Abstract
The reaction of atomic thorium cations with deuterated water as a function of kinetic energy from thermal to 10 eV was studied using guided ion beam tandem mass spectrometry. At thermal energies, both ThO+ + D2 and DThO+ + D are formed in barrierless exothermic processes and reproduce results in the literature obtained using ion cyclotron resonance mass spectrometry. As the energy is increased, the branching ratio between these two channels changes such that the dominant product changes from ThO+ to DThO+ and back to ThO+, until ThD+ + OD is energetically available and is the dominant product channel. To help understand these experimental results, a variety of theoretical approaches were tried and used to establish a potential energy surface, which compares well with previous theoretical studies. Utilizing the theoretical results, the kinetic energy dependent branching ratio between the ThO+ + D2 and DThO+ + D channels was calculated using both RRKM and phase space theory (PST). The results indicate that consideration of angular momentum conservation (as in PST) and spin-orbit corrected energies are needed to reproduce experimental results quantitatively. The PST modeling also provides relative energies for the two competing transition states that lead to the primary products, for which theory provides reasonable agreement.

Note: This data is
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Actinide chemistry is of interest for multiple reasons, including its applicability to nuclear power generation where members of the actinide series are either used as a fuel or are a byproduct from the nuclear process. Although nuclear power remains a viable and efficient source of energy, the risk of a breach or other incident during use or storage of materials remains significant. Consequently, a thorough understanding of actinide reactivity is warranted to help identify, assess, and model potential contamination dispersion in the event of a nuclear incident. The actinide-water reaction is of particular interest because water is a common coolant and neutron moderator in many reactor designs so that nuclear fuels and water are in close proximity.
Despite this interest, actinide chemistry remains difficult to study experimentally because of the extreme radioactivity of all members except Th and U, which are only mildly radioactive. Theoretical studies are a potentially promising alternative that mitigates any safety concerns regarding the handling of actinides in experimental studies. To validate such theoretical approaches, it is critical to have experimental work available for comparison. Several groups, including our own and that of Helmut Schwarz, have endeavored to establish accurate experimental benchmarks for gas-phase actinide compounds to which theoretical values can be directly and easily compared [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. This experimental information includes bond lengths, energy levels, and thermodynamic values such as bond energies and ionization energies. Another potential experimental benchmark to which theory can be compared is the rates and branching ratios of chemical reactions.
One particular reaction for which experimental and theoretical results have already been compared is the activation of water by atomic thorium cations. This reaction has been studied previously in two Fourier-transform ion cyclotron resonance (FT-ICR) mass spectrometry experiments at thermal energies, first by Schwarz and coworkers [9] and later by Santos et al. [16]. In both studies, a branching ratio of 65% ThO+ + H2 to 35% [Th,O,H]+ + H was observed. A subsequent potential energy surface (PES) presented by Mazzone et al. [27] indicates that the hydrido thorium oxide, HThO+, is energetically preferred compared to the thorium hydroxide, ThOH+, and that the ThO+ and HThO+ products share a common intermediate, HThOH+. Furthermore, barriers relative to the shared intermediate for ThO+ and HThO+ for the rate-limiting steps of each reaction were determined to be 0.86–1.26 and 2.00–2.32 eV, respectively (2.83–3.94 and 1.78–2.80 eV below ground state reactants, respectively). In this study, no spin-orbit effects were considered, which could alter the conclusions as these corrections are typically large for Th+. A second theoretical study by Zhou and Schlegel [28] essentially duplicates the PES presented by Mazzone et al. and also includes a theoretical determination of the ThO+/HThO+ branching ratio by calculating the Rice–Ramsperger–Kassel–Marcus (RRKM) theory [29, 30] rate constant for each product channel restricted by their respective rate-limiting step. Despite the clear thermodynamic preference for the ThO+ + H2 products, Zhou and Schlegel found that RRKM rate constants calculated using their derived PES predict that the HThO+ + H channel should dominate, giving a 11:89 ThO+/HThO+ branching ratio in clear contradiction to the experimentally observed branching pattern. Their conclusion was that the energy available may be large enough that the reaction proceeded more rapidly than energy redistribution so that a statistical treatment was not valid. In contrast, a semi-empirical trajectory study presented in the same paper observed an ~ 80:20 ThO+/HThO+ branching ratio in better agreement with the experimental results, but still not quantitative. Notably, Zhou and Schlegel indicate that a majority of their trajectories failed to dissociate to product in the simulation timeframe so that additional time was added to the simulation of several (16) trajectories to obtain the reported branching ratio for these simulations. Such an observation suggests that the intermediate common to the products is sufficiently long lived that statistical energy redistribution throughout the modes is likely. Here, spin-orbit effects were considered (using a zero-order regular approximation with spin-orbit, ZORA-SO, approach), but the Th+ + H2O asymptote was not included (meaning that the return to reactants was not considered). Rather the trajectories were started at the transition state for insertion of Th+ into the OH bond of water. This approximation should adversely affect the distribution of angular momentum available in the trajectories and also means the efficiency of the reaction is not correctly considered, potentially biasing the branching ratio results.
In recent studies of Th+ reactions [24,25,26], we have employed a first-order, semi-empirical spin-orbit energy correction that employs the experimentally determined Th+ ζ(6d) parameter and the experimental Th+ levels to correct molecular energies. For ThH+ and ThO+, these estimated spin-orbit corrections were within 0.01 eV of theoretically calculated spin-orbit corrections [25, 26]. In our study of the activation of CH4 by Th+ [24], it was concluded that the inclusion of the spin-orbit parameter was essential to reproduce the energy of the experimentally observed barrier for C–H bond activation.
Here, we present absolute cross-sections as a function of reactant kinetic energy for the reaction of Th+ + D2O observed using guided ion beam tandem mass spectrometry (GIBMS). An analysis of these data allows the determination of the experimental energies of the rate-limiting steps relative to the reactants by modeling the kinetic energy dependences of the ThO+ + D2 and DThO+ + D reaction cross-sections using phase space theory (PST), which explicitly conserves angular momentum during the reactions. The kinetic energy dependence of the branching ratio (and related cross-sections) acts as a much more stringent test of the theoretical energies than the lone thermal energy branching ratio available in the literature. We also present quantum chemical calculations of the Th+ + H2O reaction coordinate using various levels of theory and basis sets. From these energies, we calculate the RRKM and PST ThO+/HThO+ branching ratios at thermal energies before and after explicitly including spin-orbit energy corrections. For the present reaction of Th+ + H2O, the accuracy of the calculated energy of each respective rate-limiting transition state relative to each other and the reactants is shown to be critical to successfully reproducing the experimentally observed branching ratio. Both spin-orbit corrections and consideration of angular momentum conservation are required for quantitative agreement.
Experimental and Theoretical Methods
Instrument
The GIBMS used in these experiments has been described in detail previously [31]. Briefly, ions were created in a direct current discharge/flow tube source (DC/FT) [32] where a cathode holding the thorium powder sample was held at ~ 2.5 kV. The resulting electric field ionized Ar in a 9:1 He/Ar mixture flowing over the electrode. Ar ions created in the discharge collided with the cathode, sputtering Th+ ions. The Th+ ions were swept along the flow tube by the He/Ar carrier gas mixture at a total pressure of 0.3–0.4 Torr where the Th+ is thermalized by ~ 105 collisions with the carrier gas. Previous experiments [33,34,35,36,37] have indicated that the electronic state distribution of the metal ions produced by the DC/FT source can be characterized by a temperature between 300 and 1100 K. Conservatively, we estimate an internal energy of 700 ± 400 K, such that 91.9% of ions are found in the ground level (4F3/2, 6d27s) with an average electronic energy (Eel) of 0.02 ± 0.03 eV. In the present and previous experiments with thorium cations [24,25,26], no evidence of excited electronic states was observed.
After exiting the source, ions were focused through a magnetic momentum analyzer where the reactant 232Th+ ion beam was mass selected. These ions were decelerated to a well-defined kinetic energy and passed into a radio frequency (rf) octopole ion guide [38, 39] that constrained the ions radially. The octopole passes through a static pressure reaction cell that contained the neutral reaction partner D2O. Prior to use, nitrogen was bubbled through the D2O for ~ 20 min in order to remove oxygen, and the D2O was subsequently held under low vacuum for ~ 1 h. D2O was introduced into the reaction cell by mild heating of the liquid’s container with a room temperature water bath. To ensure that the probability of multiple collisions between Th+ and the neutral gas were sufficiently small, the pressure in the reaction cell was maintained at typical pressures of 0.05–0.20 mTorr. Independent measurements at several pressures were performed to determine the reaction cross-section dependence on the neutral reactant pressures. Reaction cross-sections were calculated from product ion intensities relative to reactant ion intensities after correcting for background ion intensities measured when the neutral gas was no longer directed into the gas cell [40]. Cross-sections were extrapolated to rigorous single collision conditions (zero pressure conditions) using the determined cross-section dependence on reactant gas pressures. Uncertainties in the calculated absolute cross-sections are estimated to be ± 20%, with relative uncertainties of ± 5%.
Laboratory ion energies (lab) were converted to the center-of-mass frame (CM) using the relationship E = Elab × m/(m + M) where m and M are the masses of the reactant neutral and ion, respectively. At very low energies, the conversion includes a correction for the truncation of the ion kinetic energy distribution, as described previously [40]. Cross-sections are known to be broadened by the kinetic energy distribution of the reactant ions and the thermal (300 K) motion of the neutral reactant [41]. The absolute zero of energy and the full width at half-maximum (fwhm) of the ion beam were determined by using the octopole guide as a retarding potential analyzer, as described previously [40]. Typical fwhms of the ion kinetic energy distribution for these experiments were 0.04 ± 0.01 eV (CM). Uncertainties in the absolute energy scale are ± 0.008 eV (CM). All energies reported below are in the CM frame.
Data Analysis
Phase Space Theory
Phase space theory (PST), originally developed by Light and Nikitin [42,43,44], is a statistical model for describing reactive collisions that assumes there is a strong interaction region from which the system decomposes statistically into all energetically possible reactant and product states. Here, we perform the PST calculation using modified versions of programs originally developed by Chesnavich and Bowers [45]. These calculations assumed that the potential interaction for the bimolecular reactants and products are ion-dipole and ion-induced dipole attractions, using the locked dipole cross-section [46,47,48] or trajectory collision model [49], and then explicitly conserves both energy and angular momentum. The approach used here is described in greater detail in previous works [50].
Modified Line-of-Centers Model
Endothermic reaction cross-sections were modeled using Eq. 1 [39, 51, 52],
where σ0 is an energy-independent scaling factor, E is the relative kinetic energy of the reactants, Eel is the electronic energy of the reactant ion (as defined above), Ei is the internal energy of the neutral reactants having populations gi (Σgi = 1), n is an adjustable parameter that controls the shape of the cross-section, and E0 is the 0 K reaction threshold. Before comparison to the data, Eq. 1 was convoluted over the kinetic energy distributions of the reactants. The σ0, n, and E0 parameters were then optimized using a nonlinear least-squares method to best reproduce the experimental cross-section [40, 53]. Uncertainties in E0 were calculated from the threshold values from several independent data sets over an acceptable range of n values and were combined with the absolute uncertainties in the kinetic energy scale and electronic energies of reactant ions (Eel = 0.02 ± 0.03 eV). At high energies, cross-sections decline because enough energy is available for products to dissociate. To reproduce experimental cross-sections in this energy region, Eq. 1 is modified to include a statistical model of the dissociation probability [54], which is controlled by two adjustable parameters: p, which is similar to n, but can hold only integer values; and Ed, the energy at which product cross-sections begin to decline. The inclusion of the high-energy model in the present work does not significantly alter the threshold analyses of E0.
In the limit that the threshold for the reaction Th+ + LR → ThL+ + R corresponds to the thermodynamic onset for formation of the products, the E0 obtained from Eq. 1 can be used to determine the bond dissociation energy (BDE), D0(Th+-L), using Eq. 2.
This limit often holds for ion-molecule reactions because of the long-range attractive forces. Its validity can be tested by comparison with theoretical potential energy surfaces, as discussed further below.
Theoretical Calculations
Quantum chemical calculations were performed using the Gaussian 09 suite of programs [55]. In most calculations, polarized correlation consistent core-valence quadruple-ζ (20s17p12d11f7g4h1i)/[9s9p8d8f7g4h1i] and triple-ζ (17s16p11d10f4g1h)/[8s8p7d6f4g1h] basis sets for Th [56] were used in combination with the Stuttgart–Cologne (MDF) small core (60 electron) relativistic effective core potential (ECP) [57]. For O and H atoms, polarized correlation consistent basis sets of the same quality were used, cc-pwCVXZ (X = T, Q) [58]. Extrapolation to the complete basis set limit (CBS) for the cc-pwCVXZ (X = T, Q) basis sets [56, 59] was performed using the Karton–Martin method [60], Eq. 3, for HF energies (where x = 3 for T and x = 4 for Q).
For CCSD(T)/cc-pwCVXZ calculations, Eq. 4 [61] is used to extrapolate the correlation energy.
For correlation to previous results [24,25,26], calculations were also performed using the cc-pwCVQZ basis set for Th+ with an augmented aug-cc-pwCVQZ basis set for O and H as well as the Stuttgart–Dresden (SDD) basis set and a similar segmented Stuttgart–Dresden (Seg. SDD) basis set for Th+ [62, 63]. The latter two basis sets both employ a small core quasi-relativistic ECP (MWB) and are double-ζ and quadruple-ζ in quality, respectively. These basis sets were used with a Pople 6–311++G(3df,3p) basis set [64] for O and H.
For calculations utilizing the MWB ECP, structures were optimized using B3LYP in combination with the SDD basis set for Th+ and the 6–311++G(3df,3p) basis set for O and H, B3LYP/SDD/6–311++G(3df,3p). For calculations utilizing the MDF ECP, structures were optimized using PBE0/cc-pVQZ/cc-pVTZ. This latter approach was successfully used in the previous theoretical treatment of the Th+ + CH4 reaction [24]. Single point energies of the optimized structures were calculated using the B3LYP [65, 66], B3PW91 [67], BH and HLYP (BHLYP) [66], M06 [68], and PBE0 [69] functionals. Additional single point calculations were performed using a coupled cluster method that includes single, double, and perturbative triple excitations, CCSD(T) [70,71,72]. For calculation of the correlation energy, the Th+5s and 5p electrons and O 1s electrons were frozen. All reported energies are zero-point energy corrected using the frequencies from the respective optimized structure after scaling the frequencies by 0.989 [73, 74].
Spin-Orbit Corrections
Additionally, all calculations were corrected for spin-orbit effects using a semi-empirical model that has been described in detail previously [24, 75, 76]. Briefly, the theoretical calculations described above yield energies that are the average over all spin-orbit states. For Th+, the J = 3/2 ground level is a mixture of the 4F3/2 (6d27s) and 2D3/2 (6d7s2) levels [77, 78], with the 4F3/2 comprising the primary contribution (see Ref. [24] and its supplementary material for more detail). A nuance of the Th+ system is that although the experimental ground level is best described as 4F3/2, the ground state is 2D (6d7s2), 0.06 eV lower in energy than the 4F state (6d27s) when averaged over all spin-orbit levels. The 4F3/2 ground level lies 0.46 eV lower in energy than the average 4F state and the 2D3/2 level lies 0.18 eV lower in energy than the average 2D state. For the Th+ + H2O reactants, spin-orbit effects were included by correcting the calculated energies by the empirical difference between the 2D ground state and the 4F3/2 ground level, − 0.40 eV. With the exception of the first intermediate along the potential energy surface, discussed below, all other species along the potential energy surface are A states or otherwise singly degenerate so that no explicit first-order spin-orbit corrections according to this model are needed. The net effect is that all calculated energies shift up by 0.40 eV relative to the experimental Th+ (4F3/2) + H2O reactant asymptote compared to the Th+ (2D) + H2O asymptote.
The first intermediate, Th+(H2O), is best characterized as a non-covalent interaction between the charged Th+ and polar H2O. Previously [24], for the comparable Th+(CH4) intermediate, we hypothesized that because the interaction between Th+ and the ligand is minimal, the spin-orbit splitting of this intermediate could be estimated as similar to that of the unperturbed metal, an approximation that led to consistency with the experimental measurement of D0(Th+-CH4). Therefore, this same approximation is used here for Th+(H2O). Overall, this approach may be simplistic because a much stronger interaction between Th+(H2O) is anticipated compared to Th+(CH4); however, a more exact treatment of the spin-orbit splitting of this intermediate is beyond the scope of this work and furthermore would not change any conclusions made from the results reported therein.
Theoretical Branching Ratios
Theoretical rate constants were calculated using RRKM theory [29, 30] and PST [42,43,44,45, 51]. Frequencies and rotational constants used in these calculations were taken from the optimized structures of the appropriate transition states and their preceding intermediate after scaling the vibrational frequencies by 0.989. The number of states available in the transition states and the density of states in the intermediate were calculated using the Beyer–Swinehart–Stein–Rabinovitch algorithm [79,80,81].
Results
Experimental Results
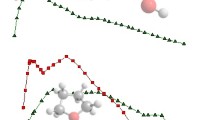
Kinetic energy dependent cross-sections for the reaction of Th+ with D2O are presented in Fig. 1. Products are formed according to the following reactions:
Product cross-sections for the reaction of Th+ with D2O at a pressure of 0.1 mTorr as a function of kinetic energy in the laboratory (upper x-axis) and center-of-mass (lower x-axis) frames. The total cross-section is shown by the black line and the Su–Chesnavich variational trajectory cross-section is shown by the black dashed line. Solid symbols show the results of Cornehl et al. [9] (to the right) and Santos et al. [16] (to the left) converted to effective cross-sections at 0.039 eV
Clearly, products formed at low energy containing two oxygen atoms must be formed in sequential reactions of the primary ThO+ and [Th,O,D]+ products. This is confirmed by the fact that the cross-sections for ThO2+ and ThO2D+ products increase linearly with D2O pressure. These sequential reactions are easily eliminated by extrapolating the cross sections to zero-pressure conditions, which affects only the lowest energy points shown in Fig. 1 for ThO+ and [Th,O,D]+. The remaining description here refers to such zero-pressure extrapolated results.
Compared with the collision cross-section, σtraj, calculated according to the trajectory model of Su and Chesnavich [49], the total reaction (i.e., the sum of reactions 5–9) proceeds with σtotal/σtraj = 0.68 ± 0.14 efficiency at 0.05 eV, equivalent to thermal energies. This value is similar to the reaction efficiency observed by Cornehl et al. [9] in FT-ICR experiments (0.57 ± 0.23), which are somewhat higher than those of Santos et al. [16] (0.20 ± 0.10). Neither of these studies reported absolute rates, but only efficiencies relative to a collision limit calculated using average dipole orientation (ADO) theory [48], which we calculate as equivalent to having a 300 K (0.039 eV) cross-section of 214 × 10−16 cm2, whereas σtraj (0.039 eV) = 340 × 10−16 cm2. When this ADO cross-section is combined with their reaction efficiencies, the effective cross-sections measured in these studies are shown in Fig. 1. The efficiency of the total reaction declines with increasing kinetic energy reaching a minimum of 0.26 ± 0.05 at 0.76 eV. This result could explain the differences in the previous FT-ICR efficiencies if those of Santos et al. were at elevated energies. Different distributions of electronic levels of the Th+ reactant might also explain such differences. It is clear from Fig. 1 that reactions 5 and 6 are dominant at all but the highest energies with a 68:32 ThO+/DThO+ product branching ratio at 0.05 eV, nearly identical to the branching ratios observed at thermal energies in the FT-ICR experiments, 65:35 [9, 16].
Above thermal energies, the ThO+ and [Th,O,D]+ cross-sections behave differently with energy such that the branching ratio shifts to increasingly favor reaction 6 as energy increases, Fig. 2. The cross-section for formation of ThO+ in reaction 5 steadily declines until it reaches ~ 1 eV where it levels out near 6 Å2. Similarly, the cross-section for formation of [Th,O,D]+ in reaction 6 declines with increasing energy until ~ 0.9 eV before increasing slightly and leveling out at ~ 8 Å2. The two cross-sections cross near 0.6 eV and the branching ratio nears 40:60 ThO+/[Th,O,D]+ from about 1–1.5 eV. Also apparent is an endothermic feature in the ThO+ cross-section that begins to rise between 1 and 2 eV. The onset of this feature corresponds to the decline in intensity of the [Th,O,D]+ cross-section, suggesting that this feature could result from decomposition of [Th,O,D]+ into ThO+ + D according to the overall reaction 10.
This hypothesis is supported by the thermochemistry discussed below. The ThO+ cross-section begins to decline sharply above about 5 eV, consistent with the threshold for Th+ + O + D2 formation at D0(O-D2) = 5.110 ± 0.001 eV [82].
Reaction 7 is endothermic with an apparent threshold near 3 eV. Although this product could begin to dissociate beginning at D0(D-OD) = 5.212 ± 0.002 eV [82], the fact that its cross-section does not peak until about 7 eV indicates that the OD product of reaction 7 carries away considerable energy. The rise in the ThD+ cross-section occurs at an energy where the [Th,O,D]+ cross-section for reaction 6 declines; however, decomposition of [Th,O,D]+ according to reaction 11 cannot occur until higher energies, 7.14 ± 0.05 eV.
Therefore, any coupling between the ThD+ + OD and [Th,O,D]+ channels would be the result of the two products sharing a common intermediate.
Thermochemical and Theoretical Results
ThO+
The energy dependence of reaction 5 below 1 eV, Fig. 1, is consistent with a barrierless, exothermic reaction indicating that D0(Th+-O) ≥ D0(O-D2) = 5.110 eV. This result is in good agreement with recent GIBMS work [26] that determined D0(Th+-O) = 8.57 ± 0.14 eV, as well as previous estimates of D0(Th+-O) = 8.74 ± 0.26 eV [21] and 8.70 ± 0.10 eV [83] derived from D0(ThO), IE(ThO), and IE(Th).
At higher energies, a second feature with an energy dependence consistent with an endothermic reaction is observed, suggested to be reaction 10 above. The enthalpy of this reaction can be calculated according to Eq. 12.
Given D0(O-D2) = 5.110 ± 0.001 eV and D0(D-D) = 4.55622 ± 0.00001 eV [84], D0(Th+-O) = 8.57 ± 0.14 eV [26] indicates the threshold for this reaction should occur at E0 = 1.10 ± 0.14 eV consistent with the apparent threshold. This feature can be modeled using the modified line-of-centers model (LOC), Eq. 1, after accounting for the exothermic pathway using a PST model, as described further below. This LOC model is shown in Fig. 3 with parameters listed in Table 1, and results in a threshold of E0 = 0.94 ± 0.30 eV, which has a large uncertainty because of the complications of accounting for the exothermic feature in the ThO+ cross-section. The threshold obtained is in reasonable agreement with that expected from the literature thermochemistry.
A full theoretical exploration of the ThO+ species can be found elsewhere [26]. Briefly, ThO+ has a 2Σ+ ground state with a triple bond and the unpaired electron located in a molecular orbital largely composed of the Th 7s-orbital. Shifting the unpaired electron to a Th 6d-orbital leads to the lowest energy excited state, 0.58 eV higher in energy [18].
[Th,O,D]+
The energy dependence of the [Th,O,D]+ cross-section at low energies shows that reaction 6 is also a barrierless, exothermic reaction, which means that D0(Th+-OD) ≥ D0(D-OD) = 5.212 ± 0.002 eV [82] (although this bond energy does not necessarily imply that the [Th,O,D]+ species is a hydroxide). The same observation made in previous reports for reaction with H2O by Cornehl et al. [9] and Santos et al. [16] led them to report that D0(Th+-OH) ≥ 5.10 ± 0.01 eV = D0(H-OH) [84]. The [Th,O,D]+ cross-section increases slightly starting near 1 eV. Identification of this feature in the cross-section requires additional information from theory and is discussed below.
Theoretical calculations indicate that the lowest energy structure of [Th,O,H]+ is a hydrido thorium oxide, HThO+, with a 1A′ ground state rather than the ThOH+ hydroxide. (Note that we will refer to [Th,O,H]+ as HThO+ for the remainder of the manuscript.) A 1Σ+ thorium hydroxide is found 0.3–1.3 eV higher in energy and a 3Δ state of ThOH+ is found 0.7–1.4 eV above the HThO+ ground state. These and additional structures of [Th,O,H]+ located theoretically are listed in Tables S1 and S2. These results are consistent with the structures calculated by Mazzone et al. [27] and Zhou and Schlegel [28], who also found a HThO+ (1A′) ground state at various levels of theory using SDD/6–311++G(d,p) basis sets. Additionally, Zhou and Schlegel report a triplet ThOH+ (specific state not provided) lying 1.1 eV higher in energy than the 1A′ ground state using PW91/SDD/6–311++G(d,p). Theoretical BDEs indicate that D0(Th+-OH) = 5.5–7.2 eV (Table S3) indicating that reaction 6 is exothermic by 0.3–2.0 eV, consistent with observation.
ThD+
Given D0(Th+-D) = 2.48 ± 0.07 eV determined in a previous GIBMS experiment of the reactions of Th+ + H2 and D2 [25] and D0(DO-D) = 5.212 ± 0.002 eV [82], reaction 7 should be endothermic by 2.73 ± 0.07 eV, roughly consistent with observation, Fig. 1. As shown in Fig. 3, the cross-section for reaction 7 was modeled using Eq. 1, with parameters in Table 1. The threshold obtained, E0 = 3.40 ± 0.31 eV, is somewhat above that predicted using the literature thermochemistry. The discrepancy is likely caused by competition of reaction 7 with the thermodynamically more favorable reactions 5 and 6, whereas the reaction with D2 has no competing channels. A similar shift in thermodynamic threshold was observed in the reaction of Th+ + CH4 forming ThH+ + CH3 where a shift of 0.21 ± 0.21 eV was observed compared to the threshold expected on the basis of the reactions of Th+ + H2 and D2. This shift was subsequently verified by a PST modeling of the competing products in the Th+ + CH4 reaction, formation of ThCH2+, ThH+, and ThCH3+ [24]. This possibility is discussed further in the section below where the competing reactions 5–7 are modeled using PST.
Potential Energy Surfaces
The potential energy surface (PES) calculated using CCSD(T)/CBS//PBE0/cc-pVQZ/cc-pVTZ for the reaction of Th+ + H2O is presented in Fig. 4, with the related structures presented in Fig. 5. Energies for all intermediates and transition states at this and additional levels of theory are tabulated in Table 2. After correcting for spin-orbit energy as described above, reported energies in Table 2 are relative to Th+ (4F3/2, 6d27s) + H2O. (Uncorrected values are also listed in parentheses relative to Th+ (2D, 6d7s2) + H2O for reference.) Energies calculated using several levels of theory and additional basis sets are listed in Table S4 in the Supplementary Material. Before correcting for spin-orbit energy, the energies for all intermediates and transition states are similar to those reported previously by Mazzone et al. [27] and Zhou and Schlegel [28]. Without the spin-orbit correction, the doublet surface lies below the quartet surface throughout the entire reaction, whereas the introduction of the semi-empirical spin-orbit correction shifts the starting asymptote to the quartet surface suggesting a crossing between the doublet and quartet surfaces, a feature not suggested previously. However, the mixed state nature of the Th+ J = 3/2 ground level (4F3/2, 2D3/2) indicates that this crossing may be an artifact introduced by the need to designate a spin state in the computations, as we have argued previously for a similar crossing in the Th+ + CH4 PESs [24]. In reality, spin is likely a poor quantum number to describe ground level Th+ and its weakly bound adducts, and both doublet and quartet spin surfaces can evolve from the J = 3/2 ground level adduct.
Potential energy surfaces for the two major products of the Th+ + H2O reaction. Single point energies calculated using CCSD(T)/CBS from PBE0/cc-pVQZ/cc-pVTZ optimized structures. Quartet spin surface in blue and doublet spin surface in red. Dotted lines represent the surfaces uncorrected for spin-orbit interactions (right-hand axis) whereas solid lines denote the spin-orbit corrected surface (left-hand axis)
Structures of intermediates and transition states along the Th+ + H2O potential energy surfaces shown in Fig. 4 as optimized at the PBE0/cc-pVQZ/cc-pVTZ level of theory. Select bond lengths (Å) and bond angles (°) are also provided
Doublet Surface
The first intermediate on the doublet surface, Th+(H2O) 21, has a 2B2 ground electronic state (2A2 for M06) and lies 1.34 eV below the Th+ (4F3/2, 6d27s) + H2O asymptote. The ∠HOH and r(O-H) of 21, Fig. 5, are largely unperturbed from those of free water, ∠HOH = 104° and r(O-H) = 0.96 Å, which suggests that 21 is an association complex between Th+ and H2O. From 21, OH bond activation occurs through 2TS1/2 (2A′). One O–H bond elongates to r(O-H) = 1.20 Å and rotates from ∠ThOH = 126° in 21 to 78° while the r(Th+-O) bond length shortens to 2.15 Å. This process forms 22, a hydrido thorium hydroxide cation, which is the global minimum lying 3.87 eV below the reactant asymptote. It has a 2A′ ground state with r(Th+-H) and r(Th+-O), Fig. 5, being nearly identical to the Th+-H bond length in the diatomic ThH+ (3Δ) ground state calculated previously, r(Th+-H) = 2.00 Å [25], and the ThOH+ (3Δ) bond length calculated here, r(Th+-OH) = 1.99 Å. These comparisons indicate that there are covalent bonds between the metal center and both ligands in HThOH+ (2A′).
22 is also a common intermediate between all three primary reaction channels, reactions 5–7. 22 is connected to the lowest energy products, ThO+ + H2, through 2TS2/3 (2A′). In 2TS2/3, both hydrogens rotate toward each other forming a four-centered ring structure, Fig. 5. 2TS2/3 lies 1.17 eV above 22 and leads to 23, (H2)ThO+. In 23, r(Th+-O) shortens to 1.80 Å, similar to r(Th+-O) = 1.79 Å in ground state ThO+ (2Σ+), with r(H-H) = 0.76 Å, similar to r(H-H) = 0.75 Å in H2 (1Σg+). Thus, 23 can be understood as a weakly bound association complex between ThO+ (2Σ+) and H2. 23 lies 3.81 eV below the reactant asymptote and readily dissociates to the ThO+ (2Σ+) + H2 (1Σg+) product asymptote, only 0.07 eV higher in energy. Given D0(O-H2) = 5.01 eV at CCSD(T)/CBS (compared to the experimental BDE, 5.0348 ± 0.0003 eV [84]), the ThO+ BDE is predicted to be D0(Th+-O) = 8.75 eV, in reasonable agreement with the experimental value of 8.57 ± 0.14 eV [26].
Alternatively, the reaction can proceed from 22 through 2TS2/4. In 2TS2/4, ∠HThO opens to 110° from 98° in 22 and r(Th+-O) contracts to 1.84 Å while r(O-H) increases from 0.96 Å in 22 to 1.57 Å. The transition state, 2TS2/4, lies 2.58 eV above the global minimum (1.41 eV higher in energy than 2TS2/3) and leads directly to the HThO+ (1A′) + H (2S) products that lie 1.95 eV below the reactants (1.92 eV above the global minimum). Given D0(HO-H) = 5.08 eV for CCSD(T)/CBS (compared to the experimental BDE, 5.1014 ± 0.0004 eV [84]), dissociation of HThO+ to Th+ + OH is predicted to require D0(Th+-OH) = 6.96 eV. Intermediate 22 can also dissociate by breaking the H-ThOH+ bond, forming ThOH+ (3Δ) + H (2S), calculated to lie 0.40 eV above 2TS2/4. Finally, at higher energies, 22 can dissociate directly to ThH+ (3Δ1) + OH (2Π), which is calculated to lie 2.36 eV above the reactants, in reasonable agreement with the experimental asymptote of 2.65 ± 0.07 eV (given D0(Th+-D) = 2.48 ± 0.07 eV [25]).
Quartet Surface
Along the quartet spin reaction surface, the first intermediate, 41, has a 4B2 ground state that lies 0.21 eV lower in energy than 21. 4A2 and 4B1 states were also found within 0.5 eV of 21 for all methods except M06, as listed in Table S4 in the Supplementary Material. The reaction along this surface evolves through 4TS1/2 to form 42. 4TS1/2 (4A″) lies 0.44 eV higher in energy than 2TS1/2. Geometrical parameters for 4TS1/2 are similar to those found for 2TS1/2, Fig. 5. 42 (4A″) lies 2.92 eV higher in energy than 22 (2A′). Unlike 22 where r(Th-H) = 2.00 Å, r(Th-H) in 42 is 3.14 Å, indicating that the bond order here is only 1/2, a consequence of the high spin state. This intermediate readily dissociates to ThOH+ (3Δ) + H (2S) with the addition of only 0.06 eV. Overall, this reaction is calculated to be exothermic by 0.89 eV. Once past the first intermediate, exploratory calculations indicate that the quartet surface is significantly higher in energy than the doublet surface (by 2.8–4.5 eV). Further, because the H atom is lost so easily from 42, channels like 4TS2/3 and 4TS2/4 cannot plausibly occur. Thus, it is unlikely that the quartet surface plays a significant role in the overall reaction at thermal energies except in the entrance channel.
Comparison to Experimental Behavior
The PESs of Fig. 4 now permit an analysis of the experimental observations in Fig. 1. Clearly, the exothermic formation of ThO+ + D2 and DThO+ + D can be explained by evolution along the doublet surface leading to the common intermediate 22, DThOD+. At low kinetic energies, products are formed by passing over 2TS2/3 and 2TS2/4, respectively. Competition between these two channels is explored more thoroughly in the next section. As the energy is increased, a new channel for reaction 6 becomes available, leading to a small increase in the [Th,O,D]+ cross-section. According to the PES shown in Fig. 4, this new channel could be associated with evolution along the quartet surface over 4TS1/2, leading to formation of ThOD+ (3Δ) + D. Although calculations indicate that 4TS1/2 lies 0.1–1.4 eV below the reactants, this entropically disfavored tight TS could lead to the appearance of an “endothermic” feature in the cross-sections, as discussed further below. Other possibilities include formation of excited states of either HThO+ or ThOH+ (as listed in Tables S1 and S2) although these were not explored fully.
Starting at about 1 eV, both the DThO+ and ThOD+ products can begin to dissociate by losing a D atom to form ThO+, resulting in the increase observed in the ThO+ cross-section and concomitant decrease in the DThO+ cross-section. This conversion is also evident in the branching ratio starting at 1 eV, Fig. 2. At still higher energies, above D0(O-D2) and D0(DO-D), both the ThO+ and DThO+ cross-sections decrease even more rapidly as these products can dissociate to form Th+ + O and Th+ + OD, respectively.
Theoretical Branching Ratios
Having characterized the potential energy surface for the competing reactions 5–7, it is now possible to model the experimental ThO+/HThO+ branching ratio. Previously, Zhou and Schlegel [28] calculated the ThO+/HThO+ branching ratio using RRKM theory and energies and molecular parameters calculated at various levels of theory. Despite ThO+ being more thermodynamically favorable, RRKM calculations favor HThO+ heavily, with a branching ratio of 11:89, in strong disagreement with the experimentally observed ratio of ThO+/HThO+ = 65:35 [9, 16]. While somewhat surprising, the result can be understood because RRKM favors reactions where the transition state has low frequencies compared to transition states with higher frequencies. As pointed out previously by Zhou and Schlegel, 2TS2/4 is much “looser” than the corresponding 2TS2/3 as observed by the vibrational frequencies of these TSs in Table 3. Figure 5 shows that this is a result of the constrained geometry of 2TS2/3 compared to the much more open structure of 2TS2/4.
Here, we calculate branching ratios according to RRKM and PST rate constants using energies calculated at multiple levels of theory. The results presented in Table 4 assume that 2TS2/3 and 2TS2/4 represent the rate-determining step (rds) to reactions 5 and 6 from 22, respectively. Energies available for reaction are taken from the energies of these transition states relative to the reactant asymptote before and after spin-orbit corrections. Molecular parameters used for 22, 2TS2/3, and 2TS2/4 were taken from the structures optimized at the B3LYP/SDD/6–311++G(3df,3p) level of theory for the smaller basis sets (SDD and Seg. SDD) and the PBE0/cc-pVQZ/cc-pVTZ level of theory for the correlation consistent basis sets. These frequencies are listed in Table 3.
Table 4 shows that RRKM ThO+/HThO+ branching ratios predict that the HThO+ product is favored in every case for energies uncorrected for spin-orbit interactions. Correcting for spin-orbit energies moves the predictions closer to experiment but still favors the HThO+ product in every case except CCSD(T)/SDD. These RRKM results are similar to those of Zhou and Schlegel, 11:89 [28]. Zhou and Schlegel explained this discrepancy as likely being a result of the large energies involved so that the competition was no longer statistical [28].
Zhou and Schlegel [28] overcame this limitation by exploring a semi-classical trajectory simulation that found a branching ratio of ThO+/HThO+ ~ 80:20, much closer to experiment, but still not quantitative. We believe that the main weakness in the RRKM analysis is that it begins at 22 so that no history of the reactants is considered. In reality, angular momentum constraints limit the number of successful trajectories for the protiated and deuterated versions of both reactions 5 and 6. More specifically, the reduced mass (μ) of the ThO+ + H2 products is 1.999 amu, whereas the HThO+ + H products are half that, 1.004 amu. In both cases, the reduced mass of the products is much smaller than that of the reactants, μ = 16.713 amu, such that both channels can only conserve angular momentum for smaller impact parameters. (Similar relationships constrain reactions 5 and 6, where the reduced masses are 3.96, 1.99, and 18.41 amu, respectively.) This tendency is overcome to some extent by the large exothermicity of both channels (even considering their rds transition states). Nevertheless, angular momentum conservation clearly favors reaction 5 and its protiated variant, in agreement with the observed behavior. Although the reduced mass argument is only semi-quantitative in nature, it definitely indicates that properly accounting for angular momentum conservation is needed to correctly predict the branching ratio.
Here, we account for this quantitatively by utilizing PST, which explicitly requires conservation of angular momentum from the reactants. Unlike the RRKM calculations, the PST branching ratios heavily favor ThO+ before correcting for spin-orbit effects. Accounting for spin-orbit energy, the PST branching ratios shift more in favor of ThO+. Now, accurately predicting the experimental branching ratio depends on the details of the energies used for the two competing transition states. PST branching ratios calculated from B3LYP, B3PW91, M06, and PBE0 energies (with all basis sets) are all within ± 11% of the observed experimental branching ratio of 65/35. These ratios result from available energies of 2.48–2.97 eV for 2TS2/3 and 1.29–1.70 eV for 2TS2/4 after including spin-orbit effects, Tables 2 and S4, with ETS2/4 − ETS2/3 = 1.08–1.29. By contrast, both CCSD(T) and BHLYP calculations are skewed heavily toward formation of ThO+. Here, the CCSD(T) calculations yield energies of 2.15–2.75 eV for 2TS2/3 and 0.72–1.33 eV for 2TS2/4 with ETS2/4 − ETS2/3 = 1.31–1.43. This suggests that an energy difference between the transition states towards the lower end of ~ 1.1 eV is needed to correctly predict the observed branching ratio.
Phase Space Theory Modeling of Cross-Sections
A much more stringent test of the theory is to model the kinetic energy dependence of reactions 5 and 6. Clearly, PST is needed and we considered two approaches, both of which use the trajectory model for the total collision probability. These models ignore any potential spin changes in the reaction; however, the mixed spin nature of the Th+ J = 3/2 ground level argues that any effects resulting from a change in spin should be minimal. In the first approach, each channel is modeled as above, using the rds for the competing reactions (i.e., 2TS2/3 and 2TS2/4, respectively). This approach is justifiable because reactions 5 and 6 pass through a common intermediate, 22, so that the only difference in reaction rates of each channel comes from their rds. Here, molecular parameters (vibrational frequencies and rotational constants) taken from B3LYP/SDD/6–311++G(3df, 3p) optimized structures of deuterated 2TS2/3 and 2TS2/4 are used in the modeling. These PST model cross-sections can be found in Fig. 6 and Fig. 2 shows the prediction of the branching ratios. In both cases, the PST model replicates the experimental results well at low energies for reactions 5 and 6 and the threshold region for reaction 7, discussed further below. Above 1 eV, the PST model fails primarily because these calculations do not account for the decomposition of DThO+ into ThO+ + D, starting at 1.10 ± 0.14 eV. This is particularly evident in Fig. 2. In addition, the minor contribution from the additional pathway forming [Th,O,D]+, postulated to proceed over 4TS1/2 above, is not included in this model. A PST model that did include competition between 2TS2/3, 2TS2/4, and 4TS1/2 was attempted and demonstrates that apparent “endothermic behavior” as observed experimentally can occur for the 4TS1/2 channel even with its energy as much as 0.5 eV below reactants, comparable to the calculations (Table 2). This model is not quantitative, however, requiring significant scaling of this channel to reproduce the data, which then interferes with the low energy behavior of the two major channels. Given the loosely bound nature of the D atom to ThOD+ along the quartet surface, this scaling could easily be a consequence of inaccurate vibrational frequencies associated with this weak interaction. As such scaling makes this interpretation even more speculative, it was not pursued further.
Product cross-sections for the reaction of Th+ with D2O (points) compared to the phase space theory model discussed in the text convoluted over the distributions of internal and translational reactant energies (solid lines) and excluding these distributions (dashed lines). The black line shows the predicted cross-section for returning to reactants
The only adjustable parameters for the PST model are the threshold energies for each channel, which correspond to the rds for each process. The models shown in Figs. 2 and 6 utilize thresholds of E0(5) = −2.0 ± 0.2 eV and E0(6) = −1.0 ± 0.1 eV in order to reproduce the experimental data. Importantly, the energy difference between these two values needs to be 0.9–1.0 eV in order to reproduce experiment. Note that this difference is in agreement with the value of about 1.1 eV obtained above from modeling the thermal branching ratio alone. Further, we can compare these energies with those calculated theoretically for 2TS2/3 and 2TS2/4 in Table 2 (ignoring the small zero-point energy differences). Values for 2TS2/3 range from − 2.26 to − 2.97 eV, 0.26–0.97 ± 0.2 eV lower than the PST modeling, whereas values for 2TS2/4 lie at − 0.96 to − 1.68 eV compared to the PST value of − 1.0 ± 0.1 eV. BHLYP values are nearly in agreement with the PST modeling. Theoretical energy differences between the two TSs range from 1.15 to 1.41 eV, slightly larger than the preferred value of ~ 1 ± 0.1 eV.
The second modeling approach tried assumes that 2TS1/2 is the rds in both reactions 5 and 6. The PST model is adapted to calculate the rate through this “tight” transition state and utilizes the molecular parameters calculated in the B3LYP/SDD/6–311++G(3df, 3p) optimized structure of deuterated 2TS1/2. Because 2TS1/2 leads to both sets of products from reactions 5 and 6, the total cross-section was modeled and led to an energy for 2TS1/2 of − 2.0 ± 0.2 eV. This value can be compared with the theoretical values in Table 2, which range from − 0.46 to − 1.58 eV, with the CCSD(T) value at − 0.68 eV. The discrepancies of 1.5–0.4 ± 0.2 eV suggest that this is not a useful means of reproducing the data.
Regardless of the approach used to model reactions 5 and 6, the PST model yields E0(7) = 3.55 ± 0.10 eV for formation of ThD+ + OD, similar to the threshold obtained by modeling with Eq. 1, Table 1. This value is much higher than the expected threshold of 2.73 ± 0.07 eV suggested by the D0(Th+-D) value determined previously [25]. The discrepancy is probably because the PST model does not account for the decomposition of DThO+ into ThO+ + D, nor for the higher energy pathway forming ThOD+ + D, such that the competition among reactions 5–7 is not accurately portrayed at energies above about 1 eV.
Discussion
A major motivation for our recent work with thorium is to provide experimental benchmarks to which theoretical values can be compared. Previous studies of the reactions of thorium with carbon monoxide [26] and hydrogen [25] indicate enthalpies of reaction for the perprotiated analogues of reactions 5 and 7 are ΔrH(5) = − 3.54 ± 0.14 eV and ΔrH(7) = 2.65 ± 0.07 eV relative to the reactants, respectively. PST modeling in this work indicates that 2TS2/3 and 2TS2/4 lie 2.0 ± 0.2 and 1.0 ± 0.1 eV below the reactants, respectively. Table 5 compares these experimental values to CCSD(T) calculated values, two other select levels of theory, and values from the literature [28]. Although Zhou and Schlegel reported results from several approaches, they considered the most reliable results to be PW91/ZORA-SO (which includes spin-orbit corrections) and CCSD(T)/SDD+ (a composite approach using a larger basis set). These two approaches give similar energy values except for 2TS2/3 (differing by 0.68 eV), hence, only the CCSD(T)/SDD+ values are listed in Table 5 as these results agree better with the present experiments. Comparison with the present calculations is hindered by the fact that Zhou and Schlegel do not report an energy for the Th+ + H2O reactants, but rather refer to the results of Mazzone et al. [27] citing a relative energy for 21 of − 0.87 eV.
A couple of trends can be observed from Table 5. The reaction forming the ThO+ + H2 products, the analogue of reaction 5, has an exothermicity that is reasonably well predicted by theory, with deviations between − 0.24 and + 0.31 eV. In contrast, the endothermicity in the formation of ThH+ + OH in the analogue of reaction 7 is systematically too low, by 0.2–0.9 eV. This latter deviation agrees with the more extensive theoretical exploration in our study of the Th+ + H2/D2 reactions [25]. Likewise, all calculations predict that 2TS2/3 is lower than found by the PST modeling, although CCSD(T)/SDD and CCSD(T)/SDD+ results are within and BHLYP/CBS results are just outside the experimental uncertainty. For 2TS2/4, the CCSD(T)/SDD and BHLYP/CBS approaches predict a higher energy than the PST model result (with the BHLYP/CBS value within experimental uncertainty, which is why this result is singled out here), whereas all other approaches listed predict lower energies (by 0.2–0.6 eV), with the CCSD(T)/SDD+ results showing the largest deviation.
The mean absolute deviations (MADs) listed in Table 5 between these four experimental energies and theoretical values indicate similar abilities to reproduce the experimental values, with BHLYP/CBS and CCSD(T)/SDD+ (which is favored because it does not include the problematic comparison with ΔrH(7)) being slightly better and CCSD(T)/Seg. SDD slightly worse than the other approaches. Although CCSD(T)/SDD and BHLYP/CBS have two values above and two values below experiment, all other levels of theory are systematically low. It should be realized that without the spin-orbit corrections (included in all the present theoretical values in Table 5), the results would be considerably worse for all levels of theory. Such deviations for actinide thermochemistry are not unprecedented with most levels of theory systematically overestimating bond energies for ThH+ (by 0.0–0.5 eV), ThC+ (by 0.0–0.6 eV), and ThO+ (by 0.0–0.4 eV) (which leads to low values on the PES for these two asymptotes) [25, 26]. In these works, more advanced theoretical approaches [85, 86] provided more accurate results, but are beyond the scope of an entire PES.
In analyzing the performance of theory, it is also instructive to examine the predicted branching ratio at thermal energies calculated using PST and the energies of 2TS2/3 and 2TS2/4 for each level of theory. This comparison is also listed in Table 5. Such an analysis indicates that although the CCSD(T)/SDD calculations yield comparable results to the correlation consistent basis sets for the thermochemical values, it deviates by ± 29% from the experimental branching ratio compared to ± 18% for the CCSD(T) using correlation consistent basis sets and ± 23% for BHLYP/CBS. M06/CBS gives the best result with a deviation of only ± 8% (and the reason this particular level of theory is included in this comparison). Note that this level has the smallest difference in energies between 2TS2/3 and 2TS2/4, 1.15 eV and similar to the experimental value of about 1.0 ± 0.1 eV, compared with 1.30–1.43 eV for the other methods in Table 5. Consistent with these observations is the fact that for the trajectory results of Zhou and Schlegel [28], the energies used were those calculated at the PW91/SDD level where the energy difference between 2TS2/3 and 2TS2/4 was 1.18 eV. Although the resulting ~ 80:20 branching ratio is in reasonable agreement with experiment, the result was obtained with only 16 trajectories that were initiated from 2TS1/2. Thus, the return to reactants, which limits the overall efficiency of the reaction and also competes with reactions 5 and 6, was not considered nor was the distribution of angular momentum available to the species likely to be accurate.
It is important to also realize that these theoretical branching ratios represent relative rates rather than absolute rates, so that a particular method can reproduce the experimental branching ratio while simultaneously incorrectly predicting the absolute rates. Therefore, a comparison of theoretical branching ratios to the experimentally observed ratios may be a valuable metric to evaluate theoretical methods and basis sets; however, without absolute rates they may be misleading. Further, the snapshot of predicting only the thermal branching ratio is clearly not as demanding as reproducing the interesting kinetic energy dependence over an extended range, such as that shown in Figs. 2 and 6.
References
Armentrout, P.B., Hodges, R.V., Beauchamp, J.L.: Endothermic reactions of uranium ions with N2, D2 and CD4. J. Chem. Phys. 66, 4683–4688 (1977)
Armentrout, P.B., Hodges, R.V., Beauchamp, J.L.: Metal atoms as super bases: the gas phase proton affinity of uranium. J. Am. Chem. Soc. 99, 3162–3263 (1977)
Armentrout, P.B., Beauchamp, J.L.: Collision induced dissociation of UO+ and UO2 +. Chem Phys. 50, 21–25 (1980)
Armentrout, P.B., Beauchamp, J.L.: Reactions of U+ and UO+ with O2, CO, CO2, COS, CS2 and D2O. Chem. Phys. 50, 27–36 (1980)
Armentrout, P.B., Beauchamp, J.L.: Thermochemistry of uranium halide ions. Reactions of U+ with CH3F, SiF4, CH3Cl, and CCl4. J. Phys. Chem. 85, 4103–4105 (1981)
Heinemann, C., Schwarz, H.: NUO+, a new species isoelectronic to the uranyl dication UO. Chem. Eur. J. 1, 7–11 (1995)
Cornehl, H.H., Heinemann, C., Marçalo, J., de Matos, A.P., Schwarz, H.: The “bare” uranyl(2+) ion, UO2 2+. Ang. Chem. Int. Ed. 35, 891–894 (1996)
Heinemann, C., Cornehl, H.H., Schwarz, H.: Hydrocarbon activation by “bare” uranium cations: formation of a cationic uranium–benzene complex from three ethylene units. J. Organomet. Chem. 501, 201–209 (1995)
Cornehl, H.H., Wesendrup, R., Diefenbach, M., Schwarz, H.: A comparative study of oxo-ligand effects in the gas-phase chemistry of atomic lanthanide and actinide cations. Chem. Eur. J. 3, 1083–1090 (1997)
Schröder, D., Diefenbach, M., Klapötke, T.M., Schwarz, H.: UF3 +—a thermochemically stable diatomic trication with a covalent bond. Ang. Chem. Int. Ed. 38, 137–140 (1999)
Gibson, J.K.: Gas-phase transuranium organometallic chemistry: reactions of Np+, Pu+, NpO+, and PuO+ with alkenes. J. Am. Chem. Soc. 120, 2633–2640 (1998)
Gibson, J.K.: Actinide gas-phase chemistry: reactions of An+ and AnO+ [An = Th, U, Np, Pu, Am] with nitriles and butylamine. Inorg. Chem. 38, 165–173 (1999)
Gibson, J. K., Gas-phase reactions of An+ and AnO+ [An = Th, U, Np, Pu, Am] with halogenated hydrocarbons [C14F24, C3F6, C2H4Cl2 and C2H4Br2]. In Radiochim. Acta, 1999; Vol. 84, p 135
Gibson, J. K., Haire, R. G., Berkelium and californium organometallic ions. In Radiochim. Acta, 2001; Vol. 89, p 363
Gibson, J.K., Haire, R.G.: Gas-phase chemistry of bare and oxo-ligated protactinium ions: a contribution to a systematic understanding of actinide chemistry. Inorg. Chem. 41, 5897–5906 (2002)
Santos, M., Marçalo, J., Matos, A.P.d., Gibson, J.K., Haire, R.G.: Gas-phase oxidation reactions of neptunium and plutonium ions investigated via Fourier transform ion cyclotron resonance mass spectrometry. J. Phys. Chem. A. 106, 7190–7194 (2002)
Santos, M., Marçalo, J., Leal, J.P., Matos, A.P.d., Gibson, J.K., Haire, R.G.: FTICR-MS study of the gas-phase thermochemistry of americium oxides. Int. J. Mass Spectrom. 228, 457–465 (2003)
Goncharov, V., Heaven, M.C.: Spectroscopy of the ground and low-lying excited states of ThO+. J. Chem. Phys. 124, 064312 (2006)
Heaven, M.C.: Probing actinide electronic structure using fluorescence and multi-photon ionization spectroscopy. Phys. Chem. Chem. Phys. 8, 4497–4509 (2006)
Gibson, J.K., Haire, R.G., Marçalo, J., Santos, M., Leal, J.P., Pires de Matos, A., Tyagi, R., Mrozik, M.K., Pitzer, R.M., Bursten, B.E.: FTICR/MS studies of gas-phase actinide ion reactions: fundamental chemical and physical properties of atomic and molecular actinide ions and neutrals. Eur. Phys. J. D. 45, 133–138 (2007)
Marçalo, J., Gibson, J.K.: Gas-phase energetics of actinide oxides: an assessment of neutral and cationic monoxides and dioxides from thorium to curium. J. Phys. Chem. A. 113, 12599–12606 (2009)
Pereira, C.C.L., Marsden, C.J., Marçalo, J., Gibson, J.K.: Actinide sulfides in the gas phase: experimental and theoretical studies of the thermochemistry of Ans (An = Ac, Th, Pa, U, Np, Pu, Am and Cm). Phys. Chem. Chem. Phys. 13, 12940–12958 (2011)
Heaven, M.C., Barker, B.J., Antonov, I.O.: Spectroscopy and structure of the simplest actinide bonds. J. Phys. Chem. A. 118, 10867–10881 (2014)
Cox, R.M., Armentrout, P.B., de Jong, W.A.: Activation of CH4 by Th+ as studied by guided ion beam mass spectrometry and quantum chemistry. Inorg. Chem. 54, 3584–3599 (2015)
Cox, R.M., Armentrout, P.B., de Jong, W.A.: Reactions of Th+ + H2, D2, and HD studied by guided ion beam tandem mass spectrometry and quantum chemical calculations. J. Phys. Chem. B. 120, 1601–1614 (2016)
Cox, R.M., Citir, M., Armentrout, P.B., Battey, S.R., Peterson, K.A.: Bond energies of ThO+ and ThC+: a guided ion beam and quantum chemical investigation of the reactions of thorium cation with O2 and CO. J. Chem. Phys. 144, 184309 (2016)
Mazzone, G., Michelini, M.d.C., Russo, N., Sicilia, E.: Mechanistic aspects of the reaction of Th+ and Th2+ with water in the gas phase. Inorg. Chem. 47, 2083–2088 (2008)
Zhou, J., Schlegel, H.B.: Ab initio molecular dynamics study of the reaction between Th+ and H2O. J. Phys. Chem. A. 114, 8613–8617 (2010)
Gilbert, R.G., Smith, S.C.: Theory of unimolecular and recombination reactions. Blackwell Scientific, London (1990)
Robinson, P.J., Holbrook, K.A.: Unimolecular reactions. Wiley Interscience, New York (1972)
Loh, S.K., Hales, D.A., Lian, L., Armentrout, P.B.: Collision-induced dissociation of Fen + (n = 2–10) with Xe: ionic and neutral iron cluster binding energies. J. Chem. Phys. 90, 5466–5485 (1989)
Schultz, R.H., Armentrout, P.B.: Reactions of N4 + with rare gases from thermal to 10 eV c.M.: collision-induced dissociation, charge transfer, and ligand exchange. Int. J. Mass Spectrom. Ion Process. 107, 29–48 (1991)
Haynes, C.L., Armentrout, P.B.: Thermochemistry and structures of CoC3H6 +: metallacycle and metal-alkene isomers. Organomet. 13, 3480–3490 (1994)
Clemmer, D.E., Chen, Y.-M., Khan, F.A., Armentrout, P.B.: State-specific reactions of Fe+(a6D, a4F) with D2O and reactions of FeO+ with D2. J. Phys. Chem. 98, 6522–6529 (1994)
Kickel, B.L., Armentrout, P.B.: Reactions of Fe+, Co+ and Ni+ with silane. Electronic state effects and M+-SiHx (x = 0–3) bond energies. J. Am. Chem. Soc. 117, 764–773 (1995)
Kickel, B.L., Armentrout, P.B.: Guided ion beam studies of the reactions of group 3 metal ions (Sc+, Y+, La+, and Lu+) with silane. Electronic state effects, comparison to reactions with methane, and M+-SiHx (x = 0–3) bond energies. J. Am. Chem. Soc. 117, 4057–4070 (1995)
Sievers, M.R., Chen, Y.-M., Elkind, J.L., Armentrout, P.B.: Reactions of Y+, Zr+, Nb+, and Mo+ with H2, HD, and D2. J. Phys. Chem. 100, 54–62 (1996)
Teloy, E., Gerlich, D.: Integral cross sections for ion-molecule reactions. 1. The guided beam technique. Chem. Phys. 4, 417–427 (1974)
Armentrout, P.B.: The kinetic energy dependence of ion-molecule reactions: guided ion beams and threshold measurements. Int. J. Mass Spectrom. 200, 219–241 (2000)
Ervin, K.M., Armentrout, P.B.: Translational energy dependence of Ar+ + XY → ArX+ + Y (XY = H2, D2, HD) from thermal to 30 eV c.m. J. Chem. Phys. 83, 166–189 (1985)
Chantry, P.J.: Doppler broadening in beam experiments. J. Chem. Phys. 55, 2746–2759 (1971)
Light, J.C.: Phase-space theory of chemical kinetics. J. Chem. Phys. 40, 3221–3229 (1964)
Pechukas, P., Light, J.C.: On detailed balancing and statistical theories of chemical kinetics. J. Chem. Phys. 42, 3281–3291 (1965)
Nikitin, E.E.: Statististical theory of exothermic ion-molecule reactions. Teor. Eksp. Khim. 1, 428–435 (1965)
Chesnavich, W.J., Bowers, M.T.: Threshold behavior of endoergic bimolecular reactions: a statistical phase space approach. J. Phys. Chem. 68, 901–910 (1978)
Langevin, P.: Une formule fondamentale de theorie cinetique. Ann. Chim. Phys. Ser. 8(5), 245–288 (1905)
Gioumousis, G., Stevenson, D.P.: Reactions of gaseous molecule ions with gaseous molecules. V. Theory. J. Chem. Phys. 29, 294–299 (1958)
Su, T., Bowers, M.T.: Classical ion-molecule collision theory. In: Bowers, M.T. (ed.) Gas phase ion chemistry, vol. 1, pp. 83–118. Academic, New York (1979)
Su, T., Chesnavich, W.J.: Parameterization of the ion-polar molecule collision rate constant by trajectory calculations. J. Chem. Phys. 76, 5183–5185 (1982)
Weber, M.E., Dalleska, N.F., Tjelta, B.L., Fisher, E.R., Armentrout, P.B.: Reaction of O2 +(X 2Πg) with H2 , D2 , and HD: guided ion beam studies, mo correlations, and statistical theory calculations. J. Chem. Phys. 98, 7855–7867 (1993)
Chesnavich, W.J., Bowers, M.T.: Theory of translationally driven reactions. J. Phys. Chem. 83, 900–905 (1979)
Muntean, F., Armentrout, P.B.: Guided ion beam study of collision-induced dissociation dynamics: integral and differential cross sections. J. Chem. Phys. 115, 1213–1228 (2001)
Aristov, N., Armentrout, P.B.: Reaction mechanisms and thermochemistry of V+ + C2H2p (p = 1,2,3). J. Am. Chem. Soc. 108, 1806–1819 (1986)
Weber, M.E., Elkind, J.L., Armentrout, P.B.: Kinetic energy dependence of Al+ + O2 → AlO+ + O. J. Chem. Phys. 84, 1521–1529 (1986)
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J. A., Peralta, J. E., Ogliaro, F., Bearpark, M. J., Heyd, J., Brothers, E. N., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A. P., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, N. J., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., Fox, D. J.: Gaussian 09, revision d.01 Gaussian, Inc.: Wallingford, CT, USA, 2009
Peterson, K. A.: Correlation consistent basis sets for actinides. I. The Th and U atoms. J. Chem. Phys. 142, 074105 (2015)
Weigand, A., Cao, X., Hangele, T., Dolg, M.: Relativistic small-core pseudopotentials for actinium, thorium, and protactinium. J. Phys. Chem. A. 118, 2519–2530 (2014)
Woon, D.E., Dunning Jr., T.H.: Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for boron through neon. J. Chem. Phys. 103, 4572–4585 (1995)
Dunning, T.H.: Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007–1023 (1989)
Karton, A., Martin, J.M.L.: Comment on: “Estimating the Hartree–Fock limit from finite basis set calculations” [Jensen F (2005) Theor Chem Acc 113:267]. Theor. Chem. Acct. 115, 330–333 (2006)
Martin, J.M.L.: Ab initio total atomization energies of small molecules—towards the basis set limit. Chem. Phys. Lett. 259, 669–678 (1996)
Küchle, W., Dolg, M., Stoll, H., Preuss, H.: Energy-adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J. Chem. Phys. 100, 7535–7542 (1994)
Cao, X., Dolg, M., Stoll, H.: Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials. J. Chem. Phys. 118, 487–496 (2003)
Krishnan, R., Binkley, J.S., Seeger, R., Pople, J.A.: Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 37, 785–789 (1988)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Perdew, J.P., Burke, K., Wang, Y.: Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B. 54, 16533–16539 (1996)
Zhao, Y., Truhlar, D.G.: The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Accounts. 120, 215–241 (2008)
Adamo, C., Barone, V.: Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999)
Purvis, G.D., Bartlett, R.J.: A full coupled-cluster singles and doubles model: the inclusion of disconnected triples. J. Chem. Phys. 76, 1910–1918 (1982)
Pople, J.A., Head-Gordon, M., Raghavachari, K.: Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 87, 5968–5975 (1987)
Scuseria, G.E., Janssen, C.L., Schaefer, H.F.: An efficient reformulation of the closed-shell coupled cluster single and double excitation (CCSD) equations. J. Chem. Phys. 89, 7382–7387 (1988)
Foresman, J.B., Frisch, A.E.: Exploring chemistry with electronic structure methods, 2nd edn. Gaussian, Inc., Pittsburgh, PA (1996)
Kesharwani, M.K., Brauer, B., Martin, J.M.L.: Frequency and zero-point vibrational energy scale factors for double-hybrid density functionals (and other selected methods): can anharmonic force fields be avoided? J. Phys. Chem. A. 119, 1701–1714 (2014)
Garcia, M.A., Morse, M.D.: Resonant two-photon ionization spectroscopy of jet-cooled OsN: 520–418 nm. J. Chem. Phys. 135, 114304 (2011)
Armentrout, P.B., Parke, L., Hinton, C., Citir, M.: Activation of methane by Os+: guided ion beam and theoretical studies. ChemPlusChem. 78, 1157–1173 (2013)
Blaise, J., Wyart, J.-F.: Energy levels and atomic spectra of actinides, Paris (1992)
Sansonetti, J.E., Martin, W.C.: Handbook of basic atomic spectroscopic data. J. Phys. Chem. Ref. Data. 34, 1559–2259 (2005)
Beyer, T.S., Swinehart, D.F.: Number of multiply-restricted partitions. Commun. ACM. 16, 379 (1973)
Stein, S.E., Rabinovitch, B.S.: Accurate evaluation of internal energy level sums and densities including anharmonic oscillators and hindered rotors. J. Chem. Phys. 58, 2438–2445 (1973)
Stein, S.E., Rabinovich, B.S.: On the use of exact state counting methods in RRKM rate calculations. Chem. Phys. Lett. 49, 183–188 (1977)
Johnson III, R. D. NIST computational chemistry comparison and benchmark database. NIST Standard Reference Database Number 101, Release 19, http://cccbdb.nist.gov/. Accessed 12 Apr 2018
Konings, R.J.M., Benes, O., Kovacs, A., Manara, D., Sedmidubsky, D., Gorokhov, L., Iorish, V.S., Yungman, V., Shenyavskaya, E., Osina, E.: The thermodynamic properties of the f-elements and their compounds. Part 2. The lanthanide and actinide oxides. J. Phys. Chem. Ref. Data. 43, 013101 (2014)
Goos, E., Burcat, A., Ruscic, B. Extended Third Millennium Ideal Gas and Condensed Phase Thermochemical Database for Combustion with Updates from Active Thermochemical Tables; ANL-05/20 and TAE 960 Technion-IIT, Aerospace Engineering, and Argonne National Laboratory, Chemistry Division: 2016. (accessed August 2017)
Feller, D., Peterson, K.A., Dixon, D.A.: A survey of factors contributing to accurate theoretical predictions of atomization energies and molecular structures. J. Chem. Phys. 129, 204105 (2008)
Vasiliu, M., Peterson, K.A., Gibson, J.K., Dixon, D.A.: Reliable potential energy surfaces for the reactions of H2O with ThO2, PaO2 +, UO2 2+, and UO2 +. J. Phys. Chem. A. 119, 11422–11431 (2015)
Acknowledgements
This work is supported by the Heavy Element Chemistry Program, Office of Basic Energy Sciences, U. S. Department of Energy, through Grant No. DE-SC0012249 (PBA). We also thank the Center for High Performance Computing at the University of Utah for the generous allocation of computer time.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Cox, R.M., Armentrout, P.B. Activation of Water by Thorium Cation: A Guided Ion Beam and Quantum Chemical Study. J. Am. Soc. Mass Spectrom. 30, 1835–1849 (2019). https://doi.org/10.1007/s13361-019-02162-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-019-02162-1