Abstract
This study proposes a new direct and fast method of analysis employing paper spray mass spectrometry (PS-MS). The paper used in the proposed method was modified with molecularly imprinted polymers (MIP) to create a specific site for cocaine analysis in oral fluid. MIP membrane was successfully synthetized and employed. The developed method showed to be linear in a concentration range from LOQ to 100 ng mL–1. The experimental value of LOQ obtained was 1 ng mL–1. The inter-day and intra-day precision and accuracy of the PS-MS method presented values lower than 15%. The total recoveries were also evaluated. The PS-MS method for the analysis of cocaine in oral fluid showed to be very promising and the validation parameters showed a good correlation with the literature.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Drug Report of 2016 [1], a total of 153 countries reported apprehension of cocaine in the period between 2009 and 2014. The traffic concentrates in the Andes region, North America and Europe. Most of 2014 seizures occurred in the Americas, which represent 90% of global seizures of cocaine (particularly in South America, 60%).
The latest interest in a fast method with high resolution is due to the recent legislation changes that were put in place in Brazil. The new law [2] determines zero tolerance to a number of psychoactive drugs present in the system of drivers while conducting a vehicle. Oral fluid is the ideal matrix to perform this kind of assays, once its analysis gives information in a short period of time [3], besides the advantages of an easy and non-invasive collection [4]. In this way, drivers may be required to do a toxicological test to verify the use of such substances.
The analysis of illicit drugs in biological fluids has gained a lot of interest in several research fields, such as toxicology, forensic chemistry and doping. However, this kind of assays can present a few challenges: drugs are often present in low concentration; they are greatly bound to proteins and there are many interfering compounds in biological matrices [5]. Cocaine analysis in complex matrices such as blood, urine, oral fluid and hair are generally performed using a LC-MS (Liquid Chromatography – Mass Spectrometry) or GC-MS (Gas Chromatography - Mass Spectrometry) with a previous derivatization step [6].
As an alternative to sample preparation or the previous separation step, a direct injection source called Paper Spray Ionization (PS) can be employed for MS. Ambient ionization techniques revolutionized MS by introducing a new concept: simplicity. The analysis is performed directly on the surface of the sample and information about its composition can be obtained in real time with an MS.
PS was introduced in 2010 by R. G. Cooks [7] and consists in the application of a high voltage in a triangular paper. The potential difference between the paper and MS inlet generates an electric field with the maximum intensity on the tip of the paper. The generation of ions by PS has the same principles as Electrospray ionization (ESI) [8].
The primary material used to perform PS experiments is chromatographic paper or filter paper. To perform a more selective analysis with higher specificity, a cellulose membrane can be modified to create specific sites to individual analytes. Molecularly Imprinted Polymers (MIP) aim to recreate synthetically the specific sites of identification using a target molecule (template), working similarly as a lock and key model [9]. MIP has been used as a biosensor [10], extraction media [11] and affinity support in the recognition of target molecules, such as drugs [12].
Different methodologies for MIP preparation are reported in the literature. The most used synthesis route consists in the copolymerization of a monomer with a cross-linker, in the presence of a template. After the polymerization step, the template is removed from the porous network via washing process. The cavities formed in the polymeric matrix can then rebind with the template in size, shape and chemical functionality [13].
Using MIP can increase the specificity of PS-MS, adding simplicity, reducing the costs of analysis and increasing the accuracy and precision of the method.
In this study, a MIP membrane was developed and employed as paper in a PS-MS source for the analysis of cocaine in oral fluid samples.
Experimental
Reagents, Standards, and Samples
Methacrylic acid, ethylene glycol and formic acid were purchased from Sigma Aldrich (St. Louis, MO, USA). Cellulose membranes (diameter of 47 mm and 0,45 μm of pore size) were purchased from Agilent Technologies (Santa Clara, CA, USA). Methanol (HPLC grade) was purchased from J.T. Baker (Phillipsburg, NJ, USA). Acetone P.A. was purchased from Scharlau (Sentmenat, Spain). Benzophenone was purchased from Vetec (Rio de Janeiro, RJ, Brazil). Ultrapure water (Millipore) was used throughout this work. Cocaine standard was also purchased from Sigma Aldrich.
Blank human oral fluid samples were collected from ten healthy volunteers that were not exposed to any drugs or medications in the last 3 months, after oral sanitation and before the first meal. The pooled material was spiked with cocaine in the calibration concentration.
MIP and NIP Membrane Synthesis
Molecularly imprinted polymer membranes were synthesized based on the free radical polymerization method described by Hande and coworkers [11]. For the synthesis, cocaine was used as the template, methacrylic acid (MAA) as the monomer, ethylene glycol dimethacrylate (EGDMA) as the cross-linker, benzophenone as the initiator and water as solvent. Cellulose membranes were washed in a Soxlhet apparatus with 400 mL of methanol. After being dried in room temperature, the membranes were put in a 0,15mol L-1 benzophenone solution prepared in acetone for 5 minutes and dried at room temperature again. Then they were submerged in an aqueous solution with 50 mmol L-1 of MAA, 150 mmol L-1 of EGDMA and 10 mmol L-1 of cocaine. The membranes were submitted to UV radiation with a wavelength of 254 nm for 30 minutes. After this procedure they were dried at room temperature and washed in a Soxlhet with 400 mL of methanol for 4 hours to remove the template. Then they were dried at room temperature before the use in PS-MS.
The synthesis of non imprinted polymer (NIP) membranes was made in a similar way to the MIP membranes, but there was no addition of the template in the process.
Morphology Analysis
The morphology of the MIP and NIP membranes were examined via SEM (scanning electron microscope) using a JSM – 6610 (JEOL Ltda., Tokyo, Japan) instrument using 5 kV as accelerating potential.
PS-MS Analysis
MIP membranes were cut in the shape of an equilateral triangle with 1.5 cm of side. To perform the extraction, the triangular membrane was submerged in 1 mL of oral fluid sample for 5 minutes then allowed to dry at room temperature. PS-MS analysis was performed in positive ionization mode, PS(+), by addition of 10 μL of a solution containing methanol with 0.1% of formic acid. All PS-MS experiments were performed on mass spectrometer LCQ Fleet (Thermo Fisher Scientific, San Jose, USA). Temperature of the MS capillary inlet was set at 275°C. The tube lens voltage was set at 50 V. The voltage used for PS was 4 V. Tandem mass spectra were acquired over the m/z 170-310 range. The PS was set by holding the membrane connected directly to the outlet probe of the ESI with a 0.5 mm wire using an alligator-type clip and applying a voltage of 4 V. Figure 1 displays the PS source prototype built in this study.
Analytical Validation
Analytical calibration curves were constructed using linear regression of the intensity of the most abundant fragment of cocaine against its concentration in oral fluid (X, ng mL−1), to evaluate linearity. Accuracy and inter-assay precision were determined using replicates (n = 5) assays of the blank oral fluid sample spiked with analytes solutions representing the entire range of the calibration curve. Accuracy values were calculated by comparing the concentrations of analytes added to the oral fluid samples (theoretical concentration) and the concentrations determined by the analytical calibration curve at three concentration levels (1,50 and 100 ng mL-1).
The selectivity of MIP for cocaine in the presence of lysergic acid diethylamide (LSD) and 3,4-methylenedioxymetham-phetamine (MDMA) as interfering compounds was studied. These chemicals were selected because they are also used as drugs of abuse and can be found in the system of drug users along with cocaine. For the experiment, a membrane of MIP or NIP cut in a triangular shape was added to 1 mL of oral fluid sample enriched with two binary mixtures cocaine/LSD or cocaine/MDMA with concentrations of 100 ng mL-1 and stirred for 5 min in room temperature. The supernatant was collected and measured by PS-MS. Distribution coefficient (Kd), selectivity coefficient (K), and relative selectivity coefficient (K′) [14, 15] were calculated according to the following Equations 1, 2, 3, and 4:
where Ci and Cf are the initial and final analyte concentrations and Vs is the volume of the solution.
Matrix effects (ME) were estimated using the following equation:
where CROF is the cocaine response in oral fluid; IROF is the LSD or MDMA response in oral fluid; CRM is the cocaine response in methanol; and IRM is the LSD or MDMA response in methanol. Matrix effect was evaluated for the samples with the LSD/cocaine and MDMA/cocaine mixtures.
The MIP membrane developed was also compared with paraffined chromatographic paper developed by Colletes and coworkers [16].
Results and Discussion
There are a few reports of PS-MS being used for the analysis of drugs of abuse. Li and coworkers [17] used PS employing chromatographic paper coupled with ion mobility to analyze cocaine residues in liquid samples. The authors found a limit of detection (LOD) of 2 μg mL–1. De Carvalho and coworkers [18] analyzed cocaine using chromatographic paper as support for PS assays. The authors found a LOD of 6 μg mL–1. Damon et al. [19] used hydrophobic paper spray mass spectrometry with a previous liquid/liquid extraction to analyze illicit drugs in biofluids. The LOD of cocaine found by the authors in the blood was 0.17 ng mL–1. In the present study, the experimental determinate LOQ was 1.0 ng mL–1, while the obtained by analytical curves data was 0.87 ng mL-1. However, it is important to emphasize that the developed procedure here had no sample preparation step and the employed MS equipment has a lower resolution, which could make developed method a possibility for routine analyses.
To increase the selectivity of the PS analysis for cocaine, a film containing molecularly imprinted polymers was synthetized on the surface of a cellulose membrane. The obtained MIP have chemically active sites for cocaine and guarantees a more selective and specific analysis. Li et al. [20] developed a similar membrane for the analysis of methotrexate in blood. But they used a different molecule (trimethoprim) as a template; this causes the analysis to not be as selective as when the target molecule is used as template.
MIP synthesis by free radical polymerization was conducted in solution under soft reaction conditions (ambient temperature and atmospheric pressure). The polymerization reaction is generally very fast and it is initiated by benzophenone, which is activated photochemically [21]. It is reported in the literature that the kinetic energy of the complex prior to polymerization decreases in photo-initiated polymerization at room temperature, which makes its stability higher and allows a greater binding ability and specificity than polymerization that has thermal initiation that requires temperatures higher than 40 °C [22,23,24].
Morphology Analysis
SEM was used to evaluate the morphology of the original cellulose membrane and membranes modified with MIP and NIP. The images were obtained with an acceleration potential of 5 kV and a magnification of 3000×. The images obtained are shown in Figure 2. It is possible to verify a significant change on the surface of the membranes modified with MIP and NIP compared with the original cellulose membrane. However, there is no significant change between the MIP and NIP membranes as expected. Several studies confirm the little change in the morphology between MIP and NIP [11, 25,26,27].
Study of Imprinting Effect and Selectivity of MIP Membrane
The results of the cross-selectivity assessment of the synthetized MIP membrane are shown in Table 1. The relative selectivity coefficient (K′) values for both mixtures are greater than 1, confirming the successful imprinting effect of the MIP membrane. Imprinting effect and selectivity of MIP were successfully achieved by a number of factors: the values of the distribution coefficient (Kd) for cocaine in the MIP membrane are higher than the ones found for the NIP membrane; the higher values of Kd for cocaine compared with the interfering drugs.
It can also be observed that the selectivity coefficient (K) of cocaine is 104.3 for mixture 1 and 438.1 for mixture 2, illustrating the high selectivity of the membrane when comparing it to NIP, and that it has a good ability to discriminate between cocaine and interfering compounds.
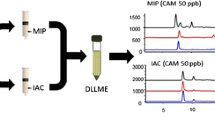
In order to compare MIP-PS-MS developed in the study and PS-MS using a paraffined chromatographic paper developed by Colletes et al. [16], an analysis of oral fluid samples spiked with cocaine, cocaine/LSD, and cocaine/MDMA was performed. The signal for the most abundant fragment of cocaine (m/z = 182) was observed in all samples. As shown in Figure 3, MIP-PS-MS has a better signal response for cocaine in all samples, as expected.
MIP-PS-MS
The triangular shape of the paper used in this study has been extensively reported in the literature [7, 8, 28, 29]. Different paper geometries cause the electric field intensity and the current on the paper to vary. Yang and coworkers [8] found the best geometry is the triangular one. One advantage of PS when comparing it to ESI is the possibility of developing disposable cartridges to automatize MS analysis [7]. Also, the intrinsic problems that are present in ESI are not present in PS, such as clogging and difficulty to form the spray from capillaries with 1 μm of diameter or less [30].
The mass spectrum obtained using the MIP cellulose membrane as substrate is shown in Figure 4a. Note that cocaine was detected as protonated ions [M + H]+ of m/z 304. Collision induced dissociation (CID) of cocaine was performed and the PS(+) MS/MS spectrum is illustrated in Figure 4b. From the PS(+) fragmentation of a precursor ion of cocaine gave m/z 182, which is formed by the alpha-cleavage next to the nitrogen atom following the loss of C7H6O2 – benzoate [31].
Analytical Validation
The most abundant fragment of cocaine, m/z 182, was used to construct the analytical calibration curve (Figure 5). Linearity of the MIP-PS-MS method was determined with oral fluid samples spiked with cocaine, which resulted in the concentrations of 1 (LOQ), 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 ng mL-1. The obtained regression equation, r2, LOD, LOQ, and ME are shown in Table 2. Each point of the calibration curve was performed in replicate (n = 5), and the coefficients of variation were lower than 5%. The matrix effect was calculated in the presence of two interfering compounds, LSD and MDMA, and in both cases it was found to be less than 10%.
To calculate LOD and LOQ, the formulas proposed by Long and Winefordner [32] were used and 10 measurements of the blank oral fluid sample were made. The values obtained for LOD and LOQ were, respectively, 0.27 ng mL–1 and 0.81 ng mL–1. The LOD value of cocaine in oral fluid is not affected by the presence of interfering compounds such as MDMA and LSD, when they are present in the same sample. MIP produces a chemical fingerprint of the chosen template when submitted to a sample, which contains a specific template molecule; it is preferentially rebounded to the polymeric array, so it results in a higher specificity of MIP polymers. El-Sharif et al. [33] recently demonstrated the ability of the MIP protein polymer to discriminate interested classes of proteins, probably due to specific hydrogen orientations between analytes and MIP polymers array. The LOQ value was also determined as the lowest concentration in which cocaine could be fragmented with accuracy and precision. The experimental value of LOQ was 1.0 ng mL–1; as expected it was higher than the calculated value.
The accuracy, inter-assay and intra-assay precision and recovery were assessed in three levels, each concentration was done in replicate (n = 5). The results are presented in Table 3.
The developed MIP-PS-MS method for cocaine analysis in oral fluid showed adequate accuracy, precision, and recovery. Sánchez-González and coworkers [34] used a micro solid phase extraction with a cocaine template MIP as an extraction phase coupled with LC-MS to analyze cocaine in plasma samples. The LOD found by the authors is smaller than the one found in the present work (0.061 ng mL–1); however, using PS-MS there is no need for sample preparation, sample extraction, the usage of solvents and reagents is very little, and the time of analysis is less than 30 s. The development of MIP for different drugs can increase the applicability of the MIP-PS-MS technique.
Conclusion
The MIP cellulose membrane developed allows the detection of cocaine with LOD of 0.27 ng mL–1. The method developed in this study creates new possibilities for paper spray ionization once it adds selectivity and specificity to the analysis, besides simplicity, low cost, low solvent consumption, speed, and efficiency.
References
UNODC: United Nations Office on Drugs and Crime. World Drug Report (2016)
Brasil: Lei n° 11.705 (2008)
Frederick, D.L.: Toxicology testing in alternative specimen matrices. Clin. Lab. Med. 32, 467–492 (2012)
Montesano, C., Simeoni, M.S., Curini, R., Sergi, M., Sterzo, C.L., Compagnone, D.: Determination of illicit drugs and metabolites in oral fluid by microextraction on packed sorbent coupled with LC-MS/MS. Anal. Bioanal. Chem. 407, 3647–3658 (2015)
Pereira, A.G., D’Avila, F.B., Ferreira, P.C.L., Holler, M.G., Limberger, R.P., Fröehlich, P.E.: Determination of cocaine, its metabolites, and pyrolytic products by LC-MS using a chemometric approach. Anal. Methods. 6, 456–462 (2014)
Chantada-Vázquez, M.P., Sánchez-González, J., Peña-Vázquez, E., Tabernero, M.J., Bermejo, A.M., Bermejo-Barrera, P., Moreda-Piñeiro, A.: Simple and sensitive molecularly imprinted polymer-Mn-Doped ZnS quantum dots based fluorescence probe for cocaine and metabolites determination in urine. Anal. Chem. 88, 2734–2741 (2016)
Liu, J., Wang, H., Manicke, N.E., Lin, J.M., Cooks, R.G., Ouyang, Z.: Development, characterization, and application of paper spray ionization. Anal. Chem. 82, 2463–2471 (2010)
Yang, Q., Wang, H., Maas, J.D., Chappell, W.J., Manicke, N.E., Cooks, R.G., Ouyang, Z.: Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. Int. J. Mass Spectrom. 312, 201–207 (2012)
Tokonami, S., Shiigi, H., Nagaoka, T.: Review: micro- and nano-sized molecularly imprinted polymers for high-throughput analytical applications. Anal. Chim. Acta. 641, 7–13 (2009)
Sun, J., Ji, J., Wang, Y., Zhao, Y., Zhang, Y., Sun, X.: Electrochemical sensor for determination of tulathromycin built with molecularly imprinted polymer film. Anal. Bioanal. Chem. 407, 1951–1959 (2015)
Hande, E.P., Samui, A.B., Kulkarni, P.S.: A molecularly imprinted polymer with flash column chromatography for the selective and continuous extraction of diphenyl amine. RSC. 5, 73434–73443 (2015)
Sellergres, B., Allender, C.J.: Molecularly imprinted polymers: a bridge to advanced drug delivery. Adv. Drug Deliv. 57, 1733–1741 (2005)
Yang, Y., Liu, X., Guo, M., Li, S., Liu, W., Xu, B.: Molecularly imprinted polymer on carbon microsphere surfaces for adsorbing dibenzothiophene. Colloids Surf. A: Physicochem. Eng. Aspects. 377, 379–385 (2011)
Andrade, F.N., Santos-Neto, A.J., Lanças, F.M.: Development of on-line molecularly imprinted solid phase extraction-liquid chromatography-mass spectrometry for triazine analysis in corn samples. J. Sep. Sci. 37, 3150–3156 (2014)
Tarley, C.R.T., Andrade, F.N., Oliveira, F.M., Corazza, M.Z., Azevedo, L.F.M., Segatelli, M.G.: Synthesis and application of imprinted polyvinylimidazole-silica hybrid copolymer for Pb2+ determination by flow-injection thermospray flame furnace atomic absorption spectrometry. Anal. Chim. Acta. 703, 145–151 (2011)
Colletes, T.C., Garcia, P.T., Campanha, R.B., Abdelnur, P.V., Romão, W., Coltro, W.K.T., Vaz, B.G.: A new insert sample approach to paper spray mass spectrometry: a paper substrate with paraffin barriers. Analyst. 141, 1707–1713 (2016)
Li, M., Zhang, J., Jiang, J., Zhang, J., Gao, J., Qiao, X.: Rapid, in situ detection of cocaine residues based on paper spray ionization coupled with ion mobility spectrometry. Analyst. 139, 1687–1691 (2014)
De Carvalho, T.C., Tosato, F., Souza, L.M., Santos, H., Merlo, B.B., Ortiz, R.S., Rodrigues, R.R.T., Filgueiras, P.R., França, H.S., Augusti, R., Romão, W., Vaz, B.G.: Thin layer chromatography couples to paper spray ionization mass spectrometry for cocaine and its adulterants analysis. Forensic Sci. Int. 262, 56–65 (2016)
Damon, D.E., Davis, K.M., Moreira, C.R., Capone, P., Cruttenden, R., Badu-Tawiah, A.K.: Direct biofluid analysis using hydrophobic paper spray mass spectrometry. Anal. Chem. 88, 1878–1884 (2016)
Li, T., Fan, L., Wang, Y., Huang, X., Xu, J., Lu, J., Zhang, M., Xu, W.: Molecularly imprinted membrane electrospray ionization for direct sample analyses. Anal. Chem. 89, 1453–1458 (2017)
Vasapollo, G., Del Sole, R., Mergola, L., Lazzoi, M.R., Scardino, A., Scorrano, S., Mele, G.: Molecularly imprinted polymers: present and future prospective. Int. J. Mol. Sci. 12, 5908–5945 (2001)
Puoci, F., Cirillo, G., Curcio, M., Iemma, F., Spizzirri, U.G., Picci, N.: Molecularly imprinted solid phase extraction for the selective HPLC determination of α-tocopherol in bay leaves. Anal. Chim. Acta. 593, 164–170 (2007)
Athikomrattanakul, U., Katterle, M., Gajovic-Eichelmann, N., Scheller, F.W.: Development of molecularly imprinted polymers for the binding of nitrofurantoin. Biosens. Bioelectron. 25, 82–87 (2009)
Spivak, D., Gilmore, M.A., Shea, K.J.: Evaluation of binding and origins of specificity of 9-ethyladenine imprinted polymers. J. Am. Chem. Soc. 119, 4388–4393 (1997)
Chaves, A.R., Queiroz, M.E.C.: In-tube solid-phase micro-extraction molecularly imprinted polymer to determine interferon alpha 2a in plasma sample by high performance liquid chromatography. J. Chromatogr. A. 1218, 43–48 (2013)
Xue, M., Wang, Y., Meng, Z., Zhang, W., Wu, Y., Jiang, S.: Extraction of shikimic acid from Chinese star anise using flash column chromatography on a molecularly imprinted polymer column. J. Liq. Chromatogr. Relat. Technol. 36, 2677–2686 (2013)
Sadegui, S., Jahani, M.: Selective solid-phase extraction using molecular imprinted polymer sorbent for the analysis of florfenicol in food samples. Food Chem. 141, 1242–1251 (2013)
Ren, Y., Chiang, S., Zhang, W., Wang, X., Lin, Z., Ouyang, Z.: Paper-capillary spray for direct mass spectrometry analysis of biofluid samples. Anal. Bioanal. Chem. 408, 1385–1390 (2016)
Wang, Q., Zheng, Y., Zhang, X., Han, X., Wang, T., Zhang, Z.: A silica coated paper substrate: development and its application in paper spray mass spectrometry for rapid analysis of pesticides in milk. Analyst. 140, 8048–8056 (2015)
Hiraoka, K., Nishidate, K.E., Mori, K., Asakawa, D., Suzuki, S.: Development of probe electrospray using a solid needle. Rapid Commun. Mass Spectrom. 21, 3139–3144 (2007)
Smith, R.M., Casale, J.F.: The mass spectrum of cocaine: deuterium labeling and MS/MS studies. Microgram. J. 7, 16–41 (2010)
Long, G.L., Winefordner, J.D.: Limit of detection a closer look at the IUPAC definition. Anal. Chem. 55, 712–724 (1983)
El-Sharif, H.F., Stevenson, D., Reddy, S.M.: MIP-based protein profiling: a method for interspecies discrimination. Sensors Actuators B Chem. 241, 33–39 (2017)
Sánchez-González, J., García-Carballal, S., Cabarcos, P., Tabernero, M.J., Bermejo-Barrera, P., Moreda-Piñeiro, A.: Determination of cocaine and its metabolites in plasma by porous membrane-protected molecularly imprinted polymer micro-solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 1451, 15–22 (2016)
Acknowledgments
This research was generously funded by FAPEG, CNPq, and CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tavares, L.S., Carvalho, T.C., Romão, W. et al. Paper Spray Tandem Mass Spectrometry Based on Molecularly Imprinted Polymer Substrate for Cocaine Analysis in Oral Fluid. J. Am. Soc. Mass Spectrom. 29, 566–572 (2018). https://doi.org/10.1007/s13361-017-1853-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-017-1853-2