Abstract
Since its invention in the 1950s, field ionization mass spectrometry (FI MS) has been, and currently is, the go-to technique employed by the petrochemical industry for the identification of the different types of nonvolatile compounds in their products. Unfortunately, FI MS has several inherent drawbacks, such as poor reproducibility. The performance of positive-ion mode atmospheric pressure chemical ionization mass spectrometry (APCI MS) with O2 gas as the sheath/auxiliary gas and a saturated hydrocarbon solvent/reagent was recently compared with that of FI MS and found to show promise as an alternative, highly reproducible method for lubricant base oil analysis. We report here on the automation of the APCI/O2/saturated hydrocarbon MS method. Isooctane was chosen as the optimal APCI solvent/reagent for base oil ionization due to the low level of fragmentation it provided for model compound mixtures. Three minutes was determined to be the shortest possible cleaning time between samples, regardless of the base oil viscosity. The total analysis time for each sample was 5 min. The reproducibility of the method was assessed by determining within-day and between-day precisions and total precision for hydrocarbon class distributions measured for three different base oils. All total precision values were found to be better than 6.2%, suggesting that the automated (+)APCI/O2/isooctane method is reproducible and robust.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lubricant base oils are manufactured from crude oil or bio-oil [1]. They are comprised of saturated hydrocarbons, including linear, branched, and one- to five-rings containing cyclic saturated hydrocarbons (or naphthenes) [2]. Knowledge of the average molecular weight, the range of the number of carbons, and the relative amount of each saturated hydrocarbon class in base oils would enable the optimization of the processes used to produce base oils for key performance qualities, such as viscosity, thermal stability, and volatility [3].
The analytical techniques currently used by the petroleum industry to characterize the chemical compositions of lubricant base oils include 13C NMR spectroscopy and infrared spectroscopy [4, 5]. However, neither one of these methods can provide information on individual compounds in the base oils. Mass spectrometry (MS), especially when coupled with some form of chromatography, is more advantageous as it can provide molecular weights and some structural information for individual saturated hydrocarbons in base oils. Gas chromatography (GC) coupled with electron ionization (EI) MS is the technique primarily used for the analysis of volatile base oil samples. However, most GC columns cannot attain high enough temperatures to be able to separate the heavier components in lubricant base oils. Furthermore, acceptable separation of even the lighter components of base oils can take several hours [6]. For these reasons, field ionization (FI) MS is widely used in the petroleum industry for chemical composition analysis of the heavier components of lubricant base oils. The benefits of FI include minimal fragmentation of saturated hydrocarbons compared with electron and chemical ionization [7], and generation of only one type of ion (molecular radical cation or M+●) per analyte [8, 9]. However, FI MS suffers from poor reproducibility, limited lifetime of FI emitters, sample carryover, and variable levels of fragmentation of large saturated hydrocarbons [8, 9].
Atmospheric pressure chemical ionization (APCI) is a soft ionization technique that has demonstrated promise for the analysis of mixtures of large saturated hydrocarbons [10,11,12,13,14]. In APCI, a high voltage is applied to the tip of a corona discharge needle, generating a high electric field [15]. In positive ion mode, gaseous molecules surrounding the tip, primarily sheath/auxiliary gas molecules (usually N2), are ionized and form a plasma containing radical cations. Ion-molecule reactions can take place within this plasma, including ionization of the APCI reagent by the molecular ions of the sheath/auxiliary gas molecules to generate reagent ions that are thought to eventually ionize the analyte molecules. Based on this thinking, the specific sheath/auxiliary gas and solvent/APCI reagent selected determine the types of reactions leading to ionization of the analyte. Therefore, a careful choice of these chemicals is critical for successful ionization of the analyte. The most desirable outcome is the generation of only one type of pseudo-molecular or molecular ion for each analyte molecule without fragmentation.
Our research group has demonstrated the generation of only one type of ion per analyte, [M-H]+ (a carbenium ion), for lubricant base oil samples upon direct injection APCI with O2 gas as the sheath/auxiliary gas and hexane as the solvent/reagent [9]. Although small amounts of fragment ions were observed, none of them overlapped with the [M-H]+ ions derived from the lubricant base oils. The APCI/O2/hexane method produced similar semi-quantitative chemical information as FI MS. The advantages of this method over FI MS include stable ion signals, great reproducibility, long life time of the APCI corona discharge needle, and easier maintenance. On the other hand, like FI MS, this method causes some fragmentation and sample carryover from experiment to experiment.
In this work, an automated method based on (+)APCI/O2/saturated hydrocarbon mass spectrometry was developed for lubricant base oil analysis. A model compound study was conducted to determine the solvent/APCI reagent that generates the lowest amount of fragmentation and the least amount of ionization bias between different compounds in base oils. Sample carryover and sample analysis time were minimized by optimizing APCI conditions. The precision of the automated method for different saturated hydrocarbon classes of three base oils with varying viscosities was assessed.
Experimental
Chemicals
The saturated hydrocarbon solvents tested for base oil ionization were pentane, hexane, heptane, decane, dodecane, 2,3-dimethylbutane, methylpentane, isooctane, methylcyclopentane, and methylcyclohexane (all with purity ≥ 99%). The model compound mixture consisted of tetracosane (99%), hexacosane (99%), octacosane (99%), dotriacontane (97%), 5-α-cholestane (≥ 97%), and squalane (98.5%). The above 16 chemicals were purchased from Sigma-Aldrich and used as received. Pristane (95%) was purchased from TCI America and was used for assessing the linear dynamic range. Three lubricant base oil samples of different viscosities (low, middle, and high) were provided by Neste, Finland. The sheath and auxiliary gas for all APCI experiments was ultrapure oxygen (99.993%), which was purchased from Praxair, Inc. All chemicals were used as received.
Instrumentation
A Thermo Scientific isocratic pump and autosampler from the Dionex UltiMate 3000 series were used to introduce the base oil samples for 2 min into an APCI source of a Thermo Scientific LTQ XL linear quadrupole ion trap (LQIT). An injection volume of 10 μL and a flow rate of 20 μL/min were employed for all samples. Injection of five portions of 25 μL of isooctane was used to clean the syringe and injection loops after the sample was injected into the APCI source. The APCI vaporizer temperature was set at 150 °C. The sheath and auxiliary gas (O2) flow rates were 60 and 30, respectively. The corona needle, capillary, and tube lens voltages were 3.5 kV, 10 V, and 30 V, respectively. The capillary was kept at a temperature of 50 °C.
The APCI source was flushed with isooctane at a flow rate of 20 μL/min for 3 min at the end of each measurement. The APCI operating conditions for the “wash” were a source vaporizer temperature of 250 °C and a sheath and auxiliary gas flow rates of 10 and 5, respectively. Due to the use of oxygen gas, a higher temperature was not used for safety reasons. Low gas flow rates were utilized to conserve oxygen.
The isocratic pump and autosampler were configured to the LQIT via a peripheral control connection. The Chromeleon Chromatography Data System (CDS) and Xcalibur 4.0 were used to program the experimental sequence for analysis of base oil samples and isooctane blanks. Xcalibur 4.0 software was used for acquisition of the mass spectra. Analysis of each sample took 5 min. Ion abundances were monitored and averaged over the whole experiment. The averaged mass spectra were used for chemical analysis.
Preparation of Non-spiked and Spiked Samples
Isooctane was used as the solvent for all automated sample analyses. The low and middle viscosity base oil samples were prepared at a concentration of 10 mg/mL in isooctane. The heavy viscosity base sample was prepared at a concentration of 20 mg/mL in isooctane to improve the signals of the heavier analytes.
Pristane was spiked into the base oil samples to assess the linear dynamic range of the automated APCI method for the lubricant base oils. A 4 mg/mL pristane stock solution in isooctane was prepared by adding 1 mL of an 8 mg/mL solution of pristane in isooctane into a 20 mg/mL solution of the middle viscosity base oil dissolved in 1 mL of isooctane. A 2 mg/mL sample concentration was prepared by adding 1 mL of the stock solution into a 10 mg/mL solution of the middle viscosity base oil. The next four concentrations of spiked samples were prepared by taking half the volume of the previously made solution and adding it into 10 mg/mL solutions of the middle viscosity base oil. The concentrations of pristane spiked into the middle viscosity base oil sample ranged from 0.1 up to 4 mg/mL.
Precision
Method precision was expressed as the relative standard deviation (RSD). Within-day precision was determined separately for each saturated hydrocarbon class and for each day by using percent abundances of ions derived from individual mass spectra. Pooled within-day precision was determined using Eq. (1). The final calculation combines the relative standard deviations (RSD) measured for each of the 6 days (N) into a weighted average, which is referred to as the “pooled within-day relative standard deviation” (1):
where k is the RSD for the kth day. Between-day precision (RSDBD) was determined by taking into account the contribution of within-day variation on the daily means and subtracting it from the RSD of daily means (RSDDM) [16]. RSDDM was determined using daily averages of percent abundances.
The total precision calculation (Eq. (3)) sums these two components.
Results and Discussion
The main objective of this study was to automate the APCI/O2/saturated hydrocarbon MS method developed for the analysis of the chemical compositions of lubricant base oils [9]. In parallel, ten different solvents/APCI reagents were tested to determine which one(s) generated the lowest level of fragmentation for the ionized compounds derived from lubricant base oils and which one(s) ionized the different types of compounds with the least bias. Using the optimal solvent/reagent system, sample carryover tests were conducted with the automated (+)APCI/O2/isooctane MS set up to find the experimental conditions that enabled using the shortest possible washing time between different samples. The reproducibility of the measurements was tested. Finally, the precision of the automated method to provide semi-quantitative information of the hydrocarbon class distribution percentages for each base oil was evaluated by measuring the within 1 day, between different days, and the total precision.
Automation
Acquiring mass spectra for lubricant base oils by using the APCI/O2 method and the following clean-up procedure were previously conducted manually [9]. The goal here was to reduce the amount of manual work and the time required to measure mass spectra and clean the instrument. A new (+)APCI/O2 method with an isocratic pump and autosampler was developed for this task. The Dionex UltiMate 3000 autosampler from Thermo Scientific was used to automatically inject samples into the APCI source. The autosampler can hold up to 96 vials. The Chromeleon Chromatography Data System (CDS) software enabled automatic analysis of up to 95 different base oil samples (with one solvent wash sample after each base oil sample).
Selection of the Solvent/APCI Reagent
Ten different solvents/APCI reagents were tested by using direct infusion APCI to identify optimal ionization reagent for the middle viscosity lubricant base oil. All the solvents/reagents tested were saturated hydrocarbons, including linear, branched, and cyclic compounds, due to the excellent solubility of base oils in nonpolar solvents. The use of three of the ten solvents/reagents studied, namely hexane, isooctane, and methylcyclohexane, resulted in high-quality APCI mass spectra (as defined by the observation of a near Gaussian ion distribution) for the lubricant base oil (data not shown). These three reagents were selected for further studies.
The most abundant ions generated from hexane, isooctane, and methylcyclohexane upon APCI were studied by examining the APCI mass spectra measured for the neat solvents in order to identify the most likely reagent ions generated from these solvents. The most abundant ions formed upon APCI/O2 of neat n-hexane and neat methylcyclohexane were the ions of m/z 85 and m/z 97, respectively, both being carbenium ions of the type [M-H]+. In contrast, the most abundant ions for neat isooctane were fragment ions of m/z 57 ([M-C4H9]+; tert-butyl cation). Based on literature, four different ion-molecule reactions could give rise to carbenium ions upon corona discharge of saturated hydrocarbons: M + O2+•, M + NO+, M + H3O+, and M + R+ where R is a carbenium ion [17]. Previous quantum-chemical calculations have suggested that the last reaction, a hydride transfer between a saturated hydrocarbon and a carbenium ion, is barrierless and exergonic in the gas phase [18]. Indeed, different carbenium ions (R+) have been demonstrated to abstract a hydride from saturated hydrocarbons to generate [M-H]+ ions in gas-phase ion-molecule reaction experiments in the LQIT [9]. However, fragment ions were not reported in the above study. Therefore, it is unlikely that the major fragment ions of m/z 57 observed for isooctane are due to reactions of saturated hydrocarbons with carbenium ions. The generation of these ions is still not well understood due to the complex chemical environment upon APCI, and further studies are needed.
The performance of each reagent was tested by measuring the APCI/reagent mass spectra for a 2 mM equimolar mixture consisting of tetracosane, hexacosane, octacosane, dotriacontane, 5-α-cholestane, and squalane. All reagent ions reacted with each of the six analyte molecules to form the desired [M-H]+ carbenium ions albeit at different efficiencies (Figure 1). As expected, the branched and cyclic hydrocarbons were ionized at higher efficiencies than the linear hydrocarbons as they contain hydrogen atoms at tertiary carbons and therefore can generate more stable (tertiary) carbenium ions without rearrangement than linear hydrocarbons. This finding is in agreement with literature results on hydride transfer between carbenium ions and saturated hydrocarbons in a pulsed ion cyclotron resonance mass spectrometer [19]. Additionally, reactions of carbenium ions at hydrogens at secondary and tertiary carbons have been reported to be more exothermic and therefore statistically more likely to occur than reactions at primary carbons. Due to this ionization bias, the determination of the hydrocarbon class distributions for base oil samples, which are mixtures of linear, branched, and cyclic saturated hydrocarbons, can only be semi-quantitative.
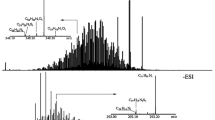
Mass spectra measured for a 2-mM equimolar mixture of six saturated model hydrocarbons dissolved in three different solvents/APCI reagents: (a) hexane, (b) methylcyclohexane, and (c) isooctane. The abundant low-mass ions of m/z 129, 138, 153, 165, 179, 193, and 212 in part B were formed from the solvent and the ions of m/z 226 were due to an impurity
The lowest level of fragmentation (~ 4% compared with the abundance of the [M-H]+ ions) was observed for isooctane (Figure 1). It is notable that, based on the mass spectra measured for the individual saturated hydrocarbons, only the cyclic and branched hydrocarbons, cholestane and squalane, produced fragment ions (Fig. S1). The linear saturated hydrocarbons yielded only minimal amounts of fragment ions (below 1% relative abundance). A study on the underlying causes for saturated hydrocarbon fragmentation upon APCI is currently underway and will be the focus of a future paper. Furthermore, the lower relative abundance of ionized squalane (a branched saturated hydrocarbon) than ionized cholestane (a cyclic saturated hydrocarbon) observed for isooctane but not for the other reagents is not understood at this time. Nevertheless, isooctane was selected as the APCI reagent for the automated method.
Carryover of Samples Between Different Experiments
Table 1 shows the experimental sequence used to assess carryover and to minimize it to an acceptable level. The extent of carryover was determined based on mass spectra measured between lubricant base oil samples for the neat solvent (isooctane) under the same conditions as used for the samples. A period of 12 min was initially chosen as the washing time as the analysis time is about 2 min, and therefore, the total analysis time (14 min) is similar to that employed in FI MS. After this washing time, the isooctane mass spectrum showed no base oil-related ions. On the other hand, after a 2-min washing time, ions derived from base oils were observed in the mass spectrum (data not shown). As no base-oil related ions were detected after a 3-min washing, this time was determined to be the ideal cleaning time.
Linear Dynamic Range
The linear dynamic range was determined to further evaluate the performance of the automated APCI/O2/isooctane method. Pristane was selected as the analyte for these experiments because it is a branched saturated hydrocarbon, like many compounds found in base oils [15]. Additionally, the m/z value (267) of the [M-H]+ ion derived from pristane does not overlap with the m/z values of the [M-H]+ ions derived from the compounds in the middle viscosity base oil sample; however, it does overlap with a fragment ion from the middle viscosity base oil sample (not shown).
Mass spectra were measured for the middle viscosity base oil spiked with pristane at six different concentrations (see preparation of non-spiked and spiked samples above) ranging from 0.1 mg of pristane in 1 mL of a base oil solution (in isooctane) up to 4.0 mg of pristane in 1 mL of a base oil solution (in isooctane). A linear relationship was observed between the relative abundance of the [M-H]+ ions (m/z 267) of pristane (and a fragment ion derived from base oil) and the concentration of pristane when the concentration was between approximately 0.25 and 3.00 mg/mL (Fig. S2). Based on this finding, six additional base oil solutions with pristane concentrations ranging from 0.25 up to 3.00 mg/mL (in isooctane) were prepared separately on three different days and their mass spectra measured each of these days to demonstrate that the linear behavior is reproducible between different days. Figure 2 shows an overlay of the results obtained on three different days. All plots demonstrate R2 values greater than 0.996.
Lubricant Base Oil Analysis
The range of the number of carbons and the relative amount of compounds in each hydrocarbon class were determined for each base oil by using the automated APCI/O2/isooctane method. Three replicates of each non-spiked base oil sample were analyzed each day for 6 days. Figure 3 shows an individual (not average) mass spectrum measured for each base oil sample. Based on previous analysis of these base oils [9], the first distribution of ions represents fragment ions (denoted in Figure 3). The second distribution of ions represents the [M-H]+ ions of the analyte molecules. The relative abundances of the fragment ions are the greatest for the low viscosity base oil and the lowest for the heavy viscosity base oil. This finding suggests that the amount of branched and cyclic hydrocarbons is the greatest for the low viscosity base oil as these compounds generate most abundant fragment ions.
Although the presence of fragment ions complicates the determination of the range of the number of carbons in the compounds in the analyte mixtures, the bimodal distributions of the [M-H]+ ions and fragment ions in the mass spectrum measured for each base oil enables a reasonable approximation. Each of the 18 mass spectra measured for each base oil demonstrated a similar bimodal distribution (data not shown). Based on these measurements, the approximate ranges for the number of carbons were 16–29 for compounds in the low viscosity base oil, 26–43 for compounds in the middle viscosity base oil, and 29–53 for compounds in the heavy viscosity base oil.
Table 2 provides the relative amounts of hydrocarbons in the six different hydrocarbon classes and the relevant standard deviations determined based on the 18 mass spectra measured for each lubricant base oil. The six classes of saturated hydrocarbons displayed in Table 2 were identified in a previous study [9] for the same base oil samples by using a LQIT equipped with a high-resolution orbitrap detector to determine the relative double bond equivalence (RDBE) values for the ions. Similar class distributions were determined for the low viscosity and middle viscosity base oil samples: the most abundant class corresponds to acyclic saturated hydrocarbons, followed by mono- and dinaphthenes, and then trinaphthenes, tetranaphthenes, and finally pentanaphthenes. The distribution measured for the high viscosity lubricant base oil is different from the other two in that the most abundant classes correspond to mono-, di-, and trinaphthenes, followed by the tetranaphthene and acyclic saturated hydrocarbons classes, and lastly, pentanaphthenes.
Precision
Table 3 shows the pooled within-day, between-day, and total precision results for the hydrocarbon class distributions measured for each hydrocarbon class in each of the three lubricant base oil samples. Pooled within-day and between-day precision values are below 5% for all the hydrocarbon classes and base oil types. The lowest total precision (most imprecise) for measurements performed on all three samples was associated with the measurement of the hydrocarbon class distribution for the pentanaphthene class; 6.2% for the low viscosity, 4.9% for the middle viscosity, and 5.2% for the heavy viscosity lubricant base oil. Due to the low abundances of ions derived from the compounds in this class, slight changes in the ion abundances between mass spectra have a much greater influence on the total precision than a similar change for a more concentrated compound. The greatest total precision (most precise) measurement was observed for the dinaphthene class for both the low and heavy viscosity base oil samples (0.8% and 1.4%, respectively) while the greatest total precision was observed for the mononaphthene class for the middle viscosity base oil sample (0.5%).
Ion Signal Stability
The stability of the ion signal on the same day and between days was also evaluated for the automated method. One of the major disadvantages of FI MS is the instability of the ion signal [7]. Severe fluctuations in the total ion signal have been observed for these same three base oil samples when measured by using FI MS [9]. The variations in total ion signal coincide with an inconsistent distribution of analyte ions in the mass spectra measured using FI MS for the base oil samples [9]. In sharp contrast, the total ion signals for the middle viscosity base oil that were measured on the same day and between days by using the automated APCI/O2/isooctane method demonstrate stable and consistent signals (Fig. S3). Similarly, the APCI mass spectra measured for the middle viscosity base oil (Fig. S3) display similar ion distributions.
The individual ion abundances in replicate mass spectra were examined carefully to evaluate the consistency of ionization efficiency for the different compounds in base oils. The ranges of ion abundances determined for the ions of different sizes derived from each compound class in the low, middle, and high viscosity base oil samples are displayed in Fig. S4, S5, and S6, respectively. The data obtained for the middle viscosity base oil sample (Fig. S5) do not show any overlapping error bars for any of the compounds. Additionally, all standard deviations for each class are less than ± 0.02, suggesting consistent ionization of each compound. The data obtained for the low viscosity base oil sample (Fig. S4), however, show some error bar overlap. In Fig. S4, the standard deviation of ion abundances for all compound classes, excluding the mononaphthenes and acyclic saturated hydrocarbons, was less than ± 0.04, and the standard deviations for the mononaphthenes and acyclic saturated hydrocarbons were ± 0.06 and ± 0.11, respectively, suggesting that ionization of these classes may not be consistent between measurements. The data obtained for the high viscosity base oil sample (Fig. S6) show many overlapping error bars for ion abundances of the mono-, di-, and trinaphthene classes as well as the acyclic saturated hydrocarbon and tetranaphthene classes. The standard deviation for each class is between ± 0.03 and 0.06; this finding might suggest that the ionization efficiency of the APCI method for the different compound classes in the high viscosity base oil sample is not consistent from experiment to experiment. However, consideration of additional data indicates that this is not true. For example, Figure 4 shows the distributions of ion abundances as a function of the number of carbons from 36 up to 46 for the mono-, di-, tri-, and tetranaphthene and hydrocarbon classes for this base oil. These ion abundance distributions are similar for all 18 mass spectra that were collected. The consistent distributions of the ion abundances for the five classes that showed overlapping error bars in Fig. S6 confirm that consistent mass spectra are measured in different days.
Conclusions
The APCI/O2/isooctane MS method automated for fast collection of lubricant base oil mass spectra produces reproducible chemical composition information for lubricant base oil samples of different viscosities. Utilizing isooctane as the APCI reagent and oxygen as the sheath gas reduces formation of ions other than the desired [M-H]+ carbenium ions. With a 2-min analysis time and a 3-min washing time, one of the major strengths of this method is the ability to quickly analyze many base oils with different viscosities.
The total precision determined for the automated APCI-MS method was extremely high. The insignificant variability in the hydrocarbon class distribution percentages for each class in each lubricant base oil suggests that APCI is an excellent technique for reproducible semi-quantitative information. The ability to quickly measure the relative abundances of different chemical classes in base oils will enable oil refineries to better evaluate their methods for converting the light distillates of crude oil into the desired products.
References
Erhan, S.Z., Asadauskas, S.: Lubricant basestocks from vegetable oils. Ind. Crop. Prod. 11(2), 277–282 (2000)
Duan, P., Qian, K., Habicht, S.C., Pinkston, D.S., Fu, M., Kenttämaa, H.I.: Analysis of base oil fractions by ClMn(H2O)+ chemical ionization combined with laser-induced acoustic desorption/Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 80(6), 1847–1853 (2008)
Kramer, D.C.; Ziemer, J.N.; Cheng, M.T.; Fry, C.E.; Reynolds, R.N.; Lok, B.K.; Sztenderowicz, M.L.; Krug, R.R. Influence of group II & III base oil composition on VI and oxidation stability. NLGI Spokesman. 1-36 (1999)
Sarpal, A.S., Kapur, G.S., Mukherjee, S., Jain, S.K.: Characterization by 13C NMR: Spectroscopy of base oils produced by different processes. Fuel. 76(10), 931–937 (1997)
Sastry, M.I.S., Chopra, A., Sarpal, A.S., Jain, S.K., Srivastava, S.P., Bhatnagar, A.K.: Determination of physicochemical properties and carbon-type analysis of base oils using mid-IR spectroscopy and partial least-squares regression analysis. Energy Fuel. 12(2), 304–311 (1998)
Wang, F.C.-Y., Zhang, L.: Chemical composition of group II lubricant oil studied by high-resolution gas chromatography and comprehensive two-dimensional gas chromatography. Energy Fuel. 21(6), 3477–3483 (2007)
Lattimer, R.P., Schulten, H.R.: Field ionization and field desorption mass spectrometry: past, present, and future. Anal. Chem. 61(21), 1201A–1215A (1998)
Klesper, G., Röllgen, F.W.: Field-induced ion chemistry leading to the formation of (M-2nH)+● and (2 M-2 mH)+● ions in field desorption mass spectrometry of saturated hydrocarbons. J. Mass Spectrom. 31(4), 383–388 (1996)
Jin, C., Viidanoja, J., Li, M., Zhang, Y., Ikonen, E., Root, A., Romanczyk, M., Manheim, J., Dziekonski, E., Kenttämaa, H.I.: Comparison of atmospheric pressure chemical ionization and field ionization mass spectrometry for the analysis of large saturated hydrocarbons. Anal. Chem. 88(21), 10592–10598 (2016)
Bell, S.E., Ewing, R.G., Eiceman, G.A., Karpas, Z.: Atmospheric pressure chemical ionization of alkanes, alkenes, and cycloalkanes. J. Am. Soc. Mass Spectrom. 5(3), 177–185 (1994)
Hourani, N., Kuhnert, N.: Development of a novel direct-infusion atmospheric pressure chemical ionization mass spectrometry method for the analysis of heavy hydrocarbons in light shredder waste. Anal. Methods-UK. 4(3), 730–735 (2012)
Hourani, N., Muller, H., Adam, F.M., Panda, S.K., Witt, M., Al-Hajji, A.A., Sarathy, S.M.: Structural level characterization of base oils using advanced analytical techniques. Energy Fuel. 29(5), 2962–2970 (2015)
Hourani, N., Kuhnert, N.: High molecular weight non-polar hydrocarbons as pure model substances and in motor oil samples can be ionized without fragmentation by atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 26(19), 2365–2371 (2012)
Owen, B.C., Gao, J., Borton, D.J., Amundson, L.M., Archibold, E.F., Tan, X., Azyat, K., Tykwinski, R., Gray, M., Kenttämaa, H.I.: Carbon disulfide reagent allows the characterization of nonpolar analytes by atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 25(14), 1924–1928 (2011)
The Encyclopedia of Mass Spectrometry, 1st Ed.; Gross, M. L., Caprioli, R. M., Eds.; Elsevier: Amsterdam; Boston, (2003)
Krouwer, J.S., Rabinowitz, R.: How to improve estimates of imprecision. Clin. Chem. 30(2), 290–292 (1984)
Marotta, E., Paradisi, C.: A mass spectrometry study of alkanes in air plasma at atmospheric pressure. J. Am. Soc. Mass Spectrom. 20(4), 697–707 (2009)
Frash, M.V., Solkan, V.N., Kazansky, V.B.: A quantum-chemical study of hydride transfer from alkanes to carbenium cations in the gas phase: a comparison with liquid- and solid-acid catalytic systems. J. Chem. Soc. Farad. T. 93(4), 512–520 (1997)
Lias, S.G., Eyler, J.R., Ausloos, P.: Hydride transfer reactions involving saturated hydrocarbons and CCl3 +, CCl2H+, CCl2F+, CF2Cl+, CF2H+, CF3 +, NO+, C2H5 +, sec-C3H7 + and t-C4H9 +. Int. J. Mass Spectrom. 19, 219–239 (1976)
Acknowledgements
The authors gratefully acknowledge Neste for financial support for this project. We also thank Dr. Chunfen Jin for her mentorship and inspiration. We also would like to thank Tiina Laaksonen from Neste for her guidance throughout the project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 263 kb)
Rights and permissions
About this article
Cite this article
Manheim, J., Zhang, Y., Viidanoja, J. et al. An Automated Method for Chemical Composition Analysis of Lubricant Base Oils by Using Atmospheric Pressure Chemical Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 30, 2014–2021 (2019). https://doi.org/10.1007/s13361-019-02284-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-019-02284-6