Abstract
Fibrillization of the microtubule-associated protein tau has been recognized as one of the signature pathologies of the nervous system in Alzheimer’s disease, progressive supranuclear palsy, and other tauopathies. The conformational transition of tau in the fibrillization process, tau monomer to soluble aggregates to fibrils in particular, remains unclear. Here we report on the use of hydrogen/deuterium exchange mass spectrometry (HDX-MS) in combination with other biochemical approaches, including Thioflavin S fluorescence measurements, enzyme-linked immunosorbent assay (ELISA), and Western blotting to understand the heparin-induced tau’s fibrillization. HDX-MS studies including anti-tau antibody epitope mapping experiments provided molecular level details of the full-length tau’s conformational dynamics and its regional solvent accessibility upon soluble aggregates formation. The results demonstrate that R3 region in the full-length tau’s microtubule binding repeat region (MTBR) is stabilized in the aggregation process, leaving both N and C terminal regions to be solvent exposed in the soluble aggregates and fibrils. The findings also illustrate the practical utility of orthogonal analytical methodologies for the characterization of protein higher order structure.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microtubule-associated protein tau is a neuronal protein that plays an important role in the microtubule assembly and dynamics, regulating neuron morphology. Tau is believed to modulate axonal stability by binding to microtubules via the microtubule binding repeat region (MTBR). In Alzheimer’s disease, tau may be detached from microtubules by phosphorylation resulting in the exposure of tau’s hydrophobic surface that can trigger tau aggregation [1,2,3]. The aggregation process facilitates the formation of paired helical filament (PHF), which can disrupt cytoplasmic functions, affect axonal transport, ultimately leading to neuronal cell death [4, 5]. Therefore, tau has been recognized as a potential therapeutic target especially for the treatment of Alzheimer’s disease [6, 7].
Tau is an intrinsically disordered protein as indicated by studies using nuclear magnetic resonance (NMR) and small-angle X-ray scattering methods [8, 9]. The structural properties of tau pose significant challenges for X-ray crystallography to determine its tertiary structure. Full-length tau is composed of four functionally defined domains that can be distinguished along the amino acid sequence: the N-terminal projection domain, the proline rich region, the MTBR, and the C-terminal region [2] (Figure 1a). Studies have shown that MTBR is prone to aggregation and many different human disease mutations in tau are located in this region [10, 11]. MTBR on its own can aggregate to form fibrillar structures in the presence of anionic agents, including heparin and it aggregates more rapidly than full-length tau [12]. Although the exact pathophysiological role of tau fibrils is not known in Alzheimer’s disease, it is believed that soluble oligomeric tau species, rather than filamentous tau, may be the toxic moiety in neurodegeneration [10, 11, 13].
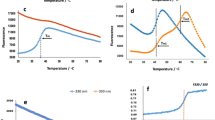
(a) Schematic representation of the functional domains of the largest tau isoform (441 amino acids). The N-terminal part is involved in signal transduction pathways and the C-terminal part, referred to as the microtubule-binding domain, regulates the rate of microtubules polymerization. Representative HDX kinetic plots for peptides from N-terminal, middle, and C-terminal regions of tau441 (b) and K18 (c). The relative deuterium uptake (%) was calculated based on the theoretical maximum deuterium uptake of each peptide
To prevent tau aggregation and the spread of tau pathology from the therapeutic point of view, it is important to obtain a molecular understanding of tau aggregation process. To date, significant efforts have been made in understanding the tau conformational properties in the fibrillization process. For example, Pavlova et al. [14] revealed a significant increment of β-sheet structured species within the early stages of tau fibrillization based on electron spin resonance (ESR) measurement. Bibow et al. [15] showed that residues in the N- and C-terminus of tau PHF are highly flexible based on NMR analysis. Eschmann et al. [16] demonstrated that the PHF6* (VQIINK) segment in the repeat region 2 (R2) is extended upon heparin-induced tau aggregation using pulsed double electron-electron resonance measurements. Recent studies by hydrogen/deuterium exchange mass spectrometry (HDX-MS) offered a means to obtain structural insight into protein conformational dynamics. For example, Ramachandran et al. [17] utilized HDX-MS for structural characterization of two tau MTBR constructs upon heparin-induced fibrillization. Their results suggested that R2 and the repeat region 3 (R3) in both constructs are stabilized in the fibrillar structures, indicative of their participation in the fibril core. Another HDX-MS study on tau was described by Zhu et al. [18]. They compared the HDX profiles of hyperphosphorylated tau, an amyloidogenic version of tau, and the native tau. Combined results from HDX and computer modeling allowed them to generate experimentally parameterized structures of the aggregation-prone conformational ensemble of tau. Their dataset revealed that hyperphosphorylation greatly increases solvent exposure of tau, particularly at the N- and C-termini and in the R3 region of MTBR. These studies provided a general picture of tau fibrils in which the N- and C-terminal domains are structurally flexible and the critical rule of MTBR in tau fibrillization. However, there are still gaps in knowledge regarding the conformational transition of the native, full-length tau in the fibrillization process, i.e., tau monomer to soluble aggregates and to fibrils.
In the present study, molecular details of tau’s conformational dynamics upon heparin-induced fibrillization were evaluated for two constructs, the full-length tau (tau441) and the MTBR construct (K18 peptide, residues 244–372). First, the fibrillized tau proteins were subjected to centrifugation, and the extent of fibrillization in the supernatant and pellet, which are composed of soluble aggregates and fibrils, respectively, were characterized by thioflavin S fluorescence measurements. Next, differential HDX-MS experiments were performed on the supernatant and monomeric tau to characterize the conformational changes of tau upon soluble aggregates formation. Complementary information regarding the local solvent exposure of tau soluble aggregates was obtained by conducting HDX-MS epitope mapping experiments with commercial anti-tau antibodies. ELISA using a panel of antibodies as well as Western blotting were applied on both supernatant and pellet fractions to gain additional conformational insight into the differences between soluble aggregates and fibrils. The results demonstrate that repeat region 3 in full-length tau becomes solvent-protected and/or structurally stabilized upon the formation of soluble aggregates. Furthermore, the N- and C-terminal domains remained solvent exposed from monomeric state to soluble aggregates and to fibrillary form. This study not only provides additional molecular insight into tau’s conformational properties in the fibrillization process but also exhibits the practical utility of HDX-MS methodology in combination with orthogonal biochemical methods for the detailed investigation of protein higher order structure.
Experimental
Reagents and Materials
Anti-tau mAbs tau12, tau46, BT2, and HT7 were purchased from ThermoFisher Scientific (Rockford, IL, USA); mAb 77G7 was purchased from Covance (Princeton, NJ, USA). Tau fibrils including K18 fibrils and tau441 fibrils were made in the presence of heparin at 37 °C for 8 h. K18-seeded tau441 fibrils were prepared under the same conditions except that K18 peptide was used instead of heparin. All fibrils were centrifuged at 20,000 g for 10 min and the supernatants were subjected to size exclusion chromatography with multi-angle light scattering (SEC-MALS) and then HDX-MS analysis. Supernatants and pellets were subjected to ELISA analysis. The SEC/UV data demonstrated a good purity of starting materials, K18 and tau441, as well as their soluble aggregates (Supplementary Figures S1 and S2). Tau protein concentrations were determined by UV280 nm absorbance.
Thioflavin S Fluorescence
The fibrillized samples were added to an equal volume of 25 μM thioflavin-S. Fluorescence signals were measured using an Envision multilabel plate reader (PerkinElmer; Waltham, MA, USA) with the excitation and emission wavelengths set at 430 nm and 550 nm, respectively.
SEC-MALS
SEC-MALS was performed using a Sepax Zenix-300, 4 μm 4.6 (ID) × 300 mm (L) (Sepax Technologies, Newark, DE, USA) column connected to an Agilent 1100 series (Agilent, Santa Clara, CA, USA) equipped with a diode array detector. Agilent HPLC system is connected to a Multi Angle Light Scattering (MALS) detector (Wyatt Technology, Santa Barbara, CA, USA).
ELISA
The pellet and supernatant fractions of T441 fibrils were run in 3-fold serial dilutions from 20 to 0.1 ng/mL concentration in the ELISA assay as previously described [19]. Anti-tau mAbs tau12 and HT7 were used as capture mAbs and alkaline phosphatase (AP) conjugated BT2, HT7, 77G7, and T46 were used as detection mAbs. Luminescence counts were measured using Envision plate reader (PerkinElmer, Waltham, MA, USA).
HDX
HDX was performed as previously described [20]. For hydrogen exchange labeling, 50 pmol of proteins (monomeric tau441, K18, and soluble aggregates) were incubated with 15-fold 10 mM sodium phosphate buffer made with 99% D2O (pD 7.0) at room temperature. At each deuterium exchange time point (from 10 s to 4 h) an aliquot from the exchange reaction was removed and the reaction was quenched by adding quenching buffer [4 M guanidine hydrochloride, 0.5 M Tris (2-carboxyethyl) phosphine hydrochloride (TCEP-HCl), 200 mM sodium phosphate, H2O, pH 2.5, 1:1, v/v]. Quenched samples were immediately frozen on dry ice and stored at –80 °C until analysis.
For epitope mapping, tau441 monomer and soluble aggregates were individually incubated with anti-tau mAbs tau12 and tau46. The samples were incubated at room temperature for 30 min prior to addition of deuterated buffer. The Kds for the antibodies were between 0.2 and 0.9 nM; therefore a 1:2 tau:to antibody ratio was used so that at labeling, 99.96% of the tau was bound to the antibodies. The labeling was performed as described above.
Chromatography and Mass Spectrometry
Each frozen sample was thawed rapidly and injected into a custom Waters nanoACQUITY UPLC HDX Manager [21] and analyzed on a Xevo-G2 mass spectrometer (Waters Corp., Milford, MA, USA) as previously described [22]. Protein samples were digested online at 15 °C for 30 s using a Poroszyme immobilized pepsin cartridge 2.1 mm × 30 mm (Applied Biosystems). The peptic peptides were trapped and desalted for 3 min at 100 μL/min and then separated in 6 min by an 5%–40% acetonitrile:water gradient at 40 μL/min. The separation column was a 1.0 mm × 100 mm ACQUITY UPLC C18 BEH (Waters Corp.) containing 1.7 μm particles, and the back pressure averaged 8800 psi at 0.1 °C. The average amount of back-exchange using this experimental setup was 18% to 25%, based on analysis of highly deuterated peptide standards. Mass accuracy was ensured by calibration with 500 fmol/μL GFP, and was less than 10 ppm throughout all experiments. All comparison experiments were done under identical experimental conditions such that deuterium levels were not corrected for back-exchange and are therefore reported as relative [23]. The % deuterium uptake was calculated using theoretical maximum number of exchangeable amide hydrogens, excluding the two N-terminal residues in each peptide, with the consideration of ~92.4% D2O in each incubation. All experiments were performed in duplicate. The error of measuring the mass of each peptide was ±0.20 Da in this experimental setup, consistent with previously obtained values [24,25,26].
HDX Data Analysis
Deuterium uptake was calculated by subtracting the centroid of the isotopic distribution for peptide ions from undeuterated protein from the centroid of the isotopic distribution for peptide ions from the deuterium labeled sample. The resulting relative deuterium levels were plotted versus the exchange time using Waters DynamX 3.0 software. Identification of the peptic peptides was accomplished through a combination of exact mass analysis and MSE [27] using PLGS 2.5 Software (Waters Corp., Milford, MA, USA), as previously described [28, 29]. All assignments, deuterated spectra, and data processing were manually checked and verified.
Results and Discussion
Conformational Dynamics of Tau
Protein conformational dynamics in solution can be revealed by following the HDX kinetics. Figure 1a shows the schematics of the proteins that were investigated in this study. K18 peptide corresponds to residues 244–372 from tau411 and overlaps the MTBR. The HDX-MS data on both tau441 and K18 exhibited rapid incorporation of deuterium at the earlier time points in most of the peptides, and the exchange levels stayed constant throughout the time course of the study (Figure 1b and c). Supplemental Figures S3 and S4 display all the deuterium incorporation graphs and the protein coverage for tau441 and K18. Residues 254–284 were observed in K18 but not in tau441. This is likely due to different folding or digestion patterns of full-length tau. Given that tau is an intrinsically disordered protein, the HDX experiments conducted under current conditions, e.g., pH 7, may not be able to capture the fast protein folding/unfolding events. Zhu et al. [18] utilized millisecond HDX, i.e., HDX coupled with time-resolved electrospray ionization mass spectrometry (HDX-TRESI-MS), on the monomeric tau and were able to reveal some secondary structural information as derived from a NMR study [30]. One could also implement HDX under low pH for the structural characterization of intrinsically disordered proteins [31]. Nevertheless, the fast exchange kinetics of tau observed in this study is indicative of highly dynamic and solvent-exposed nature of the protein, which is not unexpected with the intrinsically disordered property of tau.
Tau Fibrils Characterization
In this study, heparin was used to trigger tau fibrillization. For the fibrillization process, both tau441 and K18 were incubated with heparin, and the supernatants and pellets from the fibrillized samples were characterized by thioflavin S fluorescence. Thioflavin S is a widely used amyloid-specific dye for biophysical studies of amyloid plaques [32, 33]. Upon binding to beta sheet-rich structures such as amyloids, thioflavins exhibit an intensity increase and a maximum shift of their fluorescence spectra. Figure 2 illustrates the fibril characterization of supernatants and pellets of tau441 and K18 after fibrillization. An increase in thioflavin S fluorescence signal was observed for both supernatants and pellets of tau441, and for the pellets of K18, compared with starting material and buffer. The supernatants of K18 showed comparable fluorescence signal compared with the starting material. This is likely due to the minimal changes of beta-sheet content in K18 upon soluble aggregate formation owing to its smaller size. Not surprisingly, signal in the pellet fraction was significantly greater than that in the supernatant for both tau441 and K18 peptides, confirming the presence of more complex fibrillar structures of tau in the pellet. A significantly greater proportion of the K18 peptide fibrillized (96%) compared with tau441 (70%) was detected. This is consistent with literature report that MTBR on its own can aggregate to form fibrillar structures in the presence of anionic agents and it aggregates more rapidly than full-length tau [12].
tau fibril characterization. An increase in Thioflavin S fluorescence signal was observed with both K18 and tau441 peptides, following fibrillization, compared with starting material and buffer (Buf). Signal in the fibrillized pellet fraction (P) was greater than in the supernatant (S) for both K18 and tau441 peptides
The supernatants of tau441 and K18 fibrils were further characterized by SEC/MALS. The data (Supplementary Figure S2) suggest that tau441 and K18 soluble aggregates were composed of 100–500 oligomeric states of peptides based on the theoretical molar mass of monomeric tau. Although the monomeric state could still be present in the supernatant, its abundance should be low based on the SEC/MALS profile. The mixture of different oligomeric tau suggests that the conformational analysis of supernatants should provide information on the structural ensembles of tau soluble aggregates.
Tau Conformational Changes upon the Formation of Soluble Aggregates
To examine the aggregation-induced tau conformational changes, we utilized HDX-MS on the soluble tau441 aggregates and K18 aggregates and compared their peptide-level HDX profiles with the profiles of their corresponding monomeric states. Beside heparin-based aggregates, we also prepared tau soluble aggregates through seeding K18 peptide in tau441 to examine any potential differences in the conformational dynamics of tau in different fibrillization processes.
Pairwise comparison of deuteration levels of common peptides allows detailed examination of changes in conformational dynamics across different samples/conditions. The HDX-MS data indicate that the only peptide region that was significantly affected upon aggregation in both samples (tau441 fibrils and K18 fibrils) was between amino acids 308 and 315, the repeat region 3 (R3) of MTBR (Figure 3a and b). The two peptides covering this region remained solvent exposed in the monomeric state and at least two amide Hs were protected from exchange upon heparin-induced aggregation. This protection could be caused either by the local solvent protection or the hydrogen bond formation during aggregation process. The slight change in slope of the kinetic curves of tau soluble aggregates compared with the monomeric state suggests potential conformational change in R3 upon aggregation. It was also noted that there was a slight decrease in deuterium uptake as the labeling time increased for the two peptides in heparin-induced oligomeric tau. This is likely the outcome of combined contributing factors from HDX process and tau aggregation. Ramachandran et al. [17] revealed that R3 is part of the fibril core based on their HDX-MS data on the tau aggregates bearing only MTBR construct. Current HDX-MS dataset shows that the HDX reduction in this region was comparable between tau441 and K18, indicating that full-length tau and the MTBR construct share similar aggregation mechanism. The HDX reduction in R3 was greater in heparin-induced tau441 and K18 soluble aggregates compared with the K18 seeded tau441 aggregates, suggesting more rigid structure and/or hydrogen bond formation in R3 upon heparin-induced aggregation compared with MTBR-induced aggregation. A structural compaction of MTBR in tau upon heparin binding [34] has been proposed. Eschmann et al. [16] observed that R2 in MTBR is structurally extended upon heparin-induced aggregation. We did not detect significant HDX differences in regions other than R3 in MTBR upon soluble aggregates formation. This could be due to the fast dynamic of the conformational changes that are difficult to be captured under current HDX-MS conditions. The heparin-induced conformational changes in MTBR might result in the deuteration difference we observed in R3 upon different aggregation processes.
HDX kinetics of MTBR peptides in K18 and tau441 soluble aggregates reveal potential aggregation interfaces. (a) HDX-MS data for two peptides that showed decreased uptake upon soluble aggregates formation in tau441. (b) HDX-MS data for peptides that showed protection from deuteration upon soluble aggregates formation in K18 peptide. Monomeric tau is colored in black, and heparin-induced oligomeric tau and K18-induced oligomeric tau are colored in blue and red, respectively
Interestingly, in the K18 peptide, there were several peptides (347–357, 358–372) in the repeat region 4 (R4) that displayed subtle protection from deuteration upon aggregation (Figure 3b). In contrast, this trend was not observed in tau441 protein, suggesting that the lack of the C-terminal region of full-length tau441 in K18 may affect the structural stability and/or hydrogen bonding network within the R4 of the MTBR.
Peptide regions in the N- and C-terminal domains of tau441 displayed comparable deuterium uptake profiles between monomeric state and soluble aggregates (Supplementary Figure S5), indicating that these two domains remained solvent exposed upon the formation of soluble aggregates.
Solvent Accessibility of N- and C-terminal Domains by HDX-MS Epitope Mapping of Anti-Tau mAbs
The solvent accessibility of the N- and C-terminal domains of tau soluble aggregates were further examined by HDX-MS epitope mapping of two commercial anti-tau mAbs, tau12 and tau46. These two mAbs specifically target the N- and C-terminal domains of tau, respectively, based on deletion mutants or peptide-based screening approach [35, 36]. Pairwise comparison of HDX epitope profiles of these two mAbs in tau441 monomer and soluble aggregates could provide additional insight into the local conformational properties of N- and C-terminal domains in the aggregation process.
In the epitope mapping experiments of mAb tau12 targeting the N-terminal region of tau441, exchange was followed into 31 tau441 peptides covering 71% of the tau 441 sequence. As expected, peptide regions 2–8, 9–43, and 12–43, located in the N-terminal domain, showed protection from deuteration upon mAb tau12 binding (Figure 4a). Given that these peptides are in the consecutive sequence, the epitope could be linear. Similar protection in these regions was observed for tau441 soluble aggregates, indicating that the N-terminal domain, upon aggregation, was still solvent exposed and was accessible for mAb binding. The mAb binding-induced HDX reduction in region 2–8 was greater in the soluble aggregates than in the monomeric tau441, suggesting that the local conformation in this region could be slightly different between monomeric and oligomeric states. The overlapping peptides 9–43 and 12–43, on the other hand, displayed the same reduced deuterium uptake upon mAb binding, highlighting that this region was impacted in the same way upon mAb tau12 binding regardless of whether tau was oligomeric or not.
Epitope mapping of mAbs tau12 and tau46. (a) Regions displayed significant HDX reduction upon mAb tau12 binding. mAb bound and unbound monomeric tau are colored in black and orange, respectively. mAb bound and unbound oligomeric tau are colored in blue and green, respectively. (b) Regions displayed significant HDX reduction upon mAb tau46 binding. mAb bound and unbound monomeric tau are colored in black and pink, respectively. mAb bound and unbound oligomeric tau are colored in blue and purple, respectively
Similar HDX-MS epitope mapping experiments were conducted for mAb tau46, targeting the C-terminus of tau441. HDX was followed into 31 tau441 peptides covering 72% of the tau 441 sequence. The only region affected upon antibody binding is peptide 437–441, located in the C-terminal domain (Figure 4b). As shown by HDX-MS data, this antibody is effective in targeting the tau441 soluble aggregates as well, indicating that the C-terminal domain remained solvent exposed upon aggregation. Interestingly, this C-terminal region appears to adopt a unique structure upon mAb binding, as its deuteration profile is different from the profile of the unbound tau441 in solution. The degree of mAb binding-induced HDX reduction was similar between monomeric and aggregated tau, suggesting minimal changes in conformational properties in this region upon aggregation. Overall, HDX-MS epitope mapping results of anti-tau mAbs provide complementary information. It is also consistent with the findings from HDX-MS conformational studies of tau that both N- and C-terminal domains in tau441 remain solvent exposed upon the formation of soluble aggregates. To the best of our knowledge, this is the first reported application of HDX-MS for antibody epitope mapping on tau protein in support of tau conformational assessment.
Tau Fibrillization-induced Protein Conformational Changes
Interrogation of the higher order structure of insoluble species, i.e., the pellet fraction, is challenging under a typical HDX-MS workflow. To conduct a comprehensive assessment of the structural properties of tau fibrils, we developed an orthogonal method by utilizing ELISA with a panel of five commercial anti-tau mAbs for the characterization of mAb accessibility of tau fibrils. The five mAbs include tau12, HT7, BT2, 77G7, and tau46 with known binding epitopes that span the N-terminal, mid-domain, mid-MTBR, and C-terminal regions of tau (Figure 5a). Utility of tau mAbs targeting different regions of tau as capture and detection reagents allows conformational assessment of four segments of tau upon fibrillization. Figure 5a illustrates the experimental design. The combination of mAbs tau12 and HT7 as capture and detection mAbs, respectively, allows accessibility interrogation for N-terminal region of tau, whereas the combination of mAb HT7 and mAb BT2 allows mid-domain assessment. The MTBR and C-terminal assays were designed using combination of HT7-77G7 and HT7-tau46, respectively. The pellet and supernatant fractions of T441 fibrils were run in 3-fold serial dilutions from 20 to 0.1 ng/mL concentration, and the ratios of pellet to supernatant signal from ELISAs, across a range of concentrations of T441 fibrils, were compared across the different tau ELISA assays. As illustrated in Figure 5b, the signals for mid-domain and MTBR were greater for supernatant than pellet, indicating that these regions were more accessible to mAbs binding to soluble species than to insoluble species. As indicated by HDX-MS data, MTBR is prone to aggregate, and further aggregation of tau could involve additional interactions in mid-region, leading to solvent protection of both mid-domain and MTBR in the insoluble tau fibrils. In addition, the N- and C-terminal assays showed greater pellet/supernatant signal ratios compared with the mid and MTBR assays (Figure 5b), suggesting that the N- and C-terminal regions of tau remained solvent accessible during the transition from soluble aggregates to insoluble fibrils. The observed solvent exposure of N-terminal region in fibrillar state was also supported by Western blotting using N-terminal tau12 antibody (Supplementary Figure S6), demonstrating robust signals in both pellet and supernatant following tau fibrillization. Although the solvent exposure of N- and C-terminal domains in tau fibrils was observed in NMR studies by Bibow et al. [15], the present study provides additional structural insight in which the N- and C-terminal domains remain solvent exposed, at the similar level, from soluble aggregates to the fibrils.
tau’s N- and C-terminal regions retain their solvent accessibility in fibrillization, as assessed by ELISA. (a) tau ELISA formats to interrogate mid-domain, MTBR, N- and C-terminal regions of tau. Capture and detection antibodies indicated in red and black, respectively. (b) The ratios of pellet to supernatant signal from ELISAs, across a range of concentrations of T441 fibrils, were compared across the different tau ELISA assays. N- and C-terminal assays showed greater signals in the pellet fraction compared with the mid- and MTBR assays
Conclusions
Fibrillization of microtubule-associated protein tau is an important pathology in Alzheimer’s disease and several taupathies. The biophysical properties of tau fibrillization have been characterized extensively in the literature. However, there are still gaps in knowledge regarding the conformational transition of tau in the fibrillization process from tau monomer to soluble aggregates and to fibrils in particular. This study utilized HDX-MS methodology in combination with orthogonal biochemical methods, including thioflavin S fluorescence measurements, ELISA, and Western blotting, to assess conformational properties of tau upon heparin-induced fibrillization. HDX-MS data on tau monomer and soluble aggregates demonstrate that monomeric tau441 is intrinsically disordered and gets rapidly deuterated, and the repeat region 3 in MTBR is a critical region in the fibrillization process of full-length tau. Upon soluble aggregates formation, the solvent exposure of the N- and C- terminal domains remained at the similar deuteration level compared with monomeric state. This is supported by HDX-MS epitope mapping results using antibodies targeting the N- and C-terminal domains. For the conformational assessment of tau insoluble fibrils, an ELISA approach was developed using a panel of commercial antibodies with distinct binding epitopes. The results obtained from ELISA and Western blotting elucidate the increased level of solvent protection in MTBR during the transition of tau soluble aggregates to fibrils, whereas the N- and C-terminal domains remained solvent exposed. The present study extends our understanding in the conformational transition of full-length tau in the fibrillization process in which the N- and C-terminal domain remain solvent exposed, with similar deuteration levels from soluble aggregates to fibrils. In addition, this work demonstrates the practical utility of HDX-MS methodology in combination with orthogonal approaches for detailed molecular characterization of protein higher order structure.
References
Buée, L., Bussière, T., Buée-Scherrer, V., Delacourte, A., Hof, P.R.: tau protein isoforms, phosphorylation, and role in neurodegenerative disorders. Brain Res. Rev. 33, 95–130 (2000)
Arendt, T., Stieler, J.T., Holzer, M.: tau and tauopathies. Brain Res. Bull. 126(Part 3), 238–292 (2016)
Kadavath, H., Hofele, R.V., Biernat, J., Kumar, S., Tepper, K., Urlaub, H., Mandelkow, E., Zweckstetter, M.: tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc. Natl. Acad. Sci. 112, 7501–7506 (2015)
Kanmert, D., Cantlon, A., Muratore, C.R., Jin, M., O'Malley, T.T., Lee, G., Young-Pearse, T.L., Selkoe, D.J., Walsh, D.M.: C-terminally truncated forms of tau, but not full-length tau or its C-terminal fragments, are released from neurons independently of cell death. J. Neurosci. 35, 10851–10865 (2015)
Alonso, A.D., Beharry, C., Corbo, C.P., Cohen, L.S.: Molecular mechanism of prion-like tau-induced neurodegeneration. Alzheimers Dement.: J. Alzheimers Assoc. 12, 1090–1097 (2016)
Esteves-Villanueva, J.O., Trzeciakiewicz, H., Loeffler, D.A., Martić, S.: Effects of tau domain-specific antibodies and intravenous immunoglobulin on tau aggregation and aggregate degradation. Biochemistry. 54, 293–302 (2015)
Shimada, H., Kitamura, S., Shinotoh, H., Endo, H., Niwa, F., Hirano, S., Kimura, Y., Zhang, M.-R., Kuwabara, S., Suhara, T., Higuchi, M.: Association between Aβ and tau accumulations and their influence on clinical features in aging and Alzheimer's disease spectrum brains: a [(11)C]PBB3-PET study. Alzheimers Dement.: Diagn. Assess. Dis. Monit. 6, 11–20 (2017)
Schwalbe, M., Ozenne, V., Bibow, S., Jaremko, M., Jaremko, L., Gajda, M., Jensen, M.R., Biernat, J., Becker, S., Mandelkow, E., Zweckstetter, M., Blackledge, M.: Predictive atomic resolution descriptions of intrinsically disordered htau40 and a-synuclein in solution from NMR and small angle scattering. Structure. 22, 238–249 (2014)
Abyzov, A., Salvi, N., Schneider, R., Maurin, D., Ruigrok, R.W.H., Jensen, M.R., Blackledge, M.: Identification of dynamic modes in an intrinsically disordered protein using temperature-dependent NMR relaxation. J. Am. Chem. Soc. 138, 6240–6251 (2016)
Frost, B., Jacks, R.L., Diamond, M.I.: Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284, 12845–12852 (2009)
Binder, L.I., Guillozet-Bongaarts, A.L., Garcia-Sierra, F., Berry, R.W.: tau, tangles, and Alzheimer's disease. Biochim. Biophys. Acta Mol. Basis Dis. 1739, 216–223 (2005)
Patterson, K.R., Remmers, C., Fu, Y., Brooker, S., Kanaan, N.M., Vana, L., Ward, S., Reyes, J.F., Philibert, K., Glucksman, M.J., Binder, L.I.: Characterization of prefibrillar tau oligomers in vitro and in Alzheimer's disease. J. Biol. Chem. 286, 23063–23076 (2011)
de Calignon, A., Fox, L.M., Pitstick, R., Carlson, G.A., Bacskai, B.J., Spires-Jones, T.L., Hyman, B.T.: Caspase activation precedes and leads to tangles. Nature. 464, 1201–1204 (2010)
Pavlova, A., Cheng, C.-Y., Kinnebrew, M., Lew, J., Dahlquist, F.W., Han, S.: Protein structural and surface water rearrangement constitute major events in the earliest aggregation stages of tau. Proc. Natl. Acad. Sci. 113, E127–E136 (2016)
Bibow, S., Mukrasch, M.D., Chinnathambi, S., Biernat, J., Griesinger, C., Mandelkow, E., Zweckstetter, M.: The dynamic structure of filamentous tau. Angew.Chem. Int. Ed. 50, 11520–11524 (2011)
Eschmann, N.A., Georgieva, E.R., Ganguly, P., Borbat, P.P., Rappaport, M.D., Akdogan, Y., Freed, J.H., Shea, J.-E., Han, S.: Signature of an aggregation-prone conformation of tau. 7, 44739 (2017)
Ramachandran, G., Udgaonkar, J.B.: Difference in fibril core stability between two tau four-repeat domain proteins: a hydrogen–deuterium exchange coupled to mass spectrometry study. Biochemistry. 52, 8787–8789 (2013)
Zhu, S., Shala, A., Bezginov, A., Sljoka, A., Audette, G., Wilson, D.J.: Hyperphosphorylation of intrinsically disordered tau protein induces an amyloidogenic shift in its conformational ensemble. PLoS One. 10, e0120416 (2015)
Meredith Jr., J.E., Sankaranarayanan, S., Guss, V., Lanzetti, A.J., Berisha, F., Neely, R.J., Slemmon, J.R., Portelius, E., Zetterberg, H., Blennow, K., Soares, H., Ahlijanian, M., Albright, C.F.: Characterization of novel CSF tau and ptau biomarkers for alzheimer’s disease. PLoS One. 8, e76523 (2013)
Huang, R.Y.C., Iacob, R.E., Krystek, S.R., Jin, M., Wei, H., Tao, L., Das, T.K., Tymiak, A.A., Engen, J.R., Chen, G.: Characterization of aggregation propensity of a human Fc-fusion protein therapeutic by hydrogen/deuterium exchange mass spectrometry. J. Am. Soc. Mass Spectrom. 28, 795–802 (2017)
Wales, T.E., Fadgen, K.E., Gerhardt, G.C., Engen, J.R.: High-speed and high-resolution UPLC separation at zero degrees celsius. Anal. Chem. 80, 6815–6820 (2008)
Iacob, R.E., Bou-Assaf, G.M., Makowski, L., Engen, J.R., Berkowitz, S.A., Houde, D.: Investigating monoclonal antibody aggregation using a combination of H/DX-MS and other biophysical measurements. J. Pharm. Sci. 102, 4315–4329 (2013)
Wales, T.E., Engen, J.R.: Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom. Rev. 25, 158–170 (2006)
Burkitt, W., O'Connor, G.: Assessment of the repeatability and reproducibility of hydrogen/deuterium exchange mass spectrometry measurements. Rapid Commun. Mass Spectrom. 22, 3893–3901 (2008)
Houde, D., Berkowitz, S.A., Engen, J.R.: The utility of hydrogen/deuterium exchange mass spectrometry in biopharmaceutical comparability studies. J. Pharm. Sci. 100, 2071–2086 (2011)
Iacob, R.E., Engen, J.R.: Hydrogen exchange mass spectrometry: are we out of the quicksand? J. Am. Soc. Mass Spectrom. 23, 1003–1010 (2012)
Plumb, R.S., Johnson, K.A., Rainville, P., Smith, B.W., Wilson, I.D., Castro-Perez, J.M., Nicholson, J.K.: UPLC/MSE; a new approach for generating molecular fragment information for biomarker structure elucidation. Rapid Commun. Mass Spectrom. 20, 1989–1994 (2006)
Wales, T.E., Eggertson, M.J., Engen, J.R.: Considerations in the analysis of hydrogen exchange mass spectrometry data. In: Matthiesen, R. (Ed.). Humana Press: Totowa (2013)
Ahn, J., Cao, M.-J., Yu, Y.Q., Engen, J.R.: Accessing the reproducibility and specificity of pepsin and other aspartic proteases. Biochim. Biophys. Acta Protein Proteomics. 1834, 1222–1229 (2013)
Mukrasch, M.D., Bibow, S., Korukottu, J., Jeganathan, S., Biernat, J., Griesinger, C., Mandelkow, E., Zweckstetter, M.: Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 7, e1000034 (2009)
Goswami, D., Devarakonda, S., Chalmers, M.J., Pascal, B.D., Spiegelman, B.M., Griffin, P.R.: Time window expansion for HDX analysis of an intrinsically disordered protein. J. Am. Soc. Mass Spectrom. 24, 1584–1592 (2013)
Espargaro, A., Sabate, R., Ventura, S.: Thioflavin-S staining coupled to flow cytometry. A screening tool to detect in vivo protein aggregation. Mol. Biosyst. 8, 2839–2844 (2012)
Ferrone, F.: Analysis of protein aggregation kinetics. Methods Enzymol. 309, 256–274 (1999)
Elbaum-Garfinkle, S., Rhoades, E.: Identification of an aggregation-prone structure of tau. J. Am. Chem. Soc. 134, 16607–16613 (2012)
Ghoshal, N., Garcıa, F., Wuu, J., Leurgans, S., Bennett, D.A., Berry, R.W., Binder, L.I.: tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer's disease. Exp. Neurol.. 177, 475–493 (2002)
Petry, F.R., Pelletier, J., Bretteville, A., Morin, F., Calon, F., Hébert, S.S., Whittington, R.A., Planel, E.: Specificity of anti-tau antibodies when analyzing mice models of Alzheimer's disease: problems and solutions. PLoS One. 9, e94251 (2014)
Acknowledgments
The authors thank Drs. Reb Russell, Morrey Atkinson, and Bruce Car from Bristol-Myers Squibb Company for their support of this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1832 kb)
Rights and permissions
About this article
Cite this article
Huang, R.YC., Iacob, R.E., Sankaranarayanan, S. et al. Probing Conformational Dynamics of Tau Protein by Hydrogen/Deuterium Exchange Mass Spectrometry. J. Am. Soc. Mass Spectrom. 29, 174–182 (2018). https://doi.org/10.1007/s13361-017-1815-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-017-1815-8