Abstract
The 14- and 16-membered macrolide antibiotics are an important structural class. Ubiquitously produced by a number of bacterial strains, namely actinomycetes, purification and structure elucidation of the wide array of analogs is challenging, both for discovery efforts and methodologies to monitor for byproducts, metabolites, and contaminants. Collision-induced dissociation mass spectrometry offers an attractive solution, enabling characterization of mixtures, and providing a wealth of structural information. However, interpretation of these spectra can be difficult. We present a study of 14- and 16-membered macrolide antibiotics, including MSn analysis for unprecedented depth of coverage, and complimentary analysis with D2O and H218O labeling to elucidate fragmentation mechanisms. These analyses contrast the behaviors of varying classes of macrolides and highlight how analogues can be identified in relation to similar structures, which will provide utility for future studies of novel macrolides, as well as impurities, metabolites, and degradation products of pharmaceuticals.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 14- and 16-membered macrolide antibiotics are an important class of compounds in both human and veterinary medicine, with two appearing on the World Health Organization’s list of essential medicines, and many more in use clinically [1]. These compounds are biosynthesized by polyketide synthetases (PKS). These gene clusters generally consist of a number of PKS enzymes, each responsible for sequentially adding one additional acyl residue to the growing macrolide core in an assembly line fashion. Generally, the initial module is loaded with a starter group, often acetyl-CoA, each subsequent module adds a malonyl Co-A residue, which can be tailored by varying levels of dehydration and reductions so that each residue added may contain a ketone, hydroxyl, no functionalization, or a double bond, among other less common modifications. The final PKS enzyme cyclizes the chain into the polyketide core and saccharide moieties may be attached. Thus, while maintaining a clear relation among the macrolides, there is incredible potential for structural and functional diversity owing to the combinatorial nature of their synthesis.
Members of this family derive their antimicrobial activity by inhibiting the bacterial ribosome, specifically chain elongation of the growing polypeptide [2]. While some archetypal macrolides such as erythromycin and tylosin have been in use for decades, interest remains in the discovery of novel members of this class, as variations can provide improvements on the pharmacokinetic properties of the molecules, including activity, specificity, bioavailability, toxicity, and development of resistance [3,4,5,6]. There is no richer source for compound discovery than bacterial metabolites. It is estimated that > 50% of commonly investigated bacteria have the polyketide synthetase (PKS) genes capable of synthesizing macrolides, the majority of which are as of yet uncharacterized [7, 8]. Mass spectrometry is increasingly becoming the tool of choice for natural product discovery approaches, as it offers greater speed and throughput than traditional bioactivity-guided isolation strategies [9]. Additionally, structural characterization by mass spectrometry has the decided advantage of analyzing multiple analogs directly from a crude extract without need for purification, allowing faster and more complete investigation than traditional approaches that require pure compound [10].
To gain structural information on an unknown over the molecular formula afforded by an accurate mass measurement, collision-induced dissociation (CID) is often employed to fragment the molecule and interrogate its constitutive parts. In CID, ions are collided with a neutral buffer gas until enough internal energy is acquired to break one or more bonds, resulting in a neutral loss and a product ion. Product ions can be mass-selected again and fragmented to result in additional product ions in MSn. These studies provide a wealth of information on the interconnectivity and identity of the moieties present in a molecule, enabling partial or complete elucidation of the precursor structure. Toward an application in the elucidation of unknown structures, we have developed a generalizable set of experimental conditions to collect the greatest breadth and depth of fragmentation information from a complex mixture without the need for any prior knowledge of compound structure. We demonstrate this approach with an array of structurally distinct macrolide structures, gleaning trends among macrolide families, and highlighting how this type of analysis can help identify related structures to aid in future novel macrolide identification or that of unknown contaminants in pharmaceutical formulations.
Mass spectrometry analysis is routinely used in the study of macrolide antibiotics; for example, in the identification of glycosylated natural products [11] or for identification of previously known compounds, where fragmentation spectra of species under study are matched to a database of known analogs in order to confirm their identity. These “fingerprinting” studies are incredibly important for analysis of common contaminant ions in pharmaceutical formulations or in excluding known compounds from discovery efforts, for example. When database matching is insufficient because of ambiguity in these closely related molecules, or a molecule has no matches and is a putative unknown, researchers must turn to more detailed analysis of the structural information obtained through fragmentation.
There are myriad works studying the fragmentation behavior of the individual macrolide families under study here: erythromycin [12,13,14], clarithromycin [15], roxithromycin [16, 17], spiramycin [18], kitasamycin [19, 20], (leucomycin) tylosin [21], oleandomycin [22], and josamycin [23]. Most of these works are focused on exhaustively characterizing all of the observed fragments for the particular species under study, to make positive identification of components and facilitate quantitative studies of these molecules in relevant systems, such as agricultural runoff [24], tissues, milk, and eggs [25]. While these studies offer great utility for analyses seeking to investigate known structures, missing is an analysis focused on generalizable trends seen across species that would enable a researcher with an unknown macrolide to deduce the structure. What is needed for such an approach is to collect a breadth of structural information and to develop an understanding of what drives fragmentation at certain positions within known molecules in order to apply these guidelines to the interpretation of novel structures. We have selected eight families of macrolides for the current study shown in Scheme 1. Included in these studies are erythromycins (SI Figures 1–7), clarithromycins (SI Figures 8–12), roxithromycins (SI Figures 13–19), spiramycins (SI Figures 20–26), kitasamycins (SI Figures 27–43), josamycins (SI Figures 44–52), oleandomycins (SI Figures 53–55), and tylosins (SI Figures 56–60). They were chosen based on a combination of their clinical importance and to represent a range of possible structural variation within the macrolide class of compounds. The canonical component of each class is illustrated in Scheme 1, though most contain additional analogs with slight structural variations or isomers that will be included in our analysis of these compounds in the remainder of this text.
We developed our mass spectrometry methods to be untargeted, and thus not require any prior knowledge of the molecules under study. This is especially important in the study of macrolides, as virtually all commercially available “pure” compounds contain a number of analogs. The ability to characterize all these unexpected products from either standards or a bacterial extract can greatly improve the throughput of the structure elucidation process. To that end, we have developed a series of mass spectrometry experiments designed to maximize the information gained without needing to optimize for a certain compound family. As access to mass spectrometry facilities is often available at a premium, it was our goal that this collection of experiments could be run once and contain all the information a researcher may need to elucidate a structure. Relatedly, we have chosen to focus on CID fragmentation, which is most widely available to researchers, but additional utility could certainly be gained by leveraging the orthogonal fragmentation information gleaned from higher energy techniques. Analysis of natural products, including macrolides, by CID fragmentation is complicated by the variety in bond energies present in most molecules. This often results in relatively few fragments of high intensity, which leaves large portions of the molecule uninterrogated, in contrast to the information-rich spectra afforded by the fragmentation of molecules with many bonds of comparable energy like peptides. Multistage fragmentation, MSn analysis, is therefore necessary to sequentially interrogate isolated fragments and obtain structural information for the entirety of the molecule [10].
An additional challenge in the analysis of macrolides is the difficulty of assigning the origin of many of the isolated species. Ubiquitous water losses, and the propensity of the molecule to fragment into portions with generic CnHnOn ions, which could arise from multiple sections of the precursor structure, limit the utility of even the most exhaustive structural characterization by MSn as presented here. To alleviate these challenges and assign more rigorous putative fragmentation mechanisms, we have also performed analysis with D2O and H18O labeling of the molecules. This method has proven to be very effective in macrolide structure analysis. Though it is often reported that the amine-containing sugar on these structures is the sole charge carrier [26], and that the proton is effectively sequestered, Kearney et al. used these labeling techniques to prove that the first water loss resulting from erythromycin A is not traditional water loss. Instead, it results from a charge mediated process and loss of the ketone oxygen, a hydrogen from a neighboring hydroxyl, and the proton yielding a cation as the observed fragment (Scheme 2) [27]. Without isotopically labeling the molecule, this unique fragmentation mechanism is indistinguishable from a traditional water loss, which may arise from the loss of a hydroxyl moiety and creation of a double bond in the molecule. By incubating erythromycin A in D2O, the exchangeable hydrogens, namely those on hydroxyl groups, as well as the proton are replaced by deuterium, and enable interrogation of losses. Incubation in H218O similarly exchanges only with the ketone oxygen, through a diol-intermediate, allowing assignment of the origin of some of the water losses. In combination, these labeling approaches yield incredibly valuable complimentary information to the MSn studies. We have expanded this analysis to our suite of macrolides and extended the interpretation to interrogate the mechanisms of saccharide loss, as well as elucidate more fully the fragments observed from the macrolide core.
The goal of our characterization is to glean the greatest amount of structural information possible from the fragmentation of these molecules. To that end, we will demonstrate how these experiments can yield evidence about the sugar moieties and their positions, macrolide core structure, identity of isobaric neutral losses, and localization of differences between analogs. While characterization by mass spectrometry alone will likely not be sufficient in the study of a novel molecule, these studies can be performed much faster on less material, and total or even partial characterization before any purification can facilitate intelligent selection of lead molecules for further studies within natural products discovery platforms.
Experimental
Chemicals and Reagents
Erythromycin (> 95% purity) and roxithromycin (> 90% purity) were obtained from Sigma-Aldrich. Clarithromycin (> 98% purity) and leucomycin/kitasamycin (> 48% purity) were obtained from TCI. Tylan (no purity information available) was obtained as the veterinary antibiotic Tylan from Elanco. Spiramycin (90% purity) was obtained from Alfa Aesar. Josamycin (> 98% purity) was obtained from Chemodex. Oleandomycin (> 95%) was obtained from Chem-Cruz. Isotopically labeled D2O (99.96% D) and H218O (97% 18O) were obtained from Cambridge Isotope Laboratories. All species were analyzed without any additional purification.
Instrumentation and Analysis
Samples were analyzed on a nano-ESI(+)-C18 chromatography system coupled to an Orbitrap Elite (Thermo) capable of accurate mass and MSn analysis. Each sample was analyzed by four separate injections of 2 μL of 0.1 mg/ml solutions of each macrolide source. All analyses were performed over an identical 20-min HPLC gradient, though for more complete characterization of minor components, a more specialized or longer gradient would be required. The first run (FTMS) collects MS1 spectra using the FTMS for accurate mass characterization of the precursor ions. The second run (IT-FTMS) collects MS1 spectra in the ion trap at nominal mass accuracy, and the top 5 most intense ions MS2 spectra in the FTMS to obtain accurate mass measurements of the fragments. The third run (IT-t4-t4) collects MS1–3 spectra in the ion trap selecting the top 4 intense ions/product ions at each stage. The final run (IT-t2-t2-t2) collects MS1–4 spectra in the ion trap of the top 2 most intense ions/product ions at each stage. These experiments together yield accurate mass measurements, as well as tremendous breadth and depth of fragmentation information to MS4 levels. These analyses are performed in an untargeted manner, and generally result in total characterization of any potential analogs in a sample for additional interrogation along with the expected structures.
Full details of the source and fragmentation conditions are found in the Supporting Information. We found it paramount to perform these studies in a “depth-first” manner, whereby spectra are collected for a single precursor to the maximum level (e.g., MS4) before interrogating the next precursor, as opposed to breadth first which would collect all MS2 scans, then all MS3 scans, etc. Although sufficient signal could be obtained to allow collection of MS5 spectra in some cases, the exponential increase in the number of scans decreased our overall coverage of analogs. Additionally, the products obtained after four fragmentations of these molecules were often extremely small and their utility in structure elucidation did not overcome the loss of coverage afforded by the increased scan times.
D2O and H2 18O Labeling
Samples were dissolved in D2O at a concentration of 0.1 mg/ml, incubated at room temperature for 10 min, and infused directly at a flow of 100 μl/h. H218O labeling was accomplished by incubating each sample at room temperature in H218O for 1 h before infusion. Longer incubations and elevated temperatures were explored, but no additional exchange was achieved, and increasing time and temperature lead to greater observation of hydrolysis products. Analysis was performed on an Agilent 6540 QTOF. Ions were isolated with a narrow mass selection window of ~ 1.7 Da and collisionally activated at a variety of energies from 15 to 35 V.
Results and Discussion
Saccharide Loss Patterns
The most ubiquitous and often reported fragmentation patterns obtained for macrolide structures are the sequential losses of the terminal saccharide units from the polyketide core. As the weakest bonds are nearly always the first point of fragmentation of a molecule, it is unsurprising that the early observed fragments of the macrolides result from breaking of the glycosidic linkage. These patterns are often used for the “fingerprinting” of macrolide structures to identify previously known structures. To describe the fragmentation behavior more generally across this family of molecules, we have used a nomenclature based on the most commonly observed product ions. In most cases presented here, the macrolides have two saccharide attachment points to the core with either monosaccharides, as is the case for erythromycin, or a single disaccharide, as evidenced by tylosin A. Furthermore, all of these species have at least one nitrogen-containing sugar, which provides an attractive protonation site. Thus, the observed fragmentation pattern is generally as follows: the most abundant peak is often due to the loss of the terminal moiety from the disaccharide if present (designated F1, Scheme 1). From that product ion, the remaining non-nitrogen-containing sugar is lost (designated F2, Scheme 1). After these moieties are lost, the fragmentation continues in a compound-dependent fashion, either with the loss of the final saccharide unit or by fragmentation of the macrolide core. We have tabulated these fragmentation events for the structures under study in Table 1. The comparison of these fragments offers little information about the macrolide core but make modifications to the saccharide moieties between species simple to identify. For example, erythromycin C differs from erythromycin A (1) by a hydroxyl instead of methoxy on the cladinose sugar. The difference of 14 AMU between the 2 neutral losses of F2 indicate the CH2 group as the difference in structure and isolate the modification to the cladinose unit.
Interpretation of Saccharide and Water Losses in Isotopically Labeled Structures
The perceived proton affinity of the amine-containing sugar in macrolide antibiotics, coupled with the observation of fragments most often containing the saccharide, and even the saccharide itself, has led to speculation that the proton is effectively locked onto that moiety [26], and that all fragmentation events must therefore follow a charge-retention fragmentation (CRF) mechanism [28]. However, elegant exchange experiments by Kearney et al. demonstrated clearly that the first water loss from erythromycin A arises from the protonated ketone oxygen, undergoing a rearrangement to yield a D2O loss, which must contain the deuteron, as well as an additional deuterium from a neighboring hydroxyl [27]. This loss is in stark contrast to the loss of HDO, which would arise from a CRF process. We analyzed the fragmentation of the remainder of our suite of macrolides, and all but one showed either no substantial water losses or losses of HDO in a traditional CRF mechanism. Clarithromycin (3), however, demonstrates a loss of CH3OD, deuterated methanol (Figure S62). In a CRF mechanism, a loss of methanol would result in unlabeled CH3OH, implying again the involvement of the proton. The putative mechanism by which erythromycin A (1) loses the ketone oxygen in D2O involves cyclization with the 6-OH, and subsequent proton transfer (or deuterium in the labeled experiments). Kearney et al. demonstrated this by incubation of the compounds in H218O to allow a diol-mediated replacement of the ketone oxygen over time. We have recreated those experiments and also observed the loss of H218O (Figure S68), confirming the origin of the oxygen involved. In order to proceed via the same mechanism, the fragmentation observed from clarithromycin (3) would need to involve a methyl transfer, and the observed neutral loss of methanol would contain the 18O label if it similarly arose from the ketone oxygen. Upon incubating 3, however, the neutral loss remained unlabeled (Figure S69), implying that the methanol loss arises solely from the 6-methoxy and a proton, resulting in a carbocation-containing fragment.
While these CMF water or methanol losses exemplified by erythromycin were only observed for clarithromycin and the aforementioned erythromycin, the clear involvement of the proton in the fragmentation events implied to us that the proton in these structures is far more mobile than previously expected and that more of the fragmentation events may be charge migration fragmentation (CMF) events. To that end, we analyzed the saccharide loss pattern present in each of these structures upon D2O labeling. If the proton is indeed locked onto the amine moiety, then the fragmentation of the saccharides must go through a CRF process as shown in Scheme 3a. Under this mechanism, the resulting fragment should retain two deuteriums, from the hydroxyl and the deuteron. However, if the mechanism proceeds via a CMF mechanism, it results in an oxonium ion labeled with a single deuterium on the exchangeable hydroxyl moiety as shown in Scheme 3b. [29] Importantly, without labeling with D2O, these two competing mechanisms result in isobaric species of m/z 158; however, they are resolvable as labeled species. The fragmentation spectra of D2O labeled erythromycin A with a clear peak at m/z 159 (Figure 1a, c), supporting the CMF mechanism. Previous efforts have also highlighted the presence of m/z 158 fragments in sodiated erythromycin A, in which a CRF process whereby the amine is protonated is impossible [30].
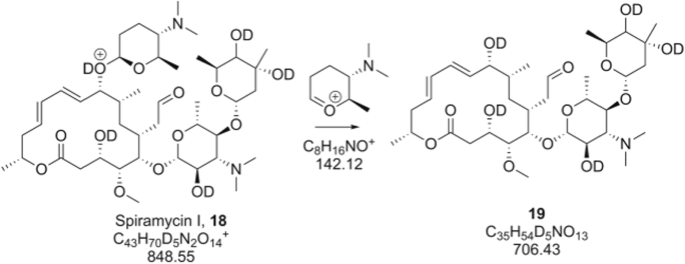
D2O-labeled erythromycin A fragmentation. (a) CID MS2 fragmentation of deuterated [M + D]+. (b) Detailed view of m/z 720, loss of D2O. (c) Detailed view of m/z 159, evidence of CMF mechanism and resulting oxonium ion. (d) MS2 fragmentation of spectrum of spiramycin detailing the presence of m/z 142, providing further evidence for the CMF mechanism
While the presence of a significant m/z 159 peak provides striking evidence for the CMF pathway, the possibility exists that H/D scrambling [31] could artificially lower the observed m/z if a deuterium on a saccharide hydroxyl moiety, for example, exchanged with the aglycone or if the same hydroxyl group was involved in the fragmentation event and the fragmentation proceeded by some mechanism resulting in the hydroxyl deuterium remaining on the macrolide core. This would lead to a fragment ion containing a m/z of one additional Dalton, as one of the deuteriums previously on a saccharide was now on the macrolide core through scrambling, and not the actual fragmentation mechanism. Perhaps the best evidence that this is not the case is the observation of the peak for forasaminyl in spiramcyin (18), which lacks a hydroxyl group on any of its saccharides. In this molecule, there is no option for H/D exchange, from saccharide units to the aglycone core, unless it proceeded through the deuteron on the tertiary amine. In addition, no hydroxyl can be involved in the fragmentation as shown in Scheme 4. Again in this case, fragmentation of the deuterated [M + D]+, shown in Figure 1d results in the observation of m/z 142, corresponding to the oxonium form, not the singly deuterated form which would arise from the CRF event necessary if the amine is the sole protonation site.
There is, however, likely a secondary mechanism to the one shown in Scheme 3b which does involve the hydroxyl group, as in each case save the aforementioned non-hydroxylated saccharide from spiramycin there is a significant population of an A-1 peak from each of the observed amino-containing sugars (e.g., m/z 158 in addition to m/z 159 in erythromycin A). As this scrambling is not universally present in every fragment, we attribute this instead to a hydroxyl involved fragmentation event [30], which would yield a population with no deuteriums on the observed fragments. This subpopulation is usually in an abundance of ~ 50% with respect to the singly-deuterated molecule, though the proportions are highly molecule dependent.
Additional evidence for a hydroxyl-mediated fragmentation mechanism is the observation that fragments F1 and F2 (Table 1), resulting from the saccharide losses, analogously have a higher proportion of A + 1 peaks. Importantly, any peaks resulting from a CRF process with the amine protonated, as has been previously proposed, would instead result in larger and smaller fragments for the saccharide and macrolide fragments, respectively.
All of this is not to claim that the tertiary amine does not have tremendous proton affinity or drive the fragmentation events. The assumption that the amine was the sole charge carrier was based on the observation of only fragments with the amine-containing saccharide. In fact, under many fragmentation voltages, this sugar residue is the base peak. In many of the molecules presented here, the other one or two saccharide units are not observable. In a CRF mechanism, this is logical, as the fragmentation event does nothing to change the protonation site, and if the other sugars were lost, they would always be neutral. However, in many of the molecules under study here, we can see fragments corresponding to the non-amine-containing sugar residues, albeit at a much lower intensity. We speculate that the amine-containing sugar is a particularly basic site on the molecule, so even though the proton is mobile, it is in closer proximity and more readily creates fragments around the amine-containing saccharide. An additional possibility is that the tertiary amine has a stabilizing effect on the oxonium ion formed and thus, increases the relative intensity of those fragments.
Isolation of Substructures
As it is our goal to obtain as much structural information as possible by fragmentation analysis, the substructures of these molecules must be isolated and fragmented during sequential fragmentation. In the first stage, we often obtain information about the first saccharide unit, as explained previously. Selection of those product ions and reinterrogating them enables isolation of the aglycone, and even individual regions of the macrolide core facilitating assignment of the differences between analogs on the ring. For example, the fragmentation scheme for 1 demonstrates that by the MS4 scans, sections of the macrolide core have been isolated and fragmented (Scheme 5). While modifications to the saccharide moieties are often straightforward to assign, as illustrated in the previous section, sequential fragmentation can serve the same purpose for the more challenging modifications to the macrolide core, which are often impossible to ascertain from MS2 analysis alone.
An alternative approach to sequential fragmentation has been used to gain information about the macrolide core by analyzing synthetic aglycones, either from pure synthetic samples [32] or by intentionally hydrolyzing the saccharide moieties from the molecule [20]. However, these approaches suffer serious drawbacks. In the case of synthetic aglycones, the detailed structural information is only useful for the molecules under study, as one cannot apply the trends and observed fragmentation pathways to other related molecules because the synthetic aglycones do not match those isolated in the mass spectrometer. For example, Roddis et al. analyzed the biosynthetic precursor to erythromycin, 6-deoxyerythronolide B [32]. Their fragmentation analysis of the sodiated precursors yielded rich structural information about the macrolide core, and allowed distinctions and characterizations of various unintentional biosynthetic byproducts. However, the sequential fragmentation of erythromycin does not result directly in any of the synthetic aglycone structures analyzed. As shown in Scheme 5, the nitrogen-containing sugar generally stays attached to the molecule even when the macrolide begins to fragment. Additionally, the fragmentation of saccharide units from the precursor structure result necessarily in either an additional double bond, ring-closure, or hydroxyl moiety on the product ion, which is not present in the artificial aglycone. Thus, drawing comparisons to any newly isolated macrolide will have little similarity to the trends gleaned from these type of isolated aglycone-based studies, as the moieties that we often observe driving the fragmentation behavior are simply not present. Hydrolysis of macrolide antibiotics more closely represents what can be achieved by sequential fragmentation studies, but characterization strategies involving chemical modifications become quite challenging with the complexity of crude bacterial extracts containing perhaps thousands of compounds, relegating these types of approaches to specific cases where purified or semi-purified products are available. Using our approach, MSn in tandem and CID studies of the deuterated species, we are able to ascertain detailed fragmentations for most molecules under study including extensive fragmentation of the macrolide core. In the following sections, we will present the key information obtained using our analysis for each class of macrolide compound. We also present the full set of all spectra obtained in each of our experiments for all molecules in Table 1 in the Supplemental Information to aid future elucidation efforts on related compounds.
Fragmentation Behavior of Erythromycin-Like Compounds
The erythromycins, and the related species clarithromycins and roxithromycin (1–3) are perhaps the most well-characterized structures under study here. These 14-membered macrolides share similar fragmentation behaviors, though the erythromycins appear to have a higher propensity for water losses than any other compounds. The MS2 spectra of each of the main components of the respective macrolide mixtures are shown in Figure 2. As discussed above, these spectra are dominated by saccharide and water losses, or losses of the methoxy or imine moieties that distinguish the clarithromycins and roxithromycins, respectively, from erythromycin. The erythromycins uniquely have a number of highly abundant water loss peaks in comparison to the other structures. It is unclear why these structures are so predisposed to water loss, but there is extensive evidence that erythromycin undergoes a number of dehydrations and ring closures spontaneously [33], making it likely that conformational stabilization of either the intermediates or resulting fragments is important.
Though this family of compounds has small differences, the clarithromycins and roxithromycin fragment in much the same way, as illustrated in Scheme 6. All of the structures lose the cladinose saccharide, followed by the modifications distinguishing them from erythromycin, resulting in shared fragment ions at m/z 558 (25) at the MS3 level. Though nominally the same, it is nearly impossible to distinguish which water residues are lost and where the newly acquired sites of unsaturation may lie on the molecule. Additionally, it is possible that the isobaric fragment resulting from each of these species is different from one another, especially in the case of roxithromycin, where significant rearrangements must occur around the site of the imine to result in the putative structure of m/z 558. The fragmentation of m/z 558 isolated in our MSn studies does indeed appear different. In most cases, the fragments observed (Figure 3) are not unique, but the relative abundance of each varies significantly supporting our assertion that the isobaric fragments originating from each precursor are not the same structure but behave similarly in subsequent fragmentations.
Deeper down the fragmentation tree, we can distinguish many ions resulting from the macrolide core and draw more meaningful structural information from these molecules. In all the following cases, we have assigned putative fragmentation pathways. These are challenging in a molecule with so many points of fragmentation resulting in isobaric ions and ubiquitous water losses, and as such are presented as a chemically reasonable interpretation, but would require far more extensive isotopic labeling and synthetic studies to definitively ascertain. The core fragmentation pathways present in the erythromycins are shown in Scheme 5. Upon saccharide loss, the molecule undergoes several water losses. We have seen from the D2O labeling that the first originates from the ketone oxygen. However, it is less clear that the first water loss resulting after the saccharide loss proceeds by the same mechanism. Fragments of m/z 581 (deuterated fragment 20) exhibit Δ19 Da, with small populations of Δ20 Da, implying that the same proton transfer-mediated loss of the ketone oxygen is not primarily involved here (Figure S61). This may be due to scrambling of the remaining deuterium labels after one fragmentation event, or the remaining waters are lost by more traditional mechanisms, losing HDO. As such, our discussion of the fragmentation of this molecule will inherently make assumptions about the origin of each water loss. Importantly, the reality is likely more complex, with competing sites of loss and rearrangements necessary to support the high degree of unsaturation present in many of the smaller fragments. Therefore, the mechanisms presented throughout this work are putative schemes, and an investigation of a novel-related structure should rely on careful analysis of the standard in tandem with the new molecule, and not necessarily the exact gas phase structures presented.
Under the conditions studied here, isolation of the intact aglycone is not possible, as it is in many of the other macrolides presented. Instead, fragmentation of the polyketide core occurs while the amine-containing sugar is still attached. It is not clear why the relatively weak glycosidic linkage stays intact while carbon-carbon bonds are cleaved on the backbone of the molecule, but these fragmentation pathways may be stabilized by the increased conjugation of the resulting species, thus offering more energetically favorable pathways than the simple loss of the saccharide. Additionally, upon loss of the ketone oxygen, the polyketide core may not have a basic enough site to retain the charge, thus the only observed fragments are those containing the amino-sugar. These erythromycin analogs all share similar fragmentation patterns, exhibiting shared isobaric fragments, though interestingly, the small differences between the precursor structures leads to isobaric, but likely different fragment structures at the MS3 level. These molecules are also unique in their retention of the saccharide, even after the seemingly more stable carbon-carbon bonds of the aglycone begin to fragment.
Fragmentation Behavior of Spiramycins
The spiramycins are 16-membered macrolides that exhibit a high degree of conjugation on the macrolide core and uniquely possess 2 amine-containing sugars, which yield doubly as well as singly charged species. The key fragments, as in the case of all the molecules under study, are the F1 and F2 fragments corresponding to the losses of the terminal saccharides in Table 1. It is easily discernable which of the molecules among this family have modifications to the saccharide moieties from these neutral losses. For example, spiramycin V has a mycarosyl in place of the forosaminyl present on spiramycin I (4). Upon incubation in D2O and MS2 analysis, the saccharide moieties are lost by a CMF mechanism as is clear from the fragment of m/z 142 shown in Scheme 4 and Figure 1d, corresponding to the forosaminyl species. This demonstrates that this species does not retain the deuterium label, which would arise from a protonated tertiary amine. Spiramycin, having just a single hydroxyl on the macrolide core, does not reliably lose a water residue from the precursor mass upon CID activation, so we were unable to assess a CMF vs CRF mechanism for losses outside of the saccharides.
In order to more fully characterize the core structure, we turn to the MSn analysis. These molecules in particular demonstrate the utility of our multi-experiment approach. In the t2-t2-t2 analysis of spiramycin I, the highest intensity fragments in the MS2 spectrum correspond to loss of the mycarose moiety, 26, and the A + 1 isotopic peak of the same molecule, thus the entire branch of the MS3–4 fragmentation tree arising from the isotope yields no structural information (Scheme 7 and Figure S24). Of course, these mis-selections of isotopic peaks, and analysis of particular ions is easily accomplished by inclusion/exclusion lists, but these untargeted scans can be performed without any prior knowledge, and are especially useful for minor, unexpected components of a mixture. In contrast, the t4-t4 of the same molecule yields further information, as in addition to ions selected under any of the t2-t2-t2 scans, MS3 fragmentation of m/z 522 and 349 (28) were also obtained. The fragmentation of the aglycone, 28, shown in Figure 4, is important as it can yield information about modifications to the core of the molecule. This fragment undergoes sequential losses of water, methanol, and C4H8, from the backbone of the macrolide (Scheme 7). The structures of the resulting ions become challenging to decipher. For example, the core macrolide can undergo sequential losses of a methanol residue followed by two waters. Given the limited remaining hydroxyl moieties, at least one of these water losses must arise from the aldehyde or ester. Additionally, the two losses of C4H8 could arise from nearly anywhere around the ring system [34]. Each of these losses necessitate an additional degree of unsaturation on the remaining fragment, so the resulting structures must be highly conjugated, or perhaps even result in new ring formations in the observed low mass ions such as m/z 225. In contrast to the erythromycin-like molecules, these species’ cores remain intact until all saccharides are lost, allowing isolation of the aglycone, and MS3 characterization. These spectra highlight the difficulty in ascertaining useful information from the macrolide core, as the additional degrees of unsaturation require extensive rearrangements to rationalize.
Fragmentation Behavior of Leucomycins and Josamycins
The kitasamycins, or leucomycins, and josamycins are an exceptionally complex mixture of related structures. While nominally two different macrolides, they share many of the same minor species, and josamycin is even referred to as leucomycin A3. Our analysis of two commercially available sources yielded a number of components of sufficient abundance to characterize fully, as well as many additional isomers [23]. The majority of these species differ at two positions: the oxygen at the four position of the terminal mycarose saccharide (Scheme 8, blue) and either a hydroxyl or acetyl moiety on the macrolide core (Scheme 8, red). The MS2 spectra of these molecules are again dominated by the loss of the terminal mycarose saccharide, yielding F1. Mycarose produces a neutral loss of 144 Da and may be functionalized at the 4 position with an ethanoyl, propanoyl, butanoyl, 3-methyl-butanoyl, or COC5H11 (likely 4-methyl-pentanoyl), giving rise to neutral losses of 186, 200, 214, 228, or 242, respectively. These modified saccharides are attached to an acetylated macrolide core, as shown in Figure 1, or a hydroxyl moiety. Therefore, the permutations of this family include the hydroxylated core, producing F1 fragments of m/z 558 and the aforementioned neutral losses 144, 186, 200, 214, 228, and 242 corresponding to Leucomycin V, A9, A7, A5, A1, and unnamed species, respectively. Similarly, the acetylated core, producing F1 fragments of m/z 600, may be attached to the same set of functionalized mycarose saccharides, producing Leucomycin U, A8, A6, A4, A3/Josamycin, respectively (the COC5H11 functionalized molecule has not been observed in literature as far as we are aware, nor do we identify it here). The structures with losses or fragments outside of these patterns represent far more challenging structures, given that modifications must arise from locations besides the saccharides, which we have seen are nearly impossible to characterize by MS2 alone, and we will highlight their interpretation using our MSn-based approach.
One of the species present, m/z 842.5 (29), has a difference in mass of 14 AMU to the major component, josamycin (6) corresponding to an additional CH2 moiety. Assigning the position of this group is not possible from the MS2 spectra alone, and the identical neutral losses arising from the saccharide unit in Table 1 indicate the additional methylene is not located there. Sequential fragmentation analysis of these species (Scheme 8) is required to pinpoint the site of modification. Both species first lose an identical functionalized mycarose saccharide unit (Scheme 8a), followed by the concurrent loss of the remaining mycaminose saccharide and two water losses. The 14 amu mass difference is maintained until the fragmentation event yielding m/z 299. Based on our characterization of the fragmentation pathways in josamycin, the transition of m/z 391 to m/z 299 (Scheme 8c) results from the loss of the ester moiety at position 3 (Scheme 8, red), as well as the loss of the 4-methoxy. Given that the mass difference in the two species is present before this transition, but the resulting fragments are isobaric at m/z 299, the difference can be assigned to the ester position, and the presence of an ethyl ester in 29 instead of a methyl in 6. This species, termed Leucomycin X3 or impurity E, is a known byproduct in the production of josamycin [23], demonstrating the ability of our approach to pinpoint structural modifications among analogues with a sequential fragmentation approach that have previously required isolation and NMR characterization in order to assign.
Fragmentation Behavior of Oleandomycin
Even with this exhaustive approach, it is not always possible to isolate the aglycone or macrolide core. In the case of oleandomycin (7), which contains a unique oxirane moiety on the macrolide backbone, activation of this molecule results in very few observed fragments, first losing the saccharide unit resulting in F2, then the nitrogen-containing sugar, which retains the charge and is virtually the only observed product ion at m/z 158 (Scheme 9, Figures S54 and S55). It is unclear why this molecule, which is quite similar to erythromycin, has such a dearth of fragmentation pathways available to it, but it is likely that the oxirane moiety imparts conformational strain that stabilizes the observed pathways (or destabilize those not observed) in much the same way that erythromycin loses a unique number of water residues in comparison to other similar structures.
Fragmentation Behavior of Tylosins
The tylosins, 16-membered macrolides used extensively as a veterinary antibiotic under the trade name Tylan, initially fragment as the rest of the macrolides under study here, losing the terminal saccharide moieties. Upon deuterium labeling, the tylosins do not exhibit a significant amount of water loss directly from the precursor ion, so it is impossible to interrogate these molecules for the abnormal behavior exhibited in the erythromycins. There are water losses from the saccharide-loss fragments that show losses of HDO (Figure S-67, m/z 776–757), which implies these losses likely proceed by the more standard CRF mechanism. As the other macrolide structures, saccharide moieties are lost with a single deuterium label, implying a CMF mechanism by which the proton is not located on the amine, but instead the observed species are oxonium ions.
Tylosin B, which lacks the terminal mycarose, can produce more useful fragments at the MS2 level, but the remaining structures yield little information since the primary fragmentation pathway proceeds via the loss of each of the three sugars (Figure S56). Under MSn conditions, however, these fragments can be isolated and interrogated. A representative pathway is shown in Scheme 10. The saccharides are sequentially lost, resulting in the isolation of the aglycone (35). Fragmentation of this ion results in a number of water losses, as well as losses of 30 and 58 (Figure S57). The loss of 58 is likely the PKS starter acid (Scheme 10, red box), and is common in many of these macrolides. The loss of 30 may correspond to either CH2O or C2H6, from the polyketide backbone. As the differences in these two species result as an absence of the saccharide units, there is very little difference in fragmentation. Interestingly, as the saccharide moieties are lost, there do not appear to be any ring fragments before the aglycone is isolated. This contrasts with erythromycin and some of the other structures, where the macrolide core begins to fragment while the amine-containing sugar is still attached. We speculate that the high degree of conjugation on the tylosins stabilizes the ring and the weaker glycosidic linkages must all be broken before any fragments arising from the ring are observed (Figure 5).
Characterizing an Unknown Macrolide
Applying the information presented here to a putatively novel macrolide will still present a significant challenge to the researcher in most cases. Fully characterizing a novel molecule by mass spectrometry alone is still a rarity in discovery approaches, but we have demonstrated cases where it can be done, especially if the compound is closely related to a known species. In the case of completely novel metabolites, even obtaining partial structural information or assigning it to a likely class of structures can be hugely informative for future studies. In our experience, the first step in the analysis of a novel structure should be to also identify any likely related structures in the mixture, searching for compounds with shared neutral losses or key fragments. Even if none of the identities of these compounds are known, detecting a suite of compounds with methylene differences, or related cores, such as the leucomycins can help to inform interpretation. Next, accurate mass measurements of both precursor and product ions should yield information about the saccharide constituents and the overall size and formula of the macrolide core. Next, the ion fragmentation trees should be interrogated, paying special attention to the shared pathways of any members of the newly identified family similar to our study of the leucomycin analog presented in Scheme 8.
If no information is available as to the broad structural class of the macrolide of interest, some general observations may help to inform a hypothesis. These trends are largely based on the structures presented here, and we have shown that even among similar classes, structural variation can have widely varied effects on fragmentation behavior so these should be taken as a guide only. The 14-membered macrolides often have significantly more water losses, presumably due to the increased stability of the resulting products. The larger 16-membered macrolides do not seem to benefit from the same stabilization, and water losses are slight or unobserved. Any single highly abundant water loss is often a result of existing conjugation, where a single additional double bond can add to the conjugated system. Isolation of the aglycone is not always possible, so any nitrogen containing fragments smaller than the F2 fragment can be assumed to result from fragmentation of the core while the final saccharide is still attached. The smaller isolated fragmentation species, such as those of the aglycone, or a ring fragment, which we often obtain at the MS3–4 level, could be used similarly to traditional fingerprinting analysis of MS2 spectra, which is commonly used to identify known compounds, in order to compare across the presented families and see if any share pathways in the fragmentation of these core constituents to draw similarities and assign a putatively similar structural class to an unknown.
Conclusions
Though it is often repeated in the analysis of macrolide fragmentation that the sole charge carrier is the amine-containing saccharide, previous work and our analysis labeling these species with D2O and H218O definitively demonstrate this not to be the case. In fact, nearly all the fragmentation events appear to be charge mediated. Fragments containing the amine-saccharide are likely more easily observed because of their high proton affinity, but the proton on these ions is undoubtedly much more mobile than is often assumed.
Though the macrolide antibiotics have been the subject of numerous works exhaustively characterizing the observed fragments, missing has been a comprehensive analysis of related structures. Our series of untargeted analyses demonstrably collect fragmentation spectra up to the MS4 level, on complex mixtures of many closely related species, without requiring any prior knowledge of the structures. This compendium of spectra has allowed us to make comparisons between the different macrolide families, and rationalize their behavior based on proposed fragmentation patterns. Our hope is that this presentation will provide a theoretical underpinning to aid the future assignment of novel macrolides or (bio) degredation products. To that end, we have made every collected spectrum available in the supporting information. We have shown that even in this highly related class of compounds, there are significant differences in fragmentation pathways, which are seemingly highly dependent on very subtle structural differences. These observed trends in known molecules such as ubiquitous water losses, retention of saccharides during fragmentation, or even a lack of observed fragments could be used in the analysis of an unknown to indicate the presence of particular structural motifs.
References
World Health Organization (WHO). WHO model list of essential medicines—20th List. Geneva, Switzerland. https://www.who.int/medicines/publications/essentialmedicines/en/ (2018)
Kannan, K., Kanabar, P., Schryer, D., Florin, T., Oh, E., Bahroos, N., Tenson, T., Weissman, J.S., Mankin, A.S.: The general mode of translation inhibition by macrolide antibiotics. Proc. Natl. Acad. Sci. 111, 15958–15963 (2014)
Zhu, Z.J., Krasnykh, O., Pan, D., Petukhova, V., Yu, G., Liu, Y., Liu, H., Hong, S., Wang, Y., Wan, B., Liang, W., Franzblau, S.G.: Structure-activity relationships of macrolides against Mycobacterium tuberculosis. Tuberculosis. 88, S49–S63 (2008)
Ma, C., Ma, S.: Various novel erythromycin deritivates obtained by different modifications: recent advance in macrolide antibiotics. Mini-Rev. Med. Chem. 10, 272–286 (2010)
Cui, W., Ma, S.: Recent advances in the field of 16-membered macrolide antibiotics. Mini-Rev. Med. Chem. 11, 1009–1018 (2011)
Clark, R.F., Ma, Z., Wang, S., Griesgraber, G., Tufano, M., Yong, H., Li, L., Zhang, X., Nilius, A.M., Chu, D.T.W., Or, Y.S.: Synthesis and antibacterial activity of novel 6-O-substituted erythromycin a derivatives. Bioorg. Med. Chem. Lett. 10, 815–819 (2000)
Ayuso-Sacido, A., Genilloud, O.: New PCR primers for the screening of NRPS and PKS-I systems in actinomycetes: detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Microb. Ecol. 49, 10–24 (2005)
Cimermancic, P., Medema, M.H., Claesen, J., Kurita, K., Wieland Brown, L.C., Mavrommatis, K., Pati, A., Godfrey, P.A., Koehrsen, M., Clardy, J., Birren, B.W., Takano, E., Sali, A., Linington, R.G., Fischbach, M.A.: Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell. 158, 412–421 (2014)
Krug, D., Muller, R.: Secondary metabolomics: the impact of mass spectrometry-based approaches on the discovery and characterization of microbial natural products. Nat. Prod. Rep. 31, 768–783 (2014)
Johnson, A.R., Carlson, E.E.: Collision-induced dissociation mass spectrometry: a powerful tool for natural product structure elucidation. Anal. Chem. 87, 10668–10678 (2015)
Kersten, R.D., Ziemert, N., Gonzalez, D.J., Duggan, B.M., Nizet, V., Dorrestein, P.C., Moore, B.S.: Glycogenomics as a mass spectrometry-guided genome-mining method for microbial glycosylated molecules. Proc. Natl. Acad. Sci. U. S. A. 110, E4407–E4416 (2013)
Crowe, M.C., Brodbelt, J.S., Goolsby, B.J., Hergenrother, P.: Characterization of erythromycin analogs by collisional activated dissociation and infrared multiphoton dissociation in a quadrupole ion trap. J. Am. Soc. Mass Spectrom. 13, 630–649 (2002)
Gates, P., Kearney, G.C., Jones, R., Leadlay, P.F., Staunton, J.: Structural elucidation studies of erythromycins by electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 13, 242–246 (1999)
Kumar Chitneni, S., Govaerts, C., Adams, E., Van Schepdael, A., Hoogmartens, J.: Identification of impurities in erythromycin by liquid chromatography–mass spectrometric detection. J. Chromatogr. A. 1056, 111–120 (2004)
Leonard, S., Ferraro, M., Adams, E., Hoogmartens, J., Van Schepdael, A.: Application of liquid chromatography/ion trap mass spectrometry to the characterization of the related substances of clarithromycin. Rapid Commun. Mass Spectrom. 20, 3101–3110 (2006)
Kwiecień, A., Krzek, J., Żmudzki, P., Matoga, U., Długosz, M., Szczubiałka, K., Nowakowska, M.: Roxithromycin degradation by acidic hydrolysis and photocatalysis. Anal. Methods. 6, 6414 (2014)
Wang, F., Zeng, H., Wang, J.: Characterization of nineteen impurities in Roxithromycin by HPLC/TOF and ion trap mass spectrometry. Chromatographia. 76, 1683–1695 (2013)
Pendela, M., Govaerts, C., Diana, J., Hoogmartens, J., Van Schepdael, A., Adams, E.: Characterization of impurities in spiramycin by liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 21, 599–613 (2007)
Govaerts, C., Chepkwony, H.K., Van Schepdael, A., Adams, E., Roets, E., Hoogmartens, J.: Application of liquid chromatography-ion trap mass spectrometry to the characterization of the 16-membered ring macrolide josamycin propionate. J. Mass Spectrom. 39, 437–446 (2004)
Gebhardt, P., Perner, A., Grafe, U.: Preparative separation and analysis of complex mixtures of leucomycins and Desmycarosyl leucomycins using HPLC and mass spectrometry. Chromatographia. 60, 229–234 (2004)
Chopra, S., Van Schepdael, A., Hoogmartens, J., Adams, E.: Characterization of impurities in tylosin using dual liquid chromatography combined with ion trap mass spectrometry. Talanta. 106, 29–38 (2013)
LeRiche, T., Osterodt, J., Volmer, D.A.: An experimental comparison of electrospray ion-trap and matrix-assisted laser desorption/ionization post-source decay mass spectra for the characterization of small drug molecules. Rapid Commun. Mass Spectrom. 15, 608–614 (2001)
Van den Bossche, L., Daidone, F., Van Schepdael, A., Hoogmartens, J., Adams, E.: Characterization of impurities in josamycin using dual liquid chromatography combined with mass spectrometry. J. Pharm. Biomed. Anal. 73, 66–76 (2013)
Yang, S., Carlson, K.H.: Solid-phase extraction-high-performance liquid chromatography-ion trap mass spectrometry for analysis of trace concentrations of macrolide antibiotics in natural and waste water matrices. J. Chrom. A. 1038, 141–155 (2004)
Dubois, M., Sior, F.E., Delahaut, P.: Identification and quantification of five macrolide antibiotics in several tissues, eggs and Milk by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. B. 753, 189–202 (2001)
Cerny, R.L., MacMillan, D.K., Gross, M.L., Mallams, A.K., Pramanik, B.N.: Fast-atom bombardment and tandem mass spectrometry of macrolide antibiotics. J. Am. Soc. Mass Specrom. 5, 151–158 (1994)
Kearney, G.C., Gates, P., Leadlay, P.F., Staunton, J., Jones, R.: Structural elucidation studies of erythromycins by electrospray tandem mass spectrometry II. Rapid Commun. Mass Spectrom. 13, 1650–1656 (1999)
Demarque, D.P., Crotti, A.E., Vessecchi, R., Lopes, J.L., Lopes, N.P.: Fragmentation reactions using electrospray ionization mass spectrometry: an important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 33, 432–455 (2016)
Bythell, B.J., Abutokaikah, M.T., Wagoner, A.R., Guan, S., Rabus, J.M.: Cationized carbohydrate gas-phase fragmentation chemistry. J. Am. Soc. Mass Spectrom. 28, 688–703 (2017)
Gembarovski, D., Markovic, V.G., Galic, N.: Structural elucidation studies of 15-membered azalide macrocycles using H/D exchange and ESI-MS(n.). J. Pharm. Biomed. Anal. 86, 1–10 (2013)
Weis, D.D.: Hydrogen exchange mass spectrometry of proteins: fundamentals, methods, and applications. John Wiley and Sons. https://doi.org/10.1002/9781118703748 (2016)
Roddis, M., Gates, P., Roddis, Y., Staunton, J.: Structure elucidation studies on 14- and 16-membered macrolide aglycones by accurate-mass electrospray sequential mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 862–874 (2002)
Cachet, T., Van den Mooter, G., Hauchecorne, R., Vinckier, C., Hoogmartens, J.: Decomposition kinetics of erythromycin a in acidic aqueous solutions. Int. J. Pharm. 55, 59–65 (1989)
Gates, P.J., Kearney, G.C., Jones, R., Leadlay, P.F., Staunton, J.: Structure elucidation studies of erythromycins by electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrom. 13, 242–246 (1999)
Acknowledgements
This work was supported by a NSF Career Award, CHE-1518379, a Sloan Research Fellow Award (E.E.C.), an Indiana University Quantitative and Chemical Biology training fellowship (A.R.J.), and the University of Minnesota.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(PDF 5922 kb)
Rights and permissions
About this article
Cite this article
Johnson, A.R., Carlson, E.E. Structure Elucidation of Macrolide Antibiotics Using MSn Analysis and Deuterium Labelling. J. Am. Soc. Mass Spectrom. 30, 1464–1480 (2019). https://doi.org/10.1007/s13361-019-02210-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-019-02210-w