 1995; Werner 2012; Vergara et al. 2017); nonetheless, wherever available, forests are selected (Virgós and Casanovas 1998; Ruiz-Gonzalez et al. 2015; Zub et al. 2018).
Habitat use by the stone marten is considered to be driven by competition with the pine marten (Martes martes), which should be able to outcompete the stone marten in forested habitats (Delibes 1983). More recently, it has been suggested that the strictly nocturnal stone marten may be more tolerant of human disturbance than the cathemeral pine marten, and thus may manage better than the latter in rural habitats (Balestrieri et al. 2019). Nonetheless, in the Iberian Peninsula, southwards of the southern edge of pine marten distribution, the stone marten is a mainly forest-dwelling species, which selects low human density areas (Virgós and Casanovas 1998).
Currently, forests are more widespread in mountainous areas than in European lowlands: on the Alps, wood cover has progressively increased since the 1960s, following the abandonment of low-intensity farming and livestock rearing (Falcucci et al. 2007), with a positive effect on forest-dwelling species (MacDonald et al. 2000). Pine- and stone marten are sympatric throughout the Eastern Italian Alps, while, according to available data, the latter would be by far most widespread than the pine marten in the central part of the mountain range (Fonda 2019).
Aiming to assess stone marten distribution, habitat selection and density in a pine marten-free, forested habitat, we focused on the Val Grande National Park, a protected area in the Lepontine Alps, at the border between the western and central sector of the mountain range, where both available data for the whole Piedmont region (Sindaco and Carpegna 2010) and park rangers’ records indicated that pine marten occurrence may be null or negligible (only two records were available: one in 1900 and one in 2006, both outside the Park). We applied two non-invasive methods, camera-trapping and faecal-DNA-based genetic sampling. Genotyping needed two steps, species identification, which was necessary to exclude the samples belonging to other sympatric mesocarnivores from further analyses, and microsatellite genotyping, to ascertain the minimum number of individuals occurring in the study area (Ruiz-Gonzalez et al. 2008, 2013).
Study area
The Val Grande National Park (Piedmont region, Verbano-Cusio-Ossola province; 46° 01′ 45″ N 8° 27′ 34″ E) is the largest wilderness area of the Alps (153.7 km2). The abandonment of traditional land use practices since the end of World War II has led to the decline of cultivated lands (meadows, pastures, chestnut orchards, crops and vineyards) from 59% of the whole area at the end of the 19th century to 5% in 1999 (Höchtl et al. 2005). Most previously cultivated areas currently show various successional stages. Woods mainly consist of beech Fagus sylvatica and chestnut Castanea sativa and cover ca. 55% of the protected area. Mean yearly temperature and yearly rainfall are respectively 6.5 °C and 2300 mm with wide variations depending on altitude above sea level, which ranges between 250 and 2300 m.
Materials and methods
Several studies have suggested that the simultaneous use of multiple survey methods may provide a more complete assessment of mammal diversity (Silveira et al. 2003; Li et al. 2012; Croose et al. 2019). Mustelids are elusive and their densities being typically low, large areas need to be sampled to assess their distribution and habitat preferences. Moreover, Martes spp. are very similar and their field signs cannot be distinguished by eye (Davison et al. 2002), making their monitoring even more challenging. To overcome these hindrances, we applied two non-invasive methods, which, based on the characteristics of both the study area and target species, were deemed to offer the best balance between cost-effectiveness and monitoring efficiency (Roberts 2011; Balestrieri et al. 2016a, 2016b).
Camera-trapping
The study area was monitored by digital scouting camera-traps (Acorn II LTL 5210 with Passive Infra-Red motion sensor), tied to trees 30–50 cm above the ground level and set to record 30-s-long videoclips, with no interval between two successive recordings. Camera-trap sites were georeferenced and superimposed on digital maps. We used an active survey design, attracting animals into the detection zone of the camera trap by placing scent lures (cat food in carnivore-proof containers) in front of camera-traps (ca. 5 m away). The use of videoclips and lures aimed to improve the opportunity of observing the distinctive morphological traits (van Maanen 2013) of martens. In monochrome images, the three more conspicuous features are the shape and position of the ears, the paler colour of chest and thighs respect to forelimbs, hocks and tail in the stone marten and its chunkier overall silhouette.
All videos of Martes spp. were subjected to a blind identification procedure by three experienced researchers (AM, FF and AB) and discordant records were discarded. Capture independence was achieved by considering consecutive records of the same species at the same site within a 30-min interval as a single event (Kelly and Holub 2008; Monterroso et al. 2014).
From October 2014 to April 2015, the whole study area, between 450 and 1720 m a.s.l., was surveyed by deploying 21 camera-traps as regularly as possible, depending on accessibility (mean inter-trap distance ± SD = 2.6 ± 1.3 km; Fig. 1). In winter (Dec-Apr), only 10 trap-sites were activated, avoiding avalanche-prone sections. Between July and November 2015, we focused on the north-south corridor formed by the valleys “de il Fiume” and “Pogallo”. Camera-traps (N = 27) were deployed within a 1 × 1 km grid superimposed on the kilometric grid of digitalized, 1:10,000 Regional Technical Maps, aiming to set each camera as much as possible in the centre of the grid mesh and sample the most representative habitat (mean inter-trap distance ± SD = 1.0 ± 0.3 km; Fig. 1).
Variation over sampling periods in each species’ encounter rates was tested by the chi-squared (χ2) test.
Faecal DNA-based species identification
To supplement the data on stone marten distribution collected by camera-trapping, non-invasive genetic sampling was conducted between July and November 2015. Fresh scats were searched for by two surveyors along linear transects coinciding with paths (N = 23; mean length = 7.2 km, min-max: 2.5–14.8 km). In the north-south corridor transects were surveyed monthly, while those in the rest of the protected area were sampled mostly once, and all samples were georeferenced by a GPS. A small portion (ca. 1 cm) of each scat suspected to belong to Martes spp. based on size and morphology (see Remonti et al. 2012) was picked up using sticks, stored in autoclaved tubes containing ethanol 96% and preserved at – 20 °C until processed.
DNA was isolated using the QIAamp DNA Stool Mini Kit (Qiagen) according to the manufacturer’s instructions and species identification was accomplished by a PCR-RFLP method. Two primers amplify the mtDNA from Martes martes, M. foina and four Mustela species; then, the simultaneous digestion of amplified mtDNA by two restriction enzymes (RsaI and HaeIII) generates different restriction patterns for each mustelid species, providing for an effective genetic identification of sympatric marten species (Ruiz-Gonzalez et al. 2008, 2013).
Individual identification by microsatellite genotyping
To assess the minimum number of stone marten individuals, a specific sampling was carried out in the lower Pogallo valley, from April to June 2017, as to record only resident adult individuals (i.e. after juvenile dispersal and before kits-of-the year start marking; Libois and Waechter 1991). By superimposing a 1 × 1 km grid on 1:10,000 maps, we identified 24, 1-km2 contiguous sub-areas with adequate paths and accessibility to the major habitats and altitude belts (500–1000, 1001–1500 and 1501–2000 m a.s.l.). Grid mesh size was based on available data on both stone- and pine marten density (0.1–3.16 ind/km2; Marchesi 1989; Zalewski and Jedrzejewski 2006; Balestrieri et al. 2016a), aiming to lower the chance of missing those individuals whose home ranges may fall in un-sampled areas (Kays and Slauson 2008). Each transect was surveyed twice (total length = 83.3 km), with a gap of 15–20 days between visits.
All samples were georeferenced and stored at – 20 °C in autoclaved tubes containing 96% ethanol until processed. Faecal DNA samples were genotyped using a multiplex panel of 17 autosomal microsatellite markers including 10 species-specific microsatellites (Mf 1.1, Mf 1.11, Mf 1.18, Mf 1.3, Mf 2.13, Mf 3.2, Mf 3.7, Mf 4.17, Mf 8.7, Mf 8.8; Basto et al. 2010) and 7 additional markers described in closely related mustelids (Ma1, Davis and Strobeck 1998; Mel1, Bijlsma et al. 2000; MLUT27, Cabria et al. 2007; Mvis072, Fleming et al. 1999; Mvi57, O’Connell et al. 1996; MP0059, Jordan et al. 2007; Lut453, Dallas et al. 2003), previously used in Martes spp. studies (Ruiz-González et al. 2013; Vergara et al. 2015) and readapted for degraded faecal nDNA analysis (Ruiz-González et al. 2013). The forward primers, labelled with the dyes 6-FAM, NED, PET and VIC, were used in four PCR multiplex reactions modified from Vergara et al. (2015) (Mult-A: Mlut27, Mel1, Mf1.1, Mf4.17; Mult-B: Lut453, Ma1, Mf1.18, Mf3.7, Mf8.8, Mp0059; Mult-C: Mf1.11, Mf2.13, Mf3.2, Mvi072; Mult-D: Mf1.3, Mf8.7, Mvi-57).
To lower the probability of retaining false homozygotes or false allele errors, a multitube-approach of 4 independent replicates was used (Taberlet et al. 1996), followed by stringent criteria to construct consensus genotypes (i.e. accepting heterozygotes if the two alleles are recorded in ≥ 2 replicates and homozygotes if a single allele is recorded in ≥ 3 replicates) (e.g. Frantz et al. 2003; Brzeski et al. 2013). Briefly, DNA quality was initially screened by PCR-amplifying each DNA sample four times at four loci (Mult-A) and only samples showing > 50% positive PCRs were further amplified four times at the remaining 12 loci. Samples with ambiguous results after four amplifications per locus or with < 50% successful amplifications across loci were not considered reliable genotypes and discarded (Ruiz-González et al. 2013). Multiplex PCR products were run on an ABI (Foster City, CA) 3130XL automated sequencer (Applied Biosystems), with the internal size standard GS500 LIZ™ (Applied Biosystems) and fragment analysis was conducted using the ABI software Genemapper 4.0. To test the discrimination power of our microsatellite set, we computed the probability of identity (PID) by GIMLET, using the unbiased equation for both small sample size and siblings. The more conservative PID for full-sibs (PID-Sib) was estimated as an upper limit to the probability that pairs of individuals would share the same genotype. Consensus genotypes from four replicates were reconstructed using GIMLET, which was also used to estimate genotyping errors: allelic dropout (ADO) and false alleles (FA) (Taberlet et al. 1996; Pompanon et al. 2005).
Estimation of population size and kinship from genetic data
Population size was assessed by capwire estimators (Miller et al. 2005), an urn model developed expressly for faecal DNA-based sampling which provides reliable estimates also for small populations and has been used to estimate population size in several species (e.g. Arrendal et al. 2007; Sugimoto et al. 2014). To obtain the maximum likelihood estimate (MLE) of population size, data were fitted to either the Equal Capture model, for which all individuals were assumed to have an equal probability of being sampled, or Two-Innate Rates model, assuming that the population contained a mixture of easy-to-capture and difficult-to-capture individuals. The fit of the two models was compared using a Likelihood Ratio Test (LRT) and the p-value was calculated by using a parametric bootstrap approach to estimate the distribution of the LRT for data simulated under the less-parameterized Equal Capture model (Pennell et al. 2013). Confidence intervals (CI) for population size were estimated using a parametric bootstrap approach (Miller et al. 2005).
Genetic relatedness and sibling analyses were calculated by ML-RELATE (Kalinowski et al. 2006) which uses a maximum likelihood method to compute pair-wise genetic relatedness (Rxy). Sibship analysis was conducted using COLONY 2.0.4 (Jones and Wang 2010), with the typing error rate set at 0.01. This approach considers the likelihood of the entire pedigree, as opposed to relatedness on a pair-wise basis.
Species distribution modelling
Land cover variables, extracted from available maps of forested areas of Piedmont region (Table 1; IPLA 2016), were re-sampled to a common resolution of 1 × 1 km cell size, using QuantumGIS 2.16.3. To test for multi-collinearity among variables, the Variance Inflation Factor (VIF) was calculated (Table 1), VIF values > 3 indicating highly correlated predictors (Fox and Monette 1992; Zuur et al. 2010; Wilson et al. 2012).
Species Distribution Models (SDMs) were developed using the MaxEnt algorithm (Phillips et al. 2006), a widely used method which applies the principle of maximum entropy to predict the potential distribution of species from presence-only data (Phillips and Dudík 2008; Elith et al. 2011), and has proved efficient for assessing habitat suitability for the stone marten (Vergara et al. 2016). Models were fitted to independent presence data, collected between October 2014 and June 2017, and an equal number of randomly selected background points (Drake 2014). To ensure more ecologically realistic response curves, MaxEnt was run using only linear and quadratic features (Bateman et al. 2012, 2016) and default values for all the other parameters (maximum number of iterations = 5000; convergence threshold = 10-5; multiplier regularization = 1). To assess the relative contribution of each variable, a jackknife test was used (Phillips et al. 2006). Particularly, we estimated the arithmetic mean between the percent contribution and permutation importance, two measures which define the contribution of each variable to the final model (Elith et al. 2011; Meyer et al. 2014). To obtain the best model, all variables with importance < 5% were removed (Brambilla et al. 2013; Warren et al. 2014). Model accuracy was analysed by the area under the curve of the receiver operating characteristic (ROC) (Pearce and Ferrier 2000; Fawcett 2006). To assess the suitable area for the stone marten, we converted the continuous suitability map into a binary (suitable/unsuitable) classification, using the “equal training sensitivity and specificity” threshold (ETSS; Lantschner et al. 2017). Mann-Whitney’s test was used to compare the environmental variables within stone marten positive cells (use) to the background (availability). All statistical analyses were carried out by R 3.4.3 packages raster (Hijmans et al. 2014), sp (Pebesma and Bivand 2011), usdm (Naimi 2017) and dismo (Hijmans et al. 2011).
Results
Distribution
A total trapping effort of 4539 camera trap-days allowed recording of stone martens 362 times (one video-clip per 12.5 trap-days). The species was recorded at 83% of trapping sites (Table 2), occurring in all available habitats, up to 1850 m above sea level (Fig. 2). The pine marten was recorded for the first time in the north of the protected area in October 2014; throughout the study period, a total of 16 independent events occurred at six different sites (12.5%; Table 2). The use of baits and videos allowed the reliable identification of the two species for 79% of trapping events. For both martens, encounter-rates did not differ among sampling periods (χ2 = 3.37, 2 df, P = 0.18 and χ2 = 1.10, 2 df, P = 0.58, respectively). The mesocarnivore community also included the red fox (one video-clip per 28.1 trap-days) and European badger (one video-clip per 135.2 trap-days).
To maximise the cost effectiveness of genetic analyses, the apparently “less fresh” faecal samples—55 out of the 167 faecal samples collected between July and September 2015—were discarded. Of the remaining 112 samples, 96 (83.9%) could be assigned to a Martes species by the PCR-RFLP analysis—namely 82 to the stone marten and 12 to the pine marten—, while 16 samples did not amplify.
Density and kinship as assessed by the genetic sampling
To assess stone marten density, 99 out of 128 faecal samples collected in spring 2017 were selected for microsatellite genetic analyses based on their “freshness”. The multiplex screening test was not passed by 55 samples, which were then discarded. Full multilocus microsatellite genotypes were obtained for the remaining 44 samples, of which 41 were assigned to the stone marten, two to the pine marten and one to Mustela sp.
The average proportion of positive PCRs (calculated from correctly and fully genotyped samples) was 82% and varied among loci from 70 to 100%. The observed average error rates across loci were ADO = 0.241 and FA = 0.021, while the number of alleles per locus ranged from 3 to 10 (mean 5.83). Average, non-biased observed and expected heterozygosities (Ho and He) were 0.6 and 0.62 respectively. PID analysis showed that the set of 17 loci would produce an identical genotype with a probability of 1.54 × 10−9, and with a probability of 3.43 × 10−4 for a full-sib.
After a regrouping procedure (i.e. pairwise comparison of the different genotypes obtained), we identified 16 stone marten individual genotypes with a complete multi-locus profile. The average number of detections (re-samplings) per individual was 2.6 (min-max 1–6), with six individuals detected only once and two individuals recorded six times. Using capwire estimators, population size was assessed as 21 (CI = 15 – 28) individuals; including only the cells for which at least one sample was genotyped (N = 22), density was assessed at 0.95 (0.7–1.3) ind/km2.
Except for individuals 2 and 4, all stone martens were related to each other (14 half-sib pairs), individuals 3 and 5, 5 and 8, 6 and 13; and 11 and 16 being first order relatives (i.e. parent/offspring or full-sib dyad). Additionally, using ML-Relate, 13 full-siblings were acknowledged among the 16 stone martens. The two pine martens identified in the area did not result related to each other.
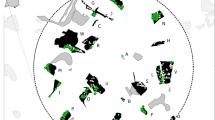
If we assume that mountain ridges that border the valleys coincide with the range limits of males, in the study area it was possible to identify six clusters consisting of 1–3 individuals (Fig. 3).
Habitat suitability
Habitat suitability, as assessed using 161 independent records of the stone marten in the protected area, depended on six environmental variables (Fig. 4), of which four provided the major contributions: rocky grasslands (31.67% importance), rocks and debris (24.63%), beech forests (16.64%) and chestnut forests (10.63%). The first had a non-linear quadratic effect, with the highest suitability at intermediate values of percent cover, although always > 0.4 (Fig. 4). Open rocky areas showed a negative trend, while suitability for the stone marten increased linearly for the other two variables (Fig. 4). The discriminatory ability of the MaxEnt model was sufficient (AUC = 0.74). Following the reclassification analysis based on ETSS = 0.47, 63.9% of the study area resulted suitable for the stone marten (Fig. 5), which, according to univariate analysis, selected beech- and other deciduous forests, while avoided rocky and scree areas (Table 3).
Discussion
Associative modelling approaches, such as SDMs, which consider the target species locations to be representative of ideal habitat conditions (in the multidimensional space described by the chosen variables), assess adequately the species potential distribution only if no other factor plays a major role in determining its occurrences (Gough and Rushton 2000). For the stone marten, interspecific competition with the strictly related pine marten has been claimed to affect both space use (Delibes 1983) and diet (Gazzola and Balestrieri 2020), suggesting that pine marten dominance may be a major biotic constraint of stone marten’s niche.
In the VGNP, the results of both camera-traps and genetic surveys were consistent with the occasional record-based framework, providing straightforward evidence of the widespread occurrence of the stone marten and patchy distribution of the pine marten. If we assume that the frequency of occurrence of records is an index of the relative abundance of both species (Gese 2001; Carbone et al. 2001), the stone marten stood out also in terms of numbers. Low pine marten abundance may depend on the recent recolonisation of this sector of the Alpine range: in the last decade, the number of roadkills has progressively increased and some camera-trapping records have been collected in the western and northern parts of the province (Mosini and Balestrieri 2017), suggesting that the pine marten may be reinforcing its occurrences on the Alps as well as it is expanding in lowland areas of NW Italy (Balestrieri et al. 2015, 2016b). Whatever the reason, the large numerical difference recorded suggests that currently interspecific competition may play a negligible role in shaping habitat use by the stone marten, although, when sympatric, the pine marten usually dominates in forested habitat as those forming the bulk of our study area.
Suitability models confirmed the preference of the stone marten for a mosaic of forested areas, particularly broad-leaved forests, which likely offer both food resources and cover from predators, and rocky grassland areas, which may offer cavities which are both safe and thermal regulated resting sites (Birks et al. 2005; Virgós et al. 2012). Pine martens, which usually nest on trees, in winter, in response to extreme cold, frequently use cavities at ground-level (Brainerd et al. 1995; Zalewski 1997). As the stone marten prefers warm climates (Vergara et al. 2016) and is also less arboreal than the pine marten (Goszczyński et al. 2007), in the study area may find suitable shelter sites in rocky crevices. Cold air temperatures and lack of cover would also explain the negative effect of “rock and debris” on habitat suitability for the stone marten, as this habitat mostly coincides with the top of mountain ridges.
Detection probability may vary with the same covariates that affect occurrence probability, leading to biased estimates of their importance in determining occupancy (Yackulic et al. 2013). As, using camera-traps, encounter-rates were constantly high throughout the study period and scat-based genetic sampling of stable marten populations has been demonstrated to provide detection probabilities next to 1 (Balestrieri et al. 2015), we are reasonably confident in the performance of our SDMs.
Besides competition with the pine marten, a further factor that has been reported to affect stone marten distribution and abundance is the availability of fleshy fruits (Mortelliti and Boitani 2008; Virgós et al. 2010), the latter being considered the most frugivorous mesocarnivore (Virgós et al. 2012). Nonetheless, recently Gazzola and Balestrieri (2020) reported that frugivory in the stone marten may depend on competition with the pine marten, the first preying mostly on rodents in areas of allopatry. In our study area, small mammals (Clethrionomys glareolus, Glis glis, Apodemus sp.) formed the bulk of stone marten diet (Balestrieri et al. 2018), suggesting that fruit availability did not affect the use of space.
In the last two decades, faecal DNA-based genotyping has proven an effective non-invasive method for estimating population size for several elusive species, including mustelids (e.g. pine marten, Ruiz-González et al. 2013; O’Mahony et al. 2012; Sheehy et al. 2014; Eurasian otter, Arrendal et al. 2007; Vergara et al. 2014). In our study area, mean stone marten density fell within the range recorded in rural Switzerland (Lachat Feller 1993) and was consistent with the density of closely related pine marten in areas on northern Europe showing similar climatic conditions (0.6-0.7 ind/km2; Zalewski and Jedrzejewski 2006). Assuming that topographical constraints drive home range size and shape, boundaries tending to coincide with ridges (Powell and Mitchell 1998; Monterroso et al. 2013), stone marten individuals may be split into six groups, each consisting of 2-3 individuals, in agreement with the species’ intra-sexual spacing pattern (Powell 1978; Genovesi et al. 1997).
As recorded for both pine martens (Balestrieri et al. 2016a) and otters (Vergara et al. 2014), most individuals were related to each other, suggesting rapid population renewal and that dispersion occurs on relatively short distances. Heterozygosity matched with average values for the pine marten in continental Europe (min-max 0.56–0.7; Kyle et al. 2003; Pertoldi et al. 2008), while was slightly higher than that reported for the stone marten (Iberian Peninsula: Ho = 0.49, Vergara et al. 2015; Eastern France: Ho = 0.55, Larroque et al. 2016; Poland: Ho = 0.52, Wereszczuk et al. 2017).
By applying two non-invasive methods, we pointed out that, as reported for feeding habits (Monterroso et al. 2016), in areas of sympatry with the pine marten patterns of habitat selection depend on the relative abundance of the two competing species. Being dominant, in the study area, the widespread stone marten selected forested areas, attaining density values like those reported for the pine marten in northern Europe. Further studies are needed to confirm the intra-sexual territorial behaviour and determine how competition with the pine marten affects stone marten density in mountainous areas.
References
Abramov AV, Kruskop SV, Lissovsky AA (2006) Distribution of the Stone Marten Martes foina (Carnivora, Mustelidae) in the European part of Russia. Russian J Theriol 5:37–41
Arrendal J, Vilà C, Björklund M (2007) Reliability of noninvasive genetic census of otters compared to field censuses. Conserv Genet 8:1097–1107
Balestrieri A, Remonti L, Ruiz-González A, Zenato M, Gazzola A, Vergara M, Dettori EE, Saino N, Capelli E, Gómez-Moliner BJ, Guidali F, Prigioni C (2015) Distribution and habitat use by pine marten Martes martes in a riparian corridor crossing intensively cultivated lowlands. Ecol Res 30:153–162
Balestrieri A, Ruiz-González A, Vergara M, Capelli E, Tirozzi P, Alfino S, Minuti G, Prigioni C, Saino N (2016a) Pine marten density in lowland riparian woods: a test for the Random Encounter Model. Mamm Biol 81:439–446
Balestrieri A, Ruiz-González A, Capelli E, Vergara M, Prigioni C, Saino N (2016b) Pine marten vs. stone marten in agricultural lowlands: a landscape-scale, genetic survey. Mammal Res 61:327–335
Balestrieri A, Mosini A, Saino N (2018) Distribuzione ed ecologia di martora e faina nel Parco Nazionale della Val Grande. Technical report, University of Milan, Italy
Balestrieri A, Mori E, Menchetti M, Ruiz-González A, Milanesi P (2019) Far from the madding crowd: tolerance toward human disturbance shapes distribution and connectivity patterns of closely related Martes spp. Popul Ecol 61:289–299
Basto MP, Rodrigues M, Santos-Reis M, Bruford MW, Fernandes CA (2010) Isolation and characterization of 13 tetranucleotide microsatellite loci in the stone marten (Martes foina). Conserv Genet Resour 2(S1):317–319
Bateman BL, Van Der Wal J, Williams SE, Johnson CN (2012) Biotic interactions influence the projected distribution of a specialist mammal under climate change. Divers Distrib 18:861–872
Bateman BL, Pidgeon AM, Radeloff VC, Flather CH, Van Der Wal J, Akçakaya HR, Thogmartin WE, Albright TP, Vavrus SJ, Heglund PJ (2016) Potential breeding distributions of U.S. birds predicted with both short-term variability and long-term average climate data. Ecol Appl 26:2720–2731
Bijlsma R, Van de Vliet M, Pertoldi C, Van Apeldoorn RC, Van de Zande L (2000) Microsatellite primers from the Eurasian badger, Meles meles. Mol Ecol 9:2216–2217
Birks JDS, Messenger JE, Halliwell EC (2005) Diversity of den sites used by pine martens Martes martes : a response to the scarcity of arboreal cavities? Mammal Rev 35:313–320
Brainerd SM, Helldin J-O, Lindström ER, Rolstad E, Rolstad J, Storch I (1995) Pine marten (Martes martes) selection of resting and denning sites in Scandinavian managed forests. Ann Zool Fenn 32:151–157
Brambilla M, Bassi E, Bergero V, Casale F, Chemollo M, Falco R, Longoni V, Saporetti F, Viganò E, Vitulano S (2013) Modelling distribution and potential overlap between Boreal Owl Aegolius funereus and Black Woodpecker Dryocopus martius: implications for management and monitoring plans. Bird Conserv Int 23:502–511
Brooke ZM, Bielby J, Nambiar K, Carbone C (2014) Correlates of research effort in carnivores: body size, range size and diet matter. PLoS One 9(4):e93195
Brzeski KE, Gunther MS, Black JM (2013) Evaluating river otter demography using noninvasive genetic methods. J Wildl Manag 77:1523–1531
Buskirk SW, Zielinski WJ (2003) Small and mid-sized carnivores. In: Zabel CJ, Anthony RG (eds) Mammal Community Dynamics. Management and Conservation in the Coniferous Forests of Western North America. Cambridge University Press, Cambridge, pp 207–249
Cabria MT, Gonzalez EG, Gomez-Moliner BJ, Zardoya R (2007) Microsatellite markers for the endangered European mink (Mustela lutreola) and closely related mustelids. Mol Ecol Notes 7:1185–1188
Carbone C, Christie S, Conforti K, Coulson T, Franklin N, Ginsberg JR, Griffiths M, Holden J, Kawanishi K, Kinnaird M, Laidlaw R, Lynam A, MacDonald DW, Martyr D, McDougal C, Nath L, O’Brien T, Seidensticker J, Smith D, Sunquist M, Tilson R, Wan Shahruddin WN (2001) The use of photographic rates to estimate densities of tigers and other cryptic mammals. Anim Conserv 4:75–79
Croose E, Birks JDS, Martin J, Ventress G, MacPherson J, O’Reilly C (2019) Comparing the efficacy and cost-effectiveness of sampling methods for estimating population abundance and density of a recovering carnivore: the European pine marten (Martes martes). Eur J Wildl Res 65:37
Dallas JF, Coxon KE, Sykes T, Chanin PRF, Marshall F, Carss DN, Bacon PJ, Piertney SB, Racey PA (2003) Similar estimates of population genetic composition and sex ratio derived from carcasses and faeces of Eurasian otter Lutra lutra. Mol Ecol 12:275–282
Davis CS, Strobeck C (1998) Isolation, variability, and cross-species amplification of polymorphic microsatellite loci in the family Mustelidae. Mol Ecol 7:1776–1778
Davison A, Birks JDS, Brookes RC, Braithwaite TC, Messenger JE (2002) On the origin of faeces: morphological versus molecular methods for surveying rare carnivores from their scats. J Zool 257:141–143
Delibes M (1983) Interspecific competition and the habitat of the stone marten Martes foina (Erxleben, 1777) in Europe. Acta Zool Fenn 174:229–231
Drake JM (2014) Ensemble algorithms for ecological niche modeling from presence-background and presence-only data. Ecosphere 5(6):1–16
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists: statistical explanation of MaxEnt. Divers Distrib 17:43–57
Falcucci A, Maiorano L, Boitani L (2007) Changes in land-use/landcover patterns in Italy and their implications for biodiversity conservation. Landsc Ecol 22:617–631
Fawcett T (2006) An introduction to ROC analysis. Pattern Recogn Lett 27:861–874
Fleming MA, Ostrander EA, Cook JA (1999) Microsatellite markers for American mink (Mustela vison) and ermine (Mustela erminea). Mol Ecol 8:1352–1354
Fonda F (2019) Idoneità ambientale per la martora (Martes martes) e la faina (Martes foina) sull’arco alpino. Degree thesis, University of Pavia, Italy.
Fox J, Monette G (1992) Generalized collinearity diagnostics. J Am Stat Assoc 87:178–183
Frantz AC, Pope LC, Carpenter PJ, Roper TJ, Wilson GJ, Delahay RJ, Burke T (2003) Reliable microsatellite genotyping of the Eurasian badger (Meles meles) using faecal DNA. Mol Ecol 12:1649–1661
Gazzola A, Balestrieri A (2020) Nutritional ecology provides insights into competitive interactions between closely related Martes species. Mammal Rev 50:82–90
Genovesi P, De Marinis AM (2003) Martes foina. In: Boitani L, Lovari S, Vigna Taglianti A (eds) Fauna d’Italia. Mammalia III, Carnivora-Artiodactyla. Calderini, Bologna, pp 113–123
Genovesi P, Sinibaldi I, Boitani L (1997) Spacing patterns and territoriality of the stone marten. Can J Zool 75:1966–1971
Gese EM (2001) Monitoring of terrestrial carnivore populations. In: Gittleman GL, Funk SM, Macdonald D, Wayne RK (eds) Carnivore conservation. Cambridge University Press, Cambridge, pp 372–396
Goszczyński J, Posłuszny M, Pilot M, Gralak B (2007) Patterns of winter locomotion and foraging in two sympatric marten species: Martes martes and Martes foina. Can J Zool 85:239–249
Gough MC, Rushton SP (2000) The application of GIS-modelling to mustelid landscape ecology. Mammal Rev 30:197–216
Herr J, Schley L, Roper TJ (2009) Socio-spatial organization of urban stone martens. J Zool 277:54–62
Herrmann M (2004) Steinmarder in unterschiedlichen Lebensräumen - Ressourcen, räumliche und soziale Organisation. Laurenti Verlag, Bielefeld
Hijmans RJ, Phillips SJ, Leathwick JR, Elith J (2011) Package dismo: Species distribution modeling. Wien: www.cran.r-project.org.
Hijmans RJ, van Etten J, Mattiuzzi M, Sumner M, Greenberg JA, Perpinan Lamigueiro O, Bevan A, Racine EB, Shortridge A (2014) Package raster: geographic data analysis and modeling. Wien: www.cran.r-project.org.
Höchtl F, Lehringer S, Konold W (2005) “Wilderness”: what it means when it becomes a reality—a case study from the southwestern Alps. Landsc Urban Plan 70:85–95
IPLA (2016) Carta forestale e delle altre coperture del territorio. Istituto per le Piante da Legno e l’Ambiente e Regione Piemonte, Torino
Jones OR, Wang J (2010) COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 10:551–555
Jordan MJ, Higley M, Matthews SM, Rhodes OE, Schwartz MK, Barrett RH, Palsbøll PJ (2007) Development of 22 new microsatellite loci for fishers (Martes pennanti) with variability results from across their range. Mol Ecol Notes 7:797–801
Kalinowski ST, Wagner AP, Taper ML (2006) ML-Relate: a computer program for maximum likelihood estimation of relatedness and relationship. Mol Ecol Notes 6:576–579
Kays R, Slauson K (2008) Remote cameras. In: Long R, MacKay P, Zielinski W, Ray J (eds) Noninvasive Survey Methods for North American Carnivores. Island Press, Washington DC, pp 110–140
Kelly MJ, Holub EL (2008) Camera trapping of carnivores: trap success among camera types and across species, and habitat selection by species, on Salt Pond Mountain, Giles County, Virginia. Northeast Nat 15:249–262
Kyle CJ, Davison A, Strobeck C (2003) Genetic structure of European pine martens (Martes martes), and evidence for introgression with M. americana in England. Conserv Genet 4:179–188
Lachat Feller N (1993) Eco-éthologie de la fouine (Martes foina Erxleben, 1777) dans le Jura suisse. Ph.D. Thesis, Université de Neuchâtel, Switzerland
Lantschner MV, Atkinson TH, Corley JC, Liebhold AM (2017) Predicting North American Scolytinae invasions in the Southern Hemisphere. Ecol Appl 27:66–77
Larroque J, Ruette S, Vandel J-M, Queney G, Devillard S (2016) Age and sex-dependent effects of landscape cover and trapping on the spatial genetic structure of the stone marten (Martes foina). Conserv Genet 17:1293–1306
Li S, McShea WJ, Wang D, Huang J, Shao L (2012) A direct comparison of camera-trapping and sign transects for monitoring wildlife in the Wanglang National Nature Reserve, China. Wildl Soc B 36:538–545
Libois R, Waechter A (1991) La fouine. Encyclopédie des carnivores de France. Société Francaise pour l’étude et la protection des Mammifères
MacDonald D, Crabtree JR, Weisinger G, Dax T, Stamou N, Fleury P, Gutierrez Lazpita J, Gibon A (2000) Agricultural abandonment in mountain areas of Europe: environmental consequences and policy response. J Environ Manag 59:47–69
Marchesi P (1989) Ecologie et comportament de la martre (Martes martes) dans le Jura Suisse. Ph.D. Thesis, University of Neuchatel, Switzerland
Mathews F, Kubasiewicz LM, Gurnell J, Harrower CA, McDonald RA, Shore RF (2018) A review of the population and conservation status of British mammals: technical summary. A report by the Mammal Society under contract to Natural England, Natural Resources Wales and Scottish Natural Heritage. Peterborough: Natural England
Mcdonald RA (2002) Resource partitioning among British and Irish mustelids. J Anim Ecol 71:185–200
Meyer ALS, Pie MR, Passos FC (2014) Assessing the exposure of Lion Tamarins (Leontopithecus spp.) to future climate change. Am J Primatol 76:551–562
Miller CR, Joyce P, Waits LP (2005) A new method for estimating the size of small populations from genetic mark-recapture data. Mol Ecol 14:1991–2005
Monterroso P, Sillero N, Rosalino LM, Loureiro F, Alves PC (2013) Estimating home-range size: when to include a third dimension? Ecol Evol 3:2285–2295
Monterroso P, Alves PC, Ferreras P (2014) Plasticity in circadian activity patterns of mesocarnivores in Southwestern Europe: implications for species coexistence. Behav Ecol Sociobiol 68:1403–1417
Monterroso P, Rebelo P, Alves PC, Ferreras P (2016) Niche partitioning at the edge of the range: a multidimensional analysis with sympatric martens. J Mammal 97:928–939
Mortelliti A, Boitani L (2008) Interaction of food resources and landscape structure in determining the probability of patch use by carnivores in fragmented landscapes. Landsc Ecol 23:285–298
Mosini A, Balestrieri A (2017) Metti una faina in Val d’Ossola. Piemonte Parchi. http://www.piemonteparchi.it/cms/index.php/natura/item/1986-metti-una-faina-in-val-d-ossola
Naimi B (2017) Package usdm: Uncertainty analysis for species distribution models. Wien: www.cran.r-project.org
O’Connell M, Wright JM, Farid A (1996) Development of PCR primers for nine polymorphic American mink Mustela vison microsatellite loci. Mol Ecol 5:311–312
Oli MK (1994) Snow leopards and blue sheep in Nepal: densities and predator:prey ratio. J Mammal 75:998–1004
O'Mahony DT, Powell C, Power J, Hannify R, Turner P, O’Reilly C (2017) National pine marten population assessment 2016. Irish Wildlife Manuals, No. 97. National Parks and Wildlife Service, Department of the Arts, Heritage, Regional, Rural and Gaeltacht Affairs, Ireland
Pearce J, Ferrier S (2000) Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Model 133:225–245
Pebesma E, Bivand R (2011) Package sp: classes and methods for spatial data. Wien: www.cran.r-project.org
Pennell MW, Stansbury CR, Waits LP, Miller CR (2013) Capwire: a R package for estimating population census size from non-invasive genetic sampling. Mol Ecol Resour 13:154–157
Pertoldi C, Barker SF, Madsen AB, Jørgensen H, Randi E, Muňoz J, Baagoe HJ, Loeschcke V (2008) Spatio-temporal population genetics of the Danish pine marten (Martes martes). Biol J Linn Soc 93:457–464
Phillips SJ, Dudík M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Pompanon F, Bonin A, Bellemain E, Taberlet P (2005) Genotyping errors: causes, consequences and solutions. Nat Rev Genet 6:847–859
Powell RA (1978) Mustelid spacing patterns: variations on a theme by Mustela. Z Tierpsychol 50:153–165
Powell RA, Mitchell MS (1998) Topographical constraints and home range quality. Ecography 21:337–341
Proulx G, Aubry KB, Birks J, Buskirk SW, Fortin C, Frost HC, Krohn WB, Mayo L, Monakhov V, Payer D, Saeki M, Santos-Reis M, Weir R, Zielinski WJ (2004) World distribution and status of the genus Martes in 2000. In: Harrison DJ, Fuller AK, Proulx G (eds) Martens and fishers (Martes) in human-altered environments: an international perspective. Springer-Verlag, New York, pp 21–76
Remonti L, Balestrieri A, Ruiz-González A, Gómez-Moliner BJ, Capelli E, Prigioni C (2012) Intraguild dietary overlap and its possible relationship to the coexistence of mesocarnivores in intensive agricultural habitats. Popul Ecol 4:521–532
Roberts NJ (2011) Investigation into survey techniques of large mammals: surveyor competence and camera-trapping vs. transect-sampling. Biosci Horiz 4:40–49
Roemer GW, Gompper ME, Van Valkenburgh B (2009) The ecological role of the mammalian mesocarnivore. BioScience 59:165–173
Ronchi G (2016) Stima della densità della faina (Martes foina) nel PLIS di San Colombano al Lambro tramite foto-trappolaggio. Degree Thesis, University of Milan, Italy
Rowcliffe JM, Field J, Turvey ST, Carbone C (2008) Estimating animal density using camera traps without the need for individual recognition. J Appl Ecol 45:1228–1236
Ruiz-Gonzalez A, Rubines J, Berdion O, Gomez-Moliner BJ (2008) A non-invasive genetic method to identify the sympatric mustelids pine marten (Martes martes) and stone marten (Martes foina): preliminary distribution survey on the northern Iberian Peninsula. Eur J Wildl Res 54:253–261
Ruiz-González A, Madeira MJ, Randi E, Urra F, Gómez-Moliner BJ (2013) Non invasive genetic sampling of sympatric marten species (Martes martes and Martes foina): assessing species and individual identification success rates on faecal DNA genotyping. Eur J Wildl Res 59:371–386
Ruiz-Gonzalez A, Cushman SA, Madeira MJ, Randi E, Gómez-Moliner BJ (2015) Isolation by distance, resistance and/or clusters? Lessons learned from a forest-dwelling carnivore inhabiting a heterogeneous landscape. Mol Ecol 24:5110–5129
Sacchi O, Meriggi A (1995) Habitat requirements of the stone marten (Martes foina) on the Tyrrhenian slopes of the northern Apennines. Hystrix 7:99–104
Sheehy E, O’Meara DB, O’Reilly C, Smart A, Lawton C (2014) A non-invasive approach to determining pine marten abundance and predation. Eur J Wildl Res 60:223–236
Silveira L, Jácomo ATA, Diniz-Filho JAF (2003) Camera trap, line transect census and track surveys: a comparative evaluation. Biol Conserv 114:351–355
Sindaco R, Carpegna F (2010) Segnalazioni Faunistiche Piemontesi. III. Dati preliminari sulla distribuzione dei Mustelidi del Piemonte (Mammalia, Carnivora, Mustelidae). Rivista piemontese di Storia naturale 31:397–422
Sugimoto T, Aramilev VV, Kerley LL, Nagata J, Miquelle DG, McCullough DR (2014) Noninvasive genetic analyses for estimating population size and genetic diversity of the remaining Far Eastern leopard (Panthera pardus orientalis) population. Conserv Genet 15:521–532
Taberlet P, Griffin S, Goossens B, Questiau S, Manceau V, Escaravage N, Waits LP, Bouvet J (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res 24:3189–3194
Van Maanen E (2013) Onderscheid tussen boom-en steenmarter in de hand, in het veld en op foto. Jaarbrief WBN van de Zoogdiervereniging over 2012 in MASTERPASSEN XIX. Zoogdiervereniging, Nijmegen
Vergara M, Ruiz-González A, López de Luzuriaga J, Gómez-Moliner BJ (2014) Individual identification and distribution assessment of otters (Lutra lutra) through non-invasive genetic sampling: Recovery of an endangered species in the Basque Country (Northern Spain). Mamm Biol 79:259–267
Vergara M, Basto MP, Madeira MJ, Gomez-Moliner BJ, Santos-Reis M, Fernandes C, Ruiz-González A (2015) Inferring population genetic structure in widely and continuously distributed carnivores: the stone marten (Martes foina) as a case study. PLoS One 10(7):e0134257
Vergara M, Cushman SA, Urra F, Ruiz-González A (2016) Shaken but not stirred: multiscale habitat suitability modeling of sympatric marten species (Martes martes and Martes foina) in the northern Iberian Peninsula. Landsc Ecol 31:1241–1260
Vergara M, Cushman SA, Madeira MJ, Ruiz-González A (2017) Living in sympatry on the edge: Assessing distribution, habitat suitability and niche partitioning for pine and stone marten (Martes martes and Martes foina) in the Iberian Peninsula. In: Zalewski A, Wierzbowska IA, Aubry KB, Birks JDS, O'Mahony DT, Proulx G (eds) The Martes complex in the 21st century: Ecology and conservation. Białowieża, Mammal Research Institute and Polish Academy of Science, pp 261–288
Virgós E, Casanovas JG (1998) Distribution patterns of the stone marten (Martes foina Erxleben, 1777) in Mediterranean mountains of central Spain. Z Saugetierkd 63(4):193–199
Virgós E, Cabezas-Díaz S, Mangas JG, Lozano J (2010) Spatial distribution models in a frugivorous carnivore, the stone marten (Martes foina): is the fleshy-fruit availability a useful predictor? Anim. Biol 60:423–436
Virgós E, Zalewski A, Rosalino LM, Mergey M (2012) Habitat ecology of genus Martes in Europe: a review of the evidences. In: Aubry KB, Zielinski WJ, Raphael MG, Proulx G, Buskirk SW (eds) Biology and Conservation of Marten, Sables, and Fisher: a new synthesis. Cornell University Press, New York, pp 255–266
Warren DL, Wright AN, Seifert SN, Shaffer HB (2014) Incorporating model complexity and spatial sampling bias into ecological niche models of climate change risks faced by 90 California vertebrate species of concern. Divers Distrib 20:334–343
Wereszczuk A, Leblois R, Zalewski A (2017) Genetic diversity and structure related to expansion history and habitat isolation: stone marten populating rural–urban habitats. BMC Ecol 17:46
Werner NY (2012) Small carnivores, big database—inferring possible small carnivore distribution and population trends in Israel from over 30 years of recorded sightings. Small Carniv Conserv 47:17–25
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations. Academic Press, San Diego
Wilson RR, Prichard AK, Parrett LS, Person BT, Carroll GM, Smith MA, Rea CL, Yokel DA (2012) Summer resource selection and identification of important habitat prior to industrial development for the Teshekpuk Caribou Herd in northern Alaska. PLoS One 7(11):e48697
Yackulic CB, Chandler R, Zipkin EF, Royle JA, Nichols JD, Campbell Grant EH, Veran S (2013) Presence-only modelling using MAXENT: when can we trust the inferences? Methods Ecol Evol 4:236–243
Zalewski A (1997) Factors affecting selection of resting site type by pine marten in primeval deciduous forests (Bialowieza National Park, Poland). Acta Theriol 42:271–288
Zalewski A, Jedrzejewski W (2006) Spatial organisation and dynamics of the pine marten Martes martes population in Bialowieza Forest (E Poland) compared with other European woodlands. Ecography 29:31–43
Zub K, Kozieł M, Siłuch M, Bednarczyk P, Zalewski A (2018) The NATURA 2000 database as a tool in the analysis of habitat selection at large scales: factors affecting the occurrence of pine and stone martens in Southern Europe. Eur J Wildl Res 64:10
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems: Data exploration. Methods Ecol Evol 1:3–14
Acknowledgements
The research was supported by the Val Grande National Park, as part of the project “Monitoraggio della biodiversità animale in ambiente alpino” (Monitoring of animal biodiversity on the Alps). We are grateful to A. Biondo, F. Canepuccia, L. Caviglia, G. Cristiani, M. Dresco, E. Galbiati, D. Morisetti, D. Ramoni, D. Sabatini, S. Torniai, F. Zucca (Carabinieri Command for Forest Protection), M. Gilardi and L. Ricci (graduate students), for their help with field work.
This paper is gratefully dedicated to the memory of the late Nicola Saino (University of Milan), who supported the research throughout the study period.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Andrzej Zalewski
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balestrieri, A., Mosini, A., Fonda, F. et al. Spatial ecology of the stone marten in an Alpine area: combining camera-trapping and genetic surveys. Mamm Res 66, 267–279 (2021). https://doi.org/10.1007/s13364-021-00564-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-021-00564-9