Abstract
Gas-phase fragmentation pathways of host-guest complexes of cyclodextrins (CDs) and polyoxometalates (POMs) were examined using collision-induced dissociation (CID). The host-guest complexes studied here were composed of two different classes of POMs—Keggin (PW12O403−) and Lindqvist (M6O192−, M = Mo, W)—and three types of CDs (α-, β-, and γ-CD) differing in the diameter of the inner cavity. The CD-POM complexes were generated either by mixing methanol solutions of POM and CD or through a one-step acidic condensation of tetraoxometalates MO42− (M = Mo, W) with CDs for complexes with Keggin and Lindqvist anions, respectively, and introduced into the gas phase using electrospray ionization (ESI). We observe distinct differences in fragmentation pathways of the complexes of Keggin and Lindqvist POMs under high- and low-energy CID conditions. Specifically, direct dissociation and proton transfer from CD to POM accompanied by the separation of fragments is observed in CID of Keggin CD-POM complexes. In contrast, dissociation of CD complexes with Lindqvist POMs is dominated by the simultaneous loss of multiple water molecules. This unusual fragmentation channel is attributed to dissociation of the POM cluster inside the CD cavity accompanied by covalent bond formation between the fragments and CD and elimination of multiple water molecules. The observed covalent coupling of metal oxide clusters opens up opportunities for derivatization of macrocyclic host molecules using collisional excitation of gaseous non-covalent complexes.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Host-guest supramolecular chemistry refers to the encapsulation of a guest molecule by the cavity of a macrocyclic host molecule via non-covalent interactions [1,2,3]. It has been widely employed for the design and construction of supramolecular assemblies for applications in catalysis [4, 5], materials sciences [6, 7], and drug delivery [8, 9]. The hydrophobic effect was originally proposed to be a major driving force for the formation of host-guest complexes in the solution phase [10, 11]. The incorporation of a hydrophobic guest into the apolar cavity of a host molecule is a thermodynamically favorable process [12].
Recent studies have demonstrated an unexpected formation of strongly bound complexes, in which a chaotropic anion is embedded in a hydrophobic cavity of a macrocyclic host molecule. Chaotropic anions are often described as hydrophilic species with a delocalized charge, which form complexes with host molecules mainly because of a strong binding of the anion to the host molecule [13]. The formation and stability of these host-guest complexes have been discussed in the context of a chaotropic effect as an alternative driving force for supramolecular assembly in an aqueous solution [12, 14]. The chaotropic effect is explained as follows: encapsulation of a chaotropic ion into the binding pocket of a host molecule allows for the recovery of the water structure initially disrupted by the ion [15]. Despite the entropic penalty resulting from the reorganization of water molecules, this process is thermodynamically favorable due to a large negative enthalpy of complexation [16]. The chaotropic effect becomes particularly pronounced for halogenated dodecaborates (B12X122−, X = F, Cl, Br, I) and small polyoxometalates (POMs) referred to as superchaotropic anions that lie far beyond the classical Hofmeister series [17, 18]. The strong binding between B12X122− anions and several host molecules have been observed in the gas phase free of solvent environment [19].
Superchaotropic POM anions [20, 21] also have been explored as guest species in host-guest supramolecular complexes with macrocyclic host molecules such as pseudorotaxanes [22], cucurbit[n]uril [23,24,25], and calix[4]arene derivatives [26, 27]. The unique redox properties of POMs make their host-guest complexes particularly attractive building blocks for the design of functional supramolecular assemblies [28,29,30]. In particular, host-guest complexes of POMs and cyclodextrins (CDs) have been extensively studied. CDs are a class of cyclic oligosaccharides composed of 6, 7, and 8 D-glucopyranosyl residues (α-, β-, and γ-CD, respectively) linked by α-1,4 glycosidic bonds [31]. The shape-persistent hydrophobic cavity of a well-defined size enables the wide application of CDs as exceptional synthetic receptors in nanotechnology [32, 33], catalysis [34, 35], and pharmaceutical science [36, 37].
The first example of CD-POM host-guest complexes was described by Stoddart and co-workers [38]. Several novel CD-POM host-guest supramolecular hybrid materials have been successfully prepared through solution-phase approaches, and the non-covalent interactions of the host-guest assemblies have been systematically characterized [39,40,41,42,43]. Nuclear magnetic resonance (NMR) and single-crystal X-ray diffraction (XRD) analyses have confirmed that hydrogen bonding between POM and CD contributes to a majority of supramolecular interactions both in solution and in the solid state [38,39,40]. Meanwhile, the intrinsic non-covalent interactions between POMs and CDs free of solvent effects are still largely unexplored.
Mass spectrometry (MS) enables the investigation of structures and stabilities of host-guest complexes as isolated species in the gas phase [44,45,46,47]. Several studies have examined gas-phase fragmentation of CD complexes with amino acids [48], peptides [49], nucleobases [50], and organometallic compounds [51, 52]. Of particular interest to this work are gas-phase fragmentation studies of CD-POM complexes using collision-induced dissociation (CID), which provide insights into the intrinsic interactions of POMs and CDs [53, 54]. Herein, we explore the effect of the cavity diameter of the host CD molecule and properties of the guest POM anion on the gas-phase fragmentation of CD-POM complexes. In particular, we examine CD-POM host-guest complexes of two archetypal POM structures, Keggin (PW12O403−, W12POM3−) and Lindqvist (M6O192−, M6POM2−, where M = Mo or W) anions with three types of CDs with different cavity diameters, α-, β-, and γ-CD. Aside from proton transfer and direct dissociation pathways, which are characteristic of CID of non-covalent complexes, we observe simultaneous loss of multiple water molecules from the complex. We attribute this unusual fragmentation pathway to covalent bond formation between the CD host and POM fragments induced by proton transfer upon collisional activation. Covalent coupling of non-covalent complexes in CID has been previously reported for complexes of crown ethers with amines [55,56,57]. Furthermore, unusual reactivity in the gas phase has been observed when the host cavity of cucurbiturils was used as a molecular reaction container [58]. However, to the best of our knowledge the reactivity observed in this study has not been previously reported. The results presented herein open up intriguing opportunities for studying proton-induced chemistry of guest anions inside host cavities and covalent modification of macrocyclic host molecules.
Experimental Section
Chemicals
Sodium phosphotungstate tribasic hydrate (Na3[PW12O40] · xH2O), sodium molybdate (Na2MoO4), sodium tungstate dihydrate (Na2WO4 · 2H2O), tetrabutylammonium bromide (TBA) (C16H36BrN), α-cyclodextrin (C36H60O30), β-cyclodextrin (C42H70O35), γ-cyclodextrin (C48H80O40), methanol-d4 (CD3OD, 99.8 at.% D), deuterium oxide (D2O, 99 at.% D), and water-18O (H218O, 97 at.% 18O) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrochloric acid (HCl, 25 wt%) was purchased from Honeywell (Mexico City, Mexico). Ethyl ether ((C2H5)2O, anhydrous), water (H2O, HPLC grade), and methanol (CH3OH) were purchased from Fisher Scientific (Fair Lawn, NJ, USA).
Preparation of Keggin [CD + W12POM]3− Host-Guest Complex Solutions
A 1 mM stock solution of W12POM3− was prepared by dissolving Na3[PW12O40] in methanol. 1 mM stock solutions of α-, β-, and γ-CD were prepared by dissolving CD in 90:10 (v/v) methanol/water solution. We found it to be necessary to include water to better solubilize the CDs. The final solution for ESI-MS was prepared by combining the Keggin POM and CD stock solutions in a 1:1 ratio and diluting the mixture with methanol to a final concentration of 10 μM.
One-Pot Synthesis of Lindqvist [CD + M6POM]2− Host-Guest Complexes
Na2[X-CD + M6O19] (where M = W or Mo, and X = α, ß, or γ) was synthesized using a one-pot method adapted from Cadot et al. [59]. Typical synthetic procedure is described here using [Na+]2[γ-CD + W6O19]2− as an example: 0.1 g (0.3 mmol) Na2WO4 · 2H2O was dissolved in 4 mL of water, followed by addition of 0.072 g (0.05 mmol) of γ-CD under stirring. The same 6:1 molar ratio of Na2MO4 and CD was used for the one-pot synthesis with both α- and β-CD. A total of six of these syntheses were performed in order to get a sample of each combination of [X-CD + M6O19]2−. These solutions were diluted with methanol to a concentration of 10 μM for MS analysis. We also verified that the same Lindqvist CD-POM complex can be made by mixing [(n-C4H9)4N]2[W6O19] and γ-CD directly in methanol in the case of [γ-CD + W6POM]2−. Synthesis of [(n-C4H9)4N]2[W6O19] was done by a procedure adapted from Klemperer et al. [60].
Low-Energy CID and HCD Conditions
ESI-MS analyses of the CD-POM complexes were performed using LTQ XL Linear Ion Trap Mass Spectrometer and Q-Exactive HF-X Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Fisher Scientific GmbH, Bremen, Germany). Samples were introduced into the MS inlet by direct infusion from a capillary (50 μm ID, 150 μm OD) at a flow rate of ~ 0.5 μL/min. Typical mass spectrometer conditions on LTQ were as follows: electrospray voltage, − 3 kV; capillary temperature, 200 °C; capillary voltage, − 10 V; tube lens, −20 V; scan range, 100–2000 m/z. In the low-energy CID experiments, negatively charged host-guest complexes were isolated in the linear ion trap and subjected to collisions with helium buffer gas. Typical isolation width was 1 m/z. The q value was set at 0.25, and the typical activation time was 30 ms. Typical mass spectrometer conditions on Q-Exactive were as follows: electrospray voltage, − 3 kV; capillary temperature, 250 °C; RF funnel level, 100. Mass spectra were acquired over 150–2000 m/z at 140000 resolution (m/z 200). In higher-energy collisional dissociation (HCD) experiments, ions were mass-selected in a quadrupole mass filter and subjected to collisions with the background gas in the HCD cell. The isolation width for all of the complexes was 0.4 m/z. Collision energies (CE) are reported in the manufacturer-specified arbitrary units.
Hydrogen/Deuterium Exchange (HDX) Experiments
The HDX experiments were conducted by diluting the solution containing [γ-CD + W6POM]2− with 50:50 (v/v) CD3OD/D2O solvent to a final concentration of ~ 10 μM. The solution was allowed to rest for 3 h. All ESI-MS and low-energy CID conditions were consistent with those described above.
18O Exchange Experiments
The 18O-enriched complex, [γ-CD + W6POM]2−, was generated using the one-pot synthesis procedure described in the previous section with H218O as a solvent. ESI-MS analysis was performed by diluting the solution containing [γ-CD + W6POM]2− in 50:50 (v/v) acetonitrile:18H2O to a final concentration of ~ 10 μM. ESI-MS and low-energy CID experiments were performed using the experimental conditions described earlier.
Results and Discussion
The CD-POM host-guest complexes examined in this study are composed of three types of CD (α, ß, and γ) and two types of POM (Keggin and Lindqvist, structures are shown in Scheme 1). They were used to generate six different host-guest complexes ([α-CD + W12POM]3−, [ß-CD + W12POM]3−, [γ-CD + W12POM]3−, [α-CD + W6POM]2−, [ß-CD + W6POM]2−, and [γ-CD + W6POM]2−. Although this study is mainly focused on W-containing POM anions (W12POM3−, W6POM2−), several experiments were performed using Mo6POM2− anion for comparison. Each of the anionic complexes was found to be an abundant species in the ESI-MS spectrum (Figure S1). Although complexes with more than one CD molecule are often produced by ESI-MS [53, 54, 59], only host-guest complexes of 1:1 stoichiometry were examined in this study.
ESI-MS of Tungsten CD-POM Complexes
Figure 1a shows a typical ESI-MS spectrum of a solution containing γ-CD and W12POM3−. Consistent with the results reported by Cao et al. [54], we observe [γ-CD + W12POM]3− and [2γ-CD + W12POM]3− as the major species in the m/z range below 2000 (Figure 1a). In addition to host-guest complexes, the spectrum contains peaks corresponding to W12POM3− and HW12POM2− anions. Similar ESI-MS spectra (Figure S1) were obtained for complexes of W12POM3− with α- and ß-CD. In this study, we examine for the first time fragmentation of [α-CD + W12POM]3− and [ß-CD + W12POM]3− anions.
CD-W6POM and CD-Mo6POM complexes were generated using the one-pot synthetic procedure reported by Cadot et al. [59]. In that study, single-crystal X-ray diffraction (XRD) analysis was used to confirm that W6O192− residing inside the γ-CD cavity has the Lindqvist-type structure [59]. A portion of the ESI-MS spectrum of the resulting solution is shown in Figure 1b. Aside from the [γ-CD + W6POM]2− anion, the spectrum contains peaks corresponding to the Cl− adduct of [γ-CD + Cl]− due to the presence of hydrochloric acid in solution, and deprotonated CD, [γ-CD − H]−.
Gas-Phase Fragmentation Pathways
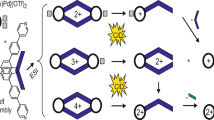
We systematically investigated the gas-phase fragmentation pathways of anionic CD-WPOM host-guest complexes using both high-energy collisional dissociation (HCD) on a QE-Orbitrap and low-energy CID on an ion trap. For both CD-W12POM and CD-W6POM complexes, we observe three major pathways shown in Scheme 2: (1) proton transfer from CD to POM, which generates peaks corresponding to protonated POM and deprotonated CD in the spectrum; (2) direct dissociation of the complex, which generates the original POM anion and undetectable neutral CD species; and (3) a series of multiple water losses from the complex. The distinctly different fragmentation patterns of the CD-POM complexes observed in HCD and low-energy CID provide insights into the kinetics of the competing fragmentation pathways, which will be discussed in detail in the following sections.
MS/MS of W12POM-CD Complexes
Fragmentation spectra of CD-W12POM complexes obtained under both HCD and low-energy CID conditions are shown in Figure 2. We observe similar fragmentation patterns for all three types of CDs under the same activation conditions and distinctly different fragmentation patterns in HCD and low-energy CID. Specifically, HCD spectra of CD-W12POM complexes (Figure 2a) contain abundant peaks corresponding to products of both the proton transfer (pathway 1 in Scheme 2) and direct dissociation (pathway 2 in Scheme 2) pathways. In contrast, pathway 1 generating HW12POM2− and [CD − H]− is dominant in low-energy CID spectra (Figure 2b). We note that the relatively low abundance of the [CD − H]− fragment ion in comparison with HW12POM2− fragment is attributed both to the lower charge state and to subsequent fragmentation of [CD − H]− (Figure S2), which reduce the observed signal intensity. Water loss (pathway 3 in Scheme 2) appears to be a minor process for CD-W12POM complexes in both HCD and low-energy CID experiments.
It is well established that slow collisional excitation in the ion trap favors low-energy dissociation pathways, while HCD experiments provide access to entropically favorable higher-energy dissociation pathways under high-energy conditions [61,62,63]. The observed dominance of direct dissociation in HCD spectra and the absence of this pathway in low-energy CID indicate that it efficiently competes with proton transfer at higher internal excitations. This observation suggests that proton transfer, which is dominant in the low-energy CID spectrum is a low-energy channel while direct dissociation of the complex is an entropically favorable process with a higher threshold energy than proton transfer.
Figure 2 also indicates that the extent of fragmentation of the complex decreases with increase in the size of the CD with the [γ-CD + W12POM]3− anion showing the smallest extent of fragmentation both in HCD and low-energy CID spectra. This may be attributed to an increase in the strength of host-guest interactions between W12POM3− and CD with increase in the cavity diameter. Crystal structure of the related [γ-CD + Mo12POM]3− complex indicates that Mo12POM3− fits deep inside the larger cavity of γ-CD (9.5 Å) and the complex is stabilized by multiple hydrogen bonding interactions with CD [38]. Similar sizes of Mo12POM3− and W12POM3− suggest that W12POM3− also fits well into the γ-CD cavity. The cavity diameters of α- and ß-CD are 5.7 Å and 7.8 Å, respectively, which are smaller in comparison with γ-CD [31]. It is reasonable to assume that fewer hydrogen bonding interactions and weaker dispersion forces between POM and smaller CDs make the corresponding complexes less stable than the complex of Keggin POM and γ-CD. Another possible explanation for the decrease in the extent of fragmentation of the complex with increase in the size of CD is that the larger γ-CD-POM complex has a larger number of vibrational degrees of freedom, which results in a decrease in the unimolecular dissociation rate and thereby reduces the extent of fragmentation [64].
The prevalence of proton transfer over other dissociation channels observed in Figure 2b may be attributed to the strong hydrogen bonding interactions between W12POM3− and CD in the complex, which facilitate proton transfer from CD to POM. A crystal structure of CD-Mo12POM complex indicates the presence of multiple hydrogen bonds between hydroxyl groups of CD and oxygen atoms of the POM anion as a major type of non-covalent interactions between POM and CD [38]. Although the energetics of proton transfer within the complex is not known, it is reasonable to assume that proton transfer from CD to W12POM3− is energetically favorable based on the substantial difference in the gas-phase basicity of the isolated W12POM3− (374–385 kcal/mol) [65] and the Brønsted acidity of CD (325–327 kcal/mol) [66]. We propose that upon collisional excitation, a proton is abstracted from the proton donor (CD) by the most basic oxygen site of the proton acceptor (W12POM3−). It also is reasonable to assume that proton abstraction is inhibited in CID of CD-POM complexes containing less basic POM anions, such as Lindqvist anion discussed in the next section. Indeed, no proton transfer was observed in CID of a host-guest complex of β-CD with another superchaotropic anion, B12F122−, with lower gas-phase basicity (322 kcal/mol) [67]. Instead, the [β-CD + B12F12]2− complex exhibited loss of a sugar unit, which was attributed to the strong binding between β-CD and B12F122− in the absence of solvent [19].
MS/MS of CD-W6POM Complexes
Gas-phase fragmentation pathways of Lindqvist CD-W6POM complexes under HCD and low-energy CID conditions are shown in Figure 3a, b, respectively. Similar to Keggin CD-W12POM complexes, we observe comparable fragmentation patterns for CD-W6POM complexes containing different CDs under the same collisional activation conditions. However, distinctly different fragmentation patterns are observed in HCD and low-energy CID experiments. In contrast with low-energy CID of CD-W12POM complexes described earlier, proton transfer from CD to W6POM2− (pathway 1 in Scheme 2) is a minor process in CID spectra of CD-W6POM complexes. Instead, HCD spectra of CD-W6POM complexes contain abundant W6O192− peaks corresponding to direct dissociation (pathway 2 in Scheme 2). The observed decrease in the extent of dissociation for different types of CDs is similar to that of CD-W12POM complexes, which has been discussed in detail in the previous section. The presence of an abundant W6POM2− fragment produced by direct dissociation of [α-CD + W6POM]2− in the low-energy CID spectrum is consistent with the relatively weak binding between the smaller α-CD cavity and W6POM2− anion.
(a) HCD (CE = 45) and (b) low-energy CID (CE = 17) spectra of Lindqvist CD-W6POM complexes. The precursor ion signal in each spectrum is marked with an asterisk. (c) Expanded m/z range of the spectra in panel (b) showing multiple water loss peaks. The red ruler on top of each spectrum shows the corresponding number of water losses
Direct dissociation of [α-CD + W6POM]2− is observed in competition with losses of multiple water molecules from the complex (pathway 3 in Scheme 2), which is the only pathway in low-energy CID spectra of [β-CD + W6POM]2− and [γ-CD + W6POM]2− anions (Figure 3b). Water losses also are observed as lower-abundance peaks in HCD spectra of CD-W6POM complexes. Figure 3c shows regions of the low-energy CID spectra of CD-W6POM complexes containing the water loss pattern observed as a distribution of peaks spaced by 9 m/z units (z = 2). For all three complexes, the most abundant fragment, [CD + W6POM − 6H2O]2−, corresponds to the loss of six water molecules. Interestingly, very low signal was obtained for a fragment ion corresponding to a single water loss and loss of three water molecules. Similar fragmentation patterns were observed in low-energy CID spectra acquired at different excitation voltages (Figure 4) indicating that the observed losses of multiple water molecules are not sequential but rather occur in parallel. Loss of seven water molecules becomes more abundant for the [γ-CD + W6POM]2− complex.
Further excitation of the fragment ions produced via loss of either single or multiple water molecules from [γ-CD + W6POM]2− in an MS3 experiment (Figure S3) results in additional losses of water molecules. The preference for the overall loss of 6 water molecules is clearly observed in the MS3 experiments. MS3 of [γ-CD + W6O19 − 6H2O]2− shows abundant losses of one and two water molecules and a minor peak corresponding to three water losses. Despite the preference for the loss of six water molecules in the MS2 experiment, an overall loss of up to ten water molecules was observed in the MS3 spectra.
The observed loss of multiple water molecules is not limited to W6POM2−. Low-energy CID spectra of CD-Mo6POM complexes shown in Figure 5 contain similar multiple water loss patterns. However, in contrast with CD-W6POM, CD-Mo6POM complexes predominantly lose five water molecules, with a single water loss almost absent in the spectra. In addition, we observed an abundant pair of fragments, HMo4O13− and [CD + Mo2O6 − H − H2O]−, as confirmed by high-resolution MS experiments. The latter is most likely produced via a loss of one water molecule from the [CD + Mo2O6 − H]− anion. Similarly, HW4O13− fragment ion is present as a minor peak in the spectrum of CD-W6POM. However, the complementary anion undergoes more substantial dehydration forming a distribution of [CD + W2O6 − H − xH2O]− (x = 1, 2, 3) fragments with two and three water losses as the dominant species (Figure S4). This observation is consistent with the greater extent of water loss from the CD-W6POM as compared to CD-Mo6POM.
Simultaneous loss of multiple water molecules is an unusual pathway in low-energy CID. We propose that this pathway is initiated by a proton transfer from the CD to the W6POM2− anion. Proton transfer is a dominant pathway in dissociation of Keggin CD-W12POM discussed earlier. However, for the Keggin CD-W12POM complex, proton transfer is followed by the separation of [CD − H]− and HW12POM2−. Comparison of the gas-phase basicities of W12POM3− (374–385 kcal/mol) [65] and W6POM2− (335–347 kcal/mol) [68] indicates that proton transfer from CD (Brønsted acidity 325–327 kcal/mol) [66] to W12POM3− is more exothermic than to W6POM2−, which could promote the separation of fragments formed through pathway 1. The absence of the HW6O19− fragment in the CID spectra of CD-W6POM complexes could be explained by assuming that the separation of [CD - H]− and HW6O19− does not efficiently compete with other dissociation pathways.
In order to rationalize simultaneous losses of multiple water molecules from the complex, we assume that HW6O19− or HMo6O19− formed through the proton transfer step decompose into several smaller fragments. We propose that POM fragments formed in this initial step subsequently attack the CD cage thereby forming covalent bonds to the host molecule and eliminating multiple water molecules. The anionic product of this dissociation pathway is a derivatized CD. MSn experiments (data not shown) demonstrate that this species undergoes subsequent loss of several water molecules followed by losses of hydrocarbon fragments. However, no losses of W-containing neutral molecules were observed in these experiments. These results confirm that POM fragments form strong covalent bonds with CD thereby yielding an unusual CD derivative.
The observed distribution of water losses from the complex may be attributed to the presence of multiple pathways for the covalent attachment of metal oxide fragments to the CD. Meanwhile, the difference between the preferred number of water losses from the CD-W6POM and CD-Mo6POM likely indicates the differences in either the type or reactivity of the fragments of HMo6POM− in comparison with HW6POM−.
The formation of the HMo4O13− and [CD + Mo2O6 − H − H2O]− pair of fragments could be rationalized using the same mechanism. However, in this case, only one covalent bond is formed between the CD and Mo2O6 fragment releasing one water molecule. In contrast, up to three water molecules are released by covalent bond formation between CD and W2O6 fragment, which indicates that W2O6 is more reactive than Mo2O6. This finding is in agreement with the more efficient water loss from CD-W6POM as compared to CD-Mo6POM. However, this pathway is only observed as a minor dissociation channel for CD-W6POM, which is consistent with the higher exothermicity of the proton transfer from the CD (Brønsted acidity 325–327 kcal/mol) [66] to Mo6POM2− (gas-phase basicity in the range of 342–355 kcal/mol) [68] as compared to W6POM2− (gas-phase basicity in the range of 335–347 kcal/mol) [68].
H/D Exchange and 18O Exchange Experiments
It is reasonable to assume that a majority of water losses involve hydroxyl groups of the CD host molecule. In order to obtain additional insights into the types of hydrogen and oxygen atoms involved in the process, we carried out H/D exchange (HDX) and 18O exchange experiments using [γ-CD + W6POM]2− as a representative species. Experimental CID spectra of the complexes were rationalized using statistical simulations.
HDX experiments were performed by diluting the stock solution of [γ-CD + W6POM]2− complex in a deuterated solvent. The extent of HDX was controlled by varying the solvent composition. Specifically, a very limited extent of HDX of the hydroxyl groups of γ-CD was observed using CD3OD as a solvent. In contrast, more extensive HDX was observed in a CD3OD/D2O mixture. γ-CD has 24 labile hydrogens of the hydroxyl groups available for HDX [69]. Each of the (α-1,4)-linked α-D-glucopyranose units on the CD structure contains one primary and two secondary hydroxyl groups on the outer rim and inner cavity of CD, respectively. The primary hydroxyl groups are flexible and often involved in intermolecular hydrogen bonding. Meanwhile, the 16 secondary hydroxyl groups on the inner CD surface are connected by intramolecular hydrogen bonds, which determine the rigidity of the CD cavity in the condensed phase [69]. More than 16 exchanges were previously observed in gas-phase HDX experiments of γ-CD indicating that the exchange is not limited to the primary hydroxyl groups [70]. The remaining hydrogen atoms in γ-CD on the C-H sites of the glucopyranose units are not exchangeable due to their lower acidity.
In the condensed phase, W6POM2− is deeply embedded into the CD cavity with the eight primary hydroxyl groups pointing away from the complex thereby optimizing the interactions with solvent molecules in solution or with other complexes in the crystalline phase [59]. However, little is known about the structure of the [γ-CD + W6POM]2− complex in the gas phase. The HDX experiments described herein were designed to probe whether only the secondary or all of the hydroxyl groups are involved in the observed water losses.
An ESI-MS spectrum of a solution of [γ-CD + W6POM]2− in 50:50 (v/v) CD3OD/D2O solvent analyzed 3 h after mixing is shown in Figure S5. We observe a series of peaks ranging from m/z = 1296 to m/z = 1320 arising from the isotopomers of [γ-CD − H]− produced by HDX. The distribution is centered at m/z = 1312 corresponding to 16 exchanged hydrogens (Figure S5). In addition, an isotopic envelope of [γ-CD + W6POM]2− is observed in a range of m/z = 1351–1365. The center of the isotopic envelope of [γ-CD + W6POM]2− after HDX is shifted to m/z = 1360 from m/z = 1352 for the original complex corresponding to an average exchange of 16 hydrogens.
We systematically examined MS2 of the isotopic peaks of [γ-CD + W6POM]2− from this distribution using an isolation window of 1 m/z. The m/z range corresponding to multiple water losses in the low-energy CID spectrum of m/z = 1365 from the high m/z side of the isotopic distribution is shown in Figure 5a. Each of the features in this region contains a distribution of isotopic peaks originating from a combined loss of H2O, HDO, and D2O due to the partial exchange of hydrogens with deuterium atoms.
The observed isotopic distributions of water losses from partially exchanged [γ-CD + W6POM]2− complexes may be rationalized using the following model. We assume that (1) the complex has a similar distribution of hydrogen and deuterium atoms as the [γ-CD − H]− detected in the ESI-MS spectrum and (2) loss of a single or multiple water molecules is a random single-step process. The latter assumption is supported by the non-sequential loss of multiple water molecules discussed earlier. First, we calculate the distribution of deuterium atoms in a selected isotopic peak of the complex. Next, we use a hypergeometric distribution to predict the number of deuterium atoms incorporated into the water molecules eliminated from this isotopic peak of the complex in CID. Finally, we compare the simulated distribution with the experimental CID data.
In the first step, we calculate all possible combinations of the isotopes of the partially exchanged γ-CD and W6POM2− that have an m/z in the ± 0.5 m/z window selected in the CID experiment. This generates a predicted distribution of hydrogen and deuterium atoms in the isolated precursor ion.
In the second step, we calculate the probability of deuterium incorporation into the products using a hypergeometric distribution by considering two scenarios: (1) when all of the 24 hydroxyl groups participate in the reaction and (2) only 16 randomly selected hydrogen or deuterium atoms can be incorporated into the products. The second scenario represents a situation when only the secondary hydroxyl groups participate in the reaction. Knowing the total number of hydrogen and deuterium atoms in the pool (N), we calculate the probability of incorporating k deuterium atoms into n water losses (n is in the range of 1–8). This probability is given by the hypergeometric distribution in Eq. (1):
where p represents the total number of available deuterium atoms in the pool. We note that Eq. (1) is also used to calculate the initial distribution of deuterium atoms in the second scenario described earlier, in which we limited the number of available hydrogen or deuterium atoms to 16. The calculated distributions of possible neutral losses from m/z = 1365 when either 24 or 16 hydroxyl groups participate in the reaction are shown in Figure 6b, c, respectively. We observe a similar bell-shaped isotopic pattern for each of the multiple water loss features in both of the simulated spectra. However, the simulated distribution in Figure 6c demonstrates a distinct shift in the center of m/z in each of the multiple water loss features with respect to the experimental isotopic distribution. The deviation of the center of m/z obtained from the simulation and the experimental value increases gradually with an increase in the number of water losses. In contrast, the experimental results are in a good agreement with the simulation, in which all of the 24 hydroxyl groups are assumed to be involved in the water loss channel (Figure 6b). We conclude that all of the 24 hydroxyl groups are involved in the observed water losses. It is unlikely that W6POM2− interacts with all the 24 hydroxyl groups prior to collisional excitation. The lack of selectivity towards specific types of hydroxyl groups in CD participating in the water loss indicates that the initial gas-phase structure of the complex may not be the determining factor in the mechanism of this fragmentation pathway.
Experimental (a) and simulated (b, c) distributions of water losses from m/z = 1365 isolated from the isotopic pattern of deuterium-exchanged [γ-CD + W6POM]2−. Panel (b) shows a simulated spectrum obtained assuming that all of the 24 –OH groups are available for the water loss channel; panel (c) shows a simulated spectrum obtained assuming that only 16 –OH groups participate in the water loss channel. The red ruler shows the corresponding number of water losses
A similar approach was used to examine fragmentation of 18O-exchanged [γ-CD + W6POM]2− anions. In these experiments, the complex was generated using the one-pot synthesis in H218O. The incorporation of 18O atoms into W6POM2− was confirmed using ESI-MS (Figure S6). We observe that the isotopic pattern of CD remains the same in H216O and H218O indicating that oxygen atoms of γ-CD do not participate in the 18O exchange. Meanwhile, the center of the isotopic distribution of W6POM2− shifts from m/z = 704 to m/z = 722 corresponding to the incorporation of 18 18O atoms into W6POM2−. Similarly, the center of the isotopic distribution of [γ-CD + W6POM]2− complex is shifted from m/z = 1352 to m/z = 1370 confirming the same extent of 18O exchange in the complex.
MS2 spectra of the isotopic peaks of [γ-CD + W6POM]2− from this distribution were acquired using an isolation window of 0.5 m/z. Figure 7a shows the low-energy CID spectrum of m/z = 1369.5 in the m/z range containing water loss fragments. The most abundant isotopic signal in each of the water loss features corresponds to losses of H218O molecules, which indicates that oxygen atoms of W6POM2− participate in this reaction.
Experimental (a) and simulated (b–d) distributions of water losses from m/z = 1369.5 isolated from the isotopic pattern of 18O-exchanged [γ-CD + W6POM]2−. (b–d) Simulated spectra obtained assuming that different number of oxygen atoms participate in the water loss channel: (b) only the oxygen atoms of W6POM2−; (c) a total of 35 oxygen atoms of both W6POM2− and 16 secondary hydroxyl groups of γ-CD; (d) a total of 43 oxygen atoms of W6POM2− and all 24 hydroxyl groups of γ-CD. The red ruler shows the corresponding number of water losses
We rationalize the observed isotopic distribution of multiple water losses from 18O-exchanged [γ-CD + W6POM]2− using the same statistical modeling described earlier. Three scenarios are considered in these simulations: (1) only 19 oxygen atoms of W6POM2− participate in the reaction and (2) 35 oxygen atoms of both W6POM2− and 16 primary hydroxyl groups of γ-CD can be incorporated into the reaction product; (3) 43 oxygen atoms of both W6POM2− and all 24 hydroxyl groups of γ-CD are involved in the process.
The calculated distributions of possible water losses from m/z = 1369.5 in the above-described scenarios are shown in Figure 7b–d, respectively. The simulated water loss spectrum shown in Figure 7b is in good agreement with the experimental result, showing substantial incorporation of 18O into the neutral products. In contrast, simulated water loss spectra (Figure 7c, d) deviate from the experimental spectrum in both the center of m/z and shape of the isotopic distribution. These results indicate that oxygen atoms of W6POM2− are predominantly incorporated into the water molecules produced in this reaction.
Based on the results of HDX and 18O exchange experiments, we conclude that the multiple water losses in the low-energy CID spectra of CD-M6POM (M = Mo, W) complexes are composed of H atoms from the hydroxyl groups of CD and O atoms of M6POM2−. The observed fragmentation pathway may be rationalized assuming that the initial proton transfer from CD to M6POM2− results in dissociation of HM6POM− producing reactive metal oxide species. These species subsequently undergo multiple condensation reactions with the hydroxyl groups of CD eliminating water molecules. Dehydration reactions between alcohols and multinuclear transition metal oxides in the gas phase resulting in a loss of one water molecule have been previously reported [71,72,73]. The reaction mechanism involves deprotonation of an alcohol by a metal oxide followed by alkoxo ligand substitution resulting in elimination of a water molecule [74]. Isotopic labeling experiments have confirmed that the oxygen atom in the resulting alkylated metal oxide product originates from the alcohol, which is consistent with our results [71]. It has been demonstrated that W-containing metal oxides are more reactive towards alcohol dehydration than their Mo-containing analogs [71]. The enhanced reactivity of W-containing metal oxides may be used to rationalize the more efficient water loss from CD-W6POM as compared to CD-Mo6POM observed in this study [75].
Conclusion
We report a systematic investigation of the gas-phase fragmentation pathways of host-guest complexes of CDs (α-, ß-, and γ-CD) and small POMs (Keggin W12POM3−, Lindqvist W6POM2− and Mo6POM2−). We observe three major fragmentation pathways under high-energy and low-energy CID conditions: proton transfer from CD to POM, direct dissociation, and a series of multiple water losses from the complex. The major dissociation channel is determined by the cavity diameter of the CD, the size of the complex, and gas-phase basicity and structural stability of the POM anion. In particular, proton transfer from CD to POM followed by the separation of [CD − H]− and HW12POM2− in CD-W12POM complexes is a major channel observed in the low-energy CID spectra and attributed to the higher gas-phase basicity and stability of the W12POM3− anion. In contrast, direct dissociation is more pronounced in HCD indicating that this is an entropically favorable pathway associated with a higher threshold energy than the proton transfer channel. An unusual fragmentation pathway observed in low-energy CID experiments of CD-W6POM and CD-Mo6POM complexes corresponds to multiple water losses from the complex. These water losses are found to occur in parallel as competing channels and are attributed to covalent coupling of the fragments of Lindqvist POM to CD accompanied by elimination of multiple water molecules upon collisional excitation. This covalent bond formation efficiently competes with other dissociation channels of the CD-W6POM and CD-Mo6POM host-guest complexes in the gas phase. The results presented in this study demonstrate the unique proton-mediated reactivity of transition metal oxide clusters for covalent modification of the macrocyclic host molecule via supramolecular gas-phase ion chemistry. Future experiments will examine the properties of the derivatized CD produced in the gas phase by depositing them onto surfaces for preparing unconventional condensed-phase materials [76,77,78].
References
Lehn, J.M.: Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed. Eng. 27, 89–112 (1988)
Steed, J.W., Turner, D.R., Wallace, K.: Core Concepts in Supramolecular Chemistry and Nanochemistry. Wiley, Hoboken (2007)
Vögtle, F., Weber, E.: Host Guest Complex Chemistry Macrocycles: Synthesis, Structures, Applications. Springer Science & Business Media, Berlin (2012)
Brown, C.J., Toste, F.D., Bergman, R.G., Raymond, K.N.: Supramolecular catalysis in metal–ligand cluster hosts. Chem. Rev. 115, 3012–3035 (2015)
Jiao, Y., Tang, B., Zhang, Y., Xu, J.F., Wang, Z., Zhang, X.: Highly efficient supramolecular catalysis by endowing the reaction intermediate with adaptive reactivity. Angew. Chem. 130, 6185–6189 (2018)
Zhang, D.S., Gao, Q., Chang, Z., Liu, X.T., Zhao, B., Xuan, Z.H., Hu, T.L., Zhang, Y.H., Zhu, J., Bu, X.H.: Rational construction of highly tunable donor–acceptor materials based on a crystalline host–guest platform. Adv. Mater. 30, 1804715 (2018)
Qu, D.-H., Wang, Q.-C., Zhang, Q.-W., Ma, X., Tian, H.: Photoresponsive host–guest functional systems. Chem. Rev. 115, 7543–7588 (2015)
Mao, D., Liang, Y., Liu, Y., Zhou, X., Ma, J., Jiang, B., Liu, J., Ma, D.: Acid-labile acyclic cucurbit [n] uril molecular containers for controlled release. Angew. Chem. Int. Ed. 56, 12614–12618 (2017)
Ma, X., Zhao, Y.: Biomedical applications of supramolecular systems based on host–guest interactions. Chem. Rev. 115, 7794–7839 (2014)
Chandler, D.: Interfaces and the driving force of hydrophobic assembly. Nature. 437, 640 (2005)
Schneider, H.J.: Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed. 48, 3924–3977 (2009)
Assaf, K.I., Nau, W.M.: The chaotropic effect as an assembly motif in chemistry. Angew. Chem. Int. Ed. 57, 13968–13981 (2018)
Kaabel, S., Adamson, J., Topić, F., Kiesilä, A., Kalenius, E., Öeren, M., Reimund, M., Prigorchenko, E., Lõokene, A., Reich, H.J.: Chiral hemicucurbit [8] uril as an anion receptor: selectivity to size, shape and charge distribution. Chem. Sci. 8, 2184–2190 (2017)
Gibb, C.L., Gibb, B.C.: Anion binding to hydrophobic concavity is central to the salting-in effects of Hofmeister chaotropes. J. Am. Chem. Soc. 133, 7344–7347 (2011)
Assaf, K.I., Ural, M.S., Pan, F., Georgiev, T., Simova, S., Rissanen, K., Gabel, D., Nau, W.M.: Water structure recovery in chaotropic anion recognition: high-affinity binding of dodecaborate clusters to γ-cyclodextrin. Angew. Chem. Int. Ed. 54, 6852–6856 (2015)
Sullivan, M.R., Yao, W., Tang, D., Ashbaugh, H.S., Gibb, B.C.: The thermodynamics of anion complexation to nonpolar pockets. J. Phys. Chem. B. 122, 1702–1713 (2018)
Assaf, K.I., Gabel, D., Zimmermann, W., Nau, W.M.: High-affinity host–guest chemistry of large-ring cyclodextrins. Org. Biomol. Chem. 14, 7702–7706 (2016)
Assaf, K.I., Hennig, A., Peng, S., Guo, D.-S., Gabel, D., Nau, W.M.: Hierarchical host–guest assemblies formed on dodecaborate-coated gold nanoparticles. Chem. Commun. 53, 4616–4619 (2017)
Warneke, J., Jenne, C., Bernarding, J., Azov, V.A., Plaumann, M.: Evidence for an intrinsic binding force between dodecaborate dianions and receptors with hydrophobic binding pockets. Chem. Commun. 52, 6300–6303 (2016)
Buchecker, T., Schmid, P., Renaudineau, S., Diat, O., Proust, A., Pfitzner, A., Bauduin, P.: Polyoxometalates in the Hofmeister series. Chem. Commun. 54, 1833–1836 (2018)
Naskar, B., Diat, O., Nardello-Rataj, V.R., Bauduin, P.: Nanometer-size polyoxometalate anions adsorb strongly on neutral soft surfaces. J. Phys. Chem. C. 119, 20985–20992 (2015)
Pang, H.-j., Peng, J., Zhang, C.-j., Li, Y.-g., Zhang, P.-p., Ma, H.-y., Su, Z.-m.: A polyoxometalate-encapsulated 3D porous metal–organic pseudo-rotaxane framework. Chem. Commun. 46, 5097–5099 (2010)
Cao, H.-L., Cai, F.-Y., Huang, H.-B., Karadeniz, B., Lü, J.: Polyoxometalate-cucurbituril molecular solid as photocatalyst for dye degradation under visible light. Inorg. Chem. Commun. 84, 164–167 (2017)
Fang, X., Kögerler, P., Isaacs, L., Uchida, S., Mizuno, N.: Cucurbit [n] uril–polyoxoanion hybrids. J. Am. Chem. Soc. 131, 432–433 (2008)
Goel, T., Barooah, N., Mallia, M.B., Bhasikuttan, A.C., Mohanty, J.: Recognition-mediated cucurbit [7] uril-heptamolybdate hybrid material: a facile supramolecular strategy for 99m Tc separation. Chem. Commun. 52, 7306–7309 (2016)
Ishii, Y., Takenaka, Y., Konishi, K.: Porous organic–inorganic assemblies constructed from Keggin polyoxometalate anions and calix [4] arene–Na+ complexes: structures and guest-sorption profiles. Angew. Chem. 116, 2756–2759 (2004)
Tian, A., Lin, X., Ying, J., Zhang, J., Lin, H., Liu, G., Zhao, D., Li, N., Wang, X.: Self-assembly of a molecular crown as a structural analogue of calix [4] arene to modify Keggin anions. Dalton Trans. 42, 9809–9812 (2013)
Su, P., Prabhakaran, V., Johnson, G.E., Laskin, J.: In situ infrared spectroelectrochemistry for understanding structural transformations of precisely defined ions at electrochemical interfaces. Anal. Chem. 90, 10935–10942 (2018)
Prabhakaran, V., Lang, Z., Clotet, A., Poblet, J.M., Johnson, G.E., Laskin, J.: Controlling the activity and stability of electrochemical interfaces using atom-by-atom metal substitution of redox species. ACS Nano. 13, 458–466 (2018)
Martin-Sabi, M., Soriano-López, J., Winter, R.S., Chen, J.-J., Vilà-Nadal, L., Long, D.-L., Galán-Mascarós, J.R., Cronin, L.: Redox tuning the Weakley-type polyoxometalate archetype for the oxygen evolution reaction. Nat. Catal. 1, 208 (2018)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Montes-García, V., Pérez-Juste, J., Pastoriza-Santos, I., Liz-Marzán, L.M.: Metal nanoparticles and supramolecular macrocycles: a tale of synergy. Chem. Eur. J. 20, 10874–10883 (2014)
Engel, S., Möller, N., Ravoo, B.J.: Stimulus-responsive assembly of nanoparticles using host–guest interactions of cyclodextrins. Chem. Eur. J. 24, 4741–4748 (2018)
Zhang, P., Meijide Suárez, J., Driant, T., Derat, E., Zhang, Y., Ménand, M., Roland, S., Sollogoub, M.: Cyclodextrin cavity-induced mechanistic switch in copper-catalyzed hydroboration. Angew. Chem. 129, 10961–10965 (2017)
Li, H., Meng, B., Chai, S.-H., Liu, H., Dai, S.: Hyper-crosslinked β-cyclodextrin porous polymer: an adsorption-facilitated molecular catalyst support for transformation of water-soluble aromatic molecules. Chem. Sci. 7, 905–909 (2016)
Jing, J., Szarpak-Jankowska, A., Guillot, R., Pignot-Paintrand, I., Picart, C., Auzély-Velty, R.: Cyclodextrin/paclitaxel complex in biodegradable capsules for breast cancer treatment. Chem. Mater. 25, 3867–3873 (2013)
Zhou, J., Yu, G., Huang, F.: Supramolecular chemotherapy based on host–guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 46, 7021–7053 (2017)
Wu, Y., Shi, R., Wu, Y.-L., Holcroft, J.M., Liu, Z., Frasconi, M., Wasielewski, M.R., Li, H., Stoddart, J.F.: Complexation of polyoxometalates with cyclodextrins. J. Am. Chem. Soc. 137, 4111–4118 (2015)
Moussawi, M.A., Leclerc-Laronze, N., Floquet, S., Abramov, P.A., Sokolov, M.N., Cordier, S., Ponchel, A., Monflier, E., Bricout, H., Landy, D.: Polyoxometalate, cationic cluster, and γ-cyclodextrin: from primary interactions to supramolecular hybrid materials. J. Am. Chem. Soc. 139, 12793–12803 (2017)
Moussawi, M.A., Haouas, M., Floquet, S., Shepard, W.E., Abramov, P.A., Sokolov, M.N., Fedin, V.P., Cordier, S., Ponchel, A., Monflier, E.: Nonconventional three-component hierarchical host–guest assembly based on Mo-blue ring-shaped giant anion, γ-cyclodextrin, and Dawson-type polyoxometalate. J. Am. Chem. Soc. 139, 14376–14379 (2017)
Zhang, B., Guan, W., Yin, F., Wang, J., Li, B., Wu, L.: Induced chirality and reversal of phosphomolybdate cluster via modulating its interaction with cyclodextrins. Dalton Trans. 47, 1388–1392 (2018)
Yang, P., Zhao, W., Shkurenko, A., Belmabkhout, Y., Eddaoudi, M., Dong, X., Alshareef, H.N., Khashab, N.M.: Polyoxometalate–cyclodextrin metal–organic frameworks: from tunable structure to customized storage functionality. J. Am. Chem. Soc. 141, 1847-1851 (2019)
Stuckart, M., Izarova, N.V., van Leusen, J., Smekhova, A., Schmitz-Antoniak, C., Bamberger, H., van Slageren, J., Santiago-Schübel, B., Kögerler, P.: Host–guest-induced environment tuning of 3d ions in a polyoxopalladate matrix. Chem. Eur. J. 24, 17767–17778 (2018)
Gabelica, V., Galic, N., De Pauw, E.: On the specificity of cyclodextrin complexes detected by electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 946–953 (2002)
Schalley, C.A.: Supramolecular chemistry goes gas phase: the mass spectrometric examination of noncovalent interactions in host–guest chemistry and molecular recognition. Int. J. Mass Spectrom. 194, 11–39 (2000)
Weimann, D.P., Schalley, C.A.: Host–guest chemistry of self-assembling supramolecular capsules in the gas phase. Supramol. Chem. 20, 117–128 (2008)
Qi, Z., Heinrich, T., Moorthy, S., Schalley, C.A.: Gas-phase chemistry of molecular containers. Chem. Soc. Rev. 44, 515–531 (2015)
Lebrilla, C.B.: The gas-phase chemistry of cyclodextrin inclusion complexes. Acc. Chem. Res. 34, 653–661 (2001)
Penn, S.G., He, F., Lebrilla, C.B.: Peptides complexed to cyclodextrin fragment rather than dissociate when subjected to blackbody infrared radiation. J. Phys. Chem. B. 102, 9119–9126 (1998)
Vrkic, A.K., O’Hair, R.A., Lebrilla, C.B.: Unusual covalent bond-breaking reactions of β-cyclodextrin inclusion complexes of nucleobases/nucleosides and related guest molecules. Eur. J. Mass Spectrom. 9, 563–577 (2003)
Ma, X., Wei, Z., Xiong, X., Jiang, Y., He, J., Zhang, S., Fang, X., Zhang, X.: Gas-phase fragmentation of host–guest complexes between β-cyclodextrin and small molecules. Talanta. 93, 252–256 (2012)
Bakhtiar, R., Kaifer, A.: Mass spectrometry studies on the complexation of several organometallic complexes by α-and β-cyclodextrins. Rapid Commun. Mass Spectrom. 12, 111–114 (1998)
Fan, Y., Lu, S., Cao, J.: A novel inorganic-organic hybrid complex between polyoxometalate and cyclodextrin: synthesis, structure and catalytic activity. Int. J. Mass Spectrom. 435, 163–167 (2019)
Fan, Y., Zhang, Y., Jia, Q., Cao, J., Wu, W.: The stabilizing role of cyclodextrins on Keggin phosphotungstic acid by complexation unveiled by electrospray mass spectrometry. Mass Spectrom. Lett. 6, 13–16 (2015)
Julian, R.R., May, J.A., Stoltz, B.M., Beauchamp, J.: Molecular mousetraps: gas-phase studies of the covalent coupling of noncovalent complexes initiated by reactive carbenes formed by controlled activation of diazo precursors. Angew. Chem. Int. Ed. 42, 1012–1015 (2003)
Julian, R.R., May, J.A., Stoltz, B.M., Beauchamp, J.: Biomimetic approaches to gas phase peptide chemistry: combining selective binding motifs with reactive carbene precursors to form molecular mousetraps. Int. J. Mass Spectrom. 228, 851–864 (2003)
Schaefer, M.: Supramolecular crown ether adducts in the gas phase: from molecular recognition of amines to the covalent coupling of host/guest molecules. Angew. Chem. Int. Ed. 42, 1896–1899 (2003)
Lee, T.-C., Kalenius, E., Lazar, A.I., Assaf, K.I., Kuhnert, N., Grün, C.H., Jänis, J., Scherman, O.A., Nau, W.M.: Chemistry inside molecular containers in the gas phase. Nat. Chem. 5, 376 (2013)
Falaise, C., Moussawi, M.A., Floquet, S., Abramov, P.A., Sokolov, M.N., Haouas, M., Cadot, E.: Probing dynamic library of metal-oxo building blocks with γ-cyclodextrin. J. Am. Chem. Soc. 140, 11198–11201 (2018)
Klemperer, W.G.: Tetrabutylammonium isopolyoxometalates. Inorg. Synth. 27, 74–85 (1990)
Fetterolf, D., Yost, R.-A.: Energy-resolved collision-induced dissociation in tandem mass spectrometry. Int. J. Mass Spectrom. Ion Phys. 44, 37–50 (1982)
Wells, J.M., McLuckey, S.A.: Collision-induced dissociation (CID) of peptides and proteins. Methods Enzymol. 402, 148–185 (2005)
Bayat, P., Gatineau, D., Lesage, D., Marhabaie, S., Martinez, A., Cole, R.B.: Investigation of activation energies for dissociation of host-guest complexes in the gas phase using low-energy collision induced dissociation. J. Mass Spectrom. 54, 437–448 (2019)
Vinokur, N., Ryzhov, V.: Using collision-induced dissociation with corrections for the ion number of degrees of freedom for quick comparisons of relative bonding strength. J. Mass Spectrom. 39, 1268–1274 (2004)
Ganapathy, S., Fournier, M., Paul, J., Delevoye, L., Guelton, M., Amoureux, J.: Location of protons in anhydrous Keggin heteropolyacids H3PMo12O40 and H3PW12O40 by 1H {31P}/31P {1H} REDOR NMR and DFT quantum chemical calculations. J. Am. Chem. Soc. 124, 7821–7828 (2002)
Li, Z., Couzijn, E.P., Zhang, X.: Intrinsic properties of α-cyclodextrin complexes with benzoate derivatives in the gas phase: an experimental and theoretical study. J. Phys. Chem. B. 116, 943–950 (2012)
Jenne, C., Keßler, M., Warneke, J.: Protic anions [H(B12X12)]−(X= F, Cl, Br, I) that act as Brønsted acids in the gas phase. Chem. Eur. J. 21, 5887–5891 (2015)
Li, S., Dixon, D.A.: Molecular and electronic structures, Brönsted basicities, and Lewis acidities of group VIB transition metal oxide clusters. J. Phys. Chem. A. 110, 6231–6244 (2006)
Price, N.P.: Oligosaccharide structures studied by hydrogen–deuterium exchange and MALDI-TOF mass spectrometry. Anal. Chem. 78, 5302–5308 (2006)
Kellersberger, K.A., Dejsupa, C., Liang, Y., Pope, R.M., Dearden, D.V.: Gas phase studies of ammonium–cyclodextrin compounds using Fourier transform ion cyclotron resonance. Int. J. Mass Spectrom. 193, 181–195 (1999)
Waters, T., O’Hair, R.A., Wedd, A.G.: Catalytic gas phase oxidation of methanol to formaldehyde. J. Am. Chem. Soc. 125, 3384–3396 (2003)
Llusar, R., Sorribes, I., Vicent, C.: Electrospray ionization based methods for the generation of polynuclear oxo-and hydroxo group 6 anions in the gas-phase. J. Clust. Sci. 20, 177–192 (2009)
Harris, B.L., Waters, T., Khairallah, G.N., O’Hair, R.A.: Gas-phase reactions of [VO2(OH)2]− and [V2O5(OH)]− with methanol: experiment and theory. J. Phys. Chem. A. 117, 1124–1135 (2012)
Jackson, P., Fisher, K.J., Willett, G.D.: The catalytic activation of primary alcohols on niobium oxide surfaces unraveled: the gas phase reactions of NbxOy − clusters with methanol and ethanol. Chem. Phys. 262, 179–187 (2000)
Waters, T., O'Hair, R.A., Wedd, A.G.: Gas-phase reactivity of heterobinuclear oxometalate anions [CrMoO6(OR)]−,[CrWO6(OR)]−, and [MoWO6(OR)]− (R= H, nBu). Inorg. Chem. 44, 3356–3366 (2005)
Laskin, J., Johnson, G.E., Warneke, J., Prabhakaran, V.: From isolated ions to multilayer functional materials using ion soft-landing. Angew. Chem. Int. Ed. 57, 16270–16284 (2018)
Warneke, J., McBriarty, M.E., Riechers, S.L., China, S., Engelhard, M.H., Apra, E., Young, R.P., Washton, N.M., Jenne, C., Johnson, G.E., Laskin, J.: Self-organizing layers from complex molecular anions. Nat. Commun. 9, 1889 (2018)
Su, P., Hu, H., Warneke, J., Belov, M.E., Anderson, G.A., Laskin, J.: Design and performance of a dual-polarity instrument for ion soft landing. Anal. Chem. 91, 5904–5912 (2019)
Acknowledgements
The authors thank Dr. Youyun Zhou (Southern University of Science and Technology, China) for the valuable discussions. The authors also thank Jessica Townsend for the help in the graphic design. J. W. acknowledges support from a Feodor Lynen Fellowship of the Alexander von Humboldt Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Andrew J. Smith was an undergraduate student researcher.
Electronic Supplementary Material
ESM 1
(DOCX 199 kb)
Rights and permissions
About this article
Cite this article
Su, P., Smith, A.J., Warneke, J. et al. Gas-Phase Fragmentation of Host-Guest Complexes of Cyclodextrins and Polyoxometalates. J. Am. Soc. Mass Spectrom. 30, 1934–1945 (2019). https://doi.org/10.1007/s13361-019-02266-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-019-02266-8