Abstract
We describe a systematic comparison of high and low resolution LC-MS/MS assays for quantification of 25-hydroxyvitamin D3 in human serum. Identical sample preparation, chromatography separations, electrospray ionization sources, precursor ion selection, and ion activation were used; the two assays differed only in the implemented final mass analyzer stage; viz. high resolution quadrupole-quadrupole-time-of-flight (QqTOF) versus low resolution triple quadrupole instruments. The results were assessed against measured concentration levels from a routine clinical chemiluminescence immunoassay. Isobaric interferences prevented the simple use of TOF-MS spectra for extraction of accurate masses and necessitated the application of collision-induced dissociation on the QqTOF platform. The two mass spectrometry assays provided very similar analytical figures of merit, reflecting the lack of relevant isobaric interferences in the MS/MS domain, and were successfully applied to determine the levels of 25-hydroxyvitamin D for patients with chronic liver disease.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reliable quantification of vitamin D in biological samples remains difficult as even selective mass spectrometry assays exhibit many possibilities for errors and interferences [1–3]. While high performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) on triple quadrupole (QqQ) instruments is currently considered the optimum method for quantifying the vitamin status marker 25-hydroxyvitamin D3 (25(OH)D3) [4, 5], the technique is not as straightforward to implement for vitamin D compounds as is the case for other endogenous compounds [1, 2]. LC-MS/MS requires skilled personnel and carefully optimized instrumental parameters to avoid interferences and to provide sufficient sensitivity. For example, ionization efficiency of the secosteroidal vitamin D compounds is generally very low in electrospray ionization (ESI) or atmospheric-pressure chemical ionization (APCI). Furthermore, endogenous isobars of 25(OH)D3 must be separated using proper chromatographic separation, combined with the application of appropriate product ions in the selected reaction monitoring (SRM) mode of the QqQ instrument. As recently demonstrated [6], there is a large number of isobaric endogenous and exogenous compounds present during the LC-MS/MS analysis of 25(OH)D3. Furthermore, additional complications arise from isomeric contributions, where the C-3 epimer of 25(OH)D3 presents a particular challenge [2]. Whether or not the epimeric species are biologically relevant is currently unknown [7]; regardless, chromatographic separation is essential to avoid bias in the analysis [1, 2, 8, 9].
Several experimental approaches have been developed to overcome some of these problems, including derivatization techniques to improve ionization efficiency [10–12], ion mobility spectrometry to remove isobars [6], isotope standards, and certified reference materials to improve intra- and inter-laboratory precision and accuracy [2], and high resolution mass spectrometry (HRMS) [2]. Mass analyzers of medium/high mass resolving power such as time-of-flight (TOF), Orbitrap, and Fourier transform ion cyclotron resonance (FTICR) have not yet routinely entered clinical practice, even though their potential has been clearly recognized [13, 14]. Furthermore, a few recent studies have successfully demonstrated the potential of HRMS in the quantification of vitamin D compounds [15–18].
The present study was aimed at a systematic comparison of the analytical performance of a previously developed triple quadrupole LC-MS/MS assay [19] for 25(OH)D3 in serum with a new high resolution mass spectrometry assay, which was implemented on a hybrid quadrupole-quadrupole-time-of-flight (QqTOF) instrument. Importantly, both assays used the same serum samples (from patients with chronic liver diseases), identical sample preparation by supported liquid extraction (SLE), and the same commercial certified vitamin D calibrator standards and QC materials. In addition, the identical chromatographic separation was implemented. To achieve a meaningful comparison, two LC-MS/MS instruments from the same manufacturer were used, with very similar electrospray sources, ion transport regions, collision cells, and collision-induced dissociation (CID) conditions. The assays differed only in the final mass analyzer stage, i.e., low resolution (LR) QqQ versus high resolution (HR) QqTOF. For accuracy assessment, measured concentrations from a routine clinical chemiluminescence immunoassay were also utilized in the comparison.
Experimental
Chemicals and Materials
Standards of 25-hydroxyvitamin D3, HPLC-MS grade methanol, acetonitrile, and formic acid were purchased from Sigma-Aldrich (Steinheim, Germany). Isotopically-labeled d 6 -25-hydroxyvitamin D3 (d-labeled at positions 26, 26, 26, 27, 27, 27) was from Chemaphor Inc. (Ottawa, ON, Canada). Stock solutions of 25(OH)D3 and d 6 -25(OH)D3 at 1 mg/mL were prepared in methanol and diluted prior to the experiments. Lyophilized ClinCal and ClinChek25-OH Vitamin D2/D3 serum calibrators (level 1–3) and quality control sera (level I and II) were obtained from Recipe (Munich, Germany) and reconstituted in water prior to analysis (ClinCal and ClinChek calibrators and serum controls are used for calibration and internal quality assurance purposes; both materials are based on calf serum). Sodium carbonate and sodium hydrogen carbonate were from Merck (Darmstadt, Germany). Organic-free water was generated by Millipore (Bedford, MA, USA) Direct-Q8 and Synergy purification systems. AC micro-extraction plates were obtained from Tecan (Männedorf, Switzerland).
Patient Samples, Calibrators, and Quality Controls
All serum calibration and quality control materials were based on lyophilized ClinCal and ClinChek 25-OH Vitamin D2/D3 materials, which were reconstituted in 1 mL of water. The patient samples were from an existing study of chronic liver diseases (CLD) at Saarland University Medical Center (Homburg, Germany) [20]. Patients with vitamin D deficiency were supplemented with vitamin D3 at 20,000 IU/wk (Dekristol, Jenapharm, Jena, Germany) for 6 mo, while patients with normal 25(OH)D3 concentrations were monitored as controls. All patients had their serum 25(OH)D3 level measured at baseline, and again after 3, 6, and 12 mo of follow-up. Ethical appoval was obtained for the study (Ärztekammer des Saarlandes, ref. 57/11), which was conducted based on the good clinical practice guidelines as per the Declaration of Helsinki. All patients provided written informed consent.
Sample Preparation
Calibrators, quality controls, and serum samples were extracted by supported liquid extraction (SLE) using Tecan AC 96-well micro-extraction plates, as previously described [19]. Briefly, the extraction process used the following reagents: (A) internal standard (50 ng/mL d 6-25(OH)D3 in acetonitrile); (B) extraction buffer (0.2 M sodium carbonate/sodium hydrogen carbonate 1:1 v/v in water/acetonitrile 95:5 v/v); (C) washing buffer (water/methanol 90:10 v/v); (D) elution buffer (water/methanol 10:90 v/v). Reagents A and B were mixed (1:2 v/v), 100 μL of the mixture transferred to the extraction well, 50 μL of serum sample added, and the plate horizontally shaken for 10 min at 1200 rpm. The supernatant was discarded, and the well was washed with buffer (C) (200 μL) for 2 min (1200 rpm). Finally, the analyte was eluted using 200 μL of buffer (D) (5 min horizontal shaking) and the eluate transferred into 1.5 mL amber glass vials; the vials were then moved to the LC-MS/MS autosampler.
Low Resolution Mass Spectrometry (QqQ)
Five microliters of each sample extract (corresponding to ~1.25 μL of the human serum sample) were injected into a Dionex UltiMate 3000 UHPLC system (Thermo, Bremen, Germany). Separations were performed on a Phenomenex (Torrance, CA, USA) Kinetex PFP 100 Å column (100 × 2.1 mm, d p = 2.6 μm) at 40 °C and flow rate of 0.4 mL/min using gradient elution. The mobile phases were (A) water (+0.1% formic acid) and (B) methanol (+0.1% formic acid). The gradient was linearly increased from 51% to 76% B within 5 min, held at 76% B for 1.75 min, increased to 99% B within 0.75 min, and held there for 2.5 min before returning to 51% within 1 min and re-equilibration for 2 min. MS experiments were performed on a Sciex (Concord, ON, Canada) QTRAP 5500 quadrupole-quadrupole-linear ion trap (QqLIT) mass spectrometer and Turbo-V electrospray ionization source operated in positive ion mode. Ion source and MS parameters were as follows: ESI voltage, 5500 V; source temperature, 550 °C; curtain gas, 55 psi; gas 1, 45 psi; gas 2, 50 psi; entrance potential, 10 V; collision exit potential, 20 V; declustering potential, 100 V. Resolving power was set to unit resolution for both Q1 and Q3. Quantification of 25(OH)D3 was performed by SRM using the [M + H]+→[M + H-H2O]+ transitions for 25(OH)D3 and d 6 -25(OH)D3 (m/z 401→383 and 407→389) with dwell times of 200 ms each, giving approximately 40 data points across the chromatographic peak for each transition.
High Resolution Mass Spectrometry (QqTOF)
A Shimadzu (Columbia, MD, USA) Nexera UHPLC system was used for HRMS experiments. Chromatography was performed as described above. A Sciex TripleTOF 5600 hybrid QqTOF instrument was used with a DuoSpray ion source in positive ion mode. Mass calibration in MS and MS/MS modes was performed after every four injections with a set of standards. Ion source and transport parameters were as follows: ESI voltage, 5500 V; source temperature, 550 °C; curtain gas, 55 psi; gas 1, 45 psi; gas 2, 50 psi; entrance potential, 10 V; collision cell exit potential, 20 V; declustering potential, 100 V; ion release delay, 66 ms; ion release width, 26 ms. TOF-MS data were obtained over m/z 120–925 with 500 ms accumulation time and 925 ms period cycle time. TOF-MS/MS was performed for precursor ions m/z 401.34 and 407.38 for 25(OH)D3 and d 6 -25(OH)D3, respectively, over m/z 100–615 using 500 ms accumulation and 1000 ms period cycle time. For MS/MS, precursor ions were isolated in Q1, which was set to unit resolution (the unit resolution settings corresponds to peak widths of ~0.7 u). The high sensitivity mode of the instrument was used throughout all experiments. The calculated resolution was ~15,000 (full-width-at-half-maximum, FWHM) for m/z 383. Quantification was achieved by integrating peak areas from extracted ion chromatograms at m/z 383.3308 and 389.3685 (±0.01 u).
Chemiluminescent Immunoassay
25(OH)D3 serum levels of the patient samples were determined at Saarland University Medical Center’s central laboratory using the LIAISON 25 OH vitamin D total chemilumiscent immunoassay (DiaSorin, Dietzenbach, Germany) [21].
Results and Discussion
In this study, a HRMS assay was developed based on our existing triple quadrupole LC-MS/MS assay for 25(OH)D3 [19]—using identical sample preparation and LC separation conditions—and systematically compared to the earlier method. We considered this comparison very important, as we previously encountered a significant co-eluting isobaric interference in the low resolution mass spectrometric (LRMS) selected reaction monitoring (SRM) analysis of 25(OH)D3 that was introduced by the HPLC system [6]. This particular isobaric interference was impossible to resolve in the triple quadrupole CID spectra and required either differential ion mobility spectrometry or increased collision energy in the CID stage for removal to avoid systematic errors [6]. We were able to reduce and eventually remove the interference signal by systematic exchange of those pump parts that were exposed to the mobile phase flow. A second HPLC system, from a different manufacturer, which was also utilized during the study (see Experimental), did not exhibit the same interference.
HRMS: TOF-MS versus TOF-MS/MS
To optimize the HRMS assay, it was initially important to investigate the presence of isobaric interferences during mass spectral analysis; that is, isobaric species formed during electrospray ion generation as well as isobaric product ions after ion activation and dissociation. This was also tested as it was initially unclear whether using accurate mass measurements of the [M + H]+ precursor ion would provide sufficient specificity in the quantitative analysis of 25(OH)D3 from serum or whether CID was required to circumvent isobaric species. This comparison also considered the generated ion currents and signal-to-noise (S/N) ratios in both modes of operation, TOF-MS and TOF-MS/MS.
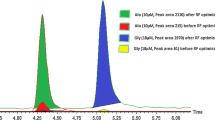
TOF-MS experiments (Figure 1a and b) showed high abundances of isobaric compounds, some of which were very close to the m/z value of the precursor ion for 25(OH)D3 (m/z 401.3414), similar to previous FTICR-MS results [6]. Several of these isobars were of an exogenous nature (Figure 1a), others originated from the serum matrix (Figure 1b). The intensities of some of these isobars were much higher than the signals obtained for 25(OH)D3 at relevant concentration levels. Although the instrumental resolving power of the QqTOF initially appeared to be sufficiently high, there was a small overlap of the main interference at m/z 401.2666 (C21H33N6O2 +; mass measurement accuracy, 1.5 ppm; the same interference was previously observed in the isobaric space of m/z 401 by FTICR analysis of serum samples [6]) from the tail-end of the peak into the 25(OH)D3 signal. This effect caused isobaric cross-talk from the slightly skewed peaks, which then irreproducibly co-contributed to signal intensities of 25(OH)D3 in some analyses, resulting in lesser precision compared with TOF-MS/MS analyses.
Electrospray TOF-MS and TOF-MS/MS spectra extracted from the chromatographic peak of 25(OH)D3 at retention time of 7.15 min (using extraction width of 0.30 min): (a) TOF mass spectrum after blank injection (90% methanol v/v), and (b) TOF-MS spectrum from QC sample (44.30 ng/mL) injection; (c) TOF-MS/MS spectrum of products of m/z 401 after blank injection, and (d) from QC sample
TOF-MS/MS measurements (Figure 1c and d) after Q1 isolation of m/z 401 exhibited significantly less signals in the isobaric space of the [M + H – H2O]+ product ion around m/z 383.3308. The [M + H – H2O]+ ion was chosen as was done for the LRMS (QqQ) analyses, as CID of the [M + H]+ ion of 25(OH)D3 yielded a very efficient neutral loss of H2O into the dehydrated species, ideal for quantification, whereas higher collision energies induced very complex product ion spectra after the initial dehydration reactions [2]. Even though a rather unspecific product ion from H2O loss was used here, the accurately measured m/z ratio of 383.3308 ([M + H – H2O]+) in the high resolution domain of TOF-MS/MS was sufficient to significantly improve specificity (Figure 1b), as other highly abundant isobars at m/z 401 only produced very minor isobaric noise in the isobaric space of m/z 383. Importantly, although the absolute ion currents for the [M + H – H2O]+ ions in TOF-MS/MS mode were lower than the [M + H]+ ion currents in TOF-MS mode, the opposite was observed for the signal-to-noise ratios because of the extremely low noise levels. Interestingly, other studies have successfully used accurate m/z values of the [M + H]+ ions from Orbitrap full scan spectra. For example, Bruce et al. [15] obtained similar analytical figures of merit from their full-scan HRMS assay in comparison to experiments using a QqQ assay. Raml et al. [18] acquired all relevant ionized species formed by atmospheric-pressure chemical ionization (APCI) of 25(OH)D3 simultaneously using a full scan Orbitrap acquisition routine and selected the [M + H]+ and [M + H – H2O]+ (from in-source CID) ions at a resolving power of 60,000. The authors pointed out that the combination of the adduct species provided maximum sensitivity; precision and accurcy of the analyses were shown to be similar to results from literature QqQ assays. Importantly, in their assay, the [M + H – H2O]+ ion from the internal standard d 6-25(OH)D2 used for 25-hydroxyvitamin D2 at m/z 401.3685 was completely separated from 25(OH)D3’s [M + H]+ ion at m/z 401.3414 and no interference occurred [18]. This separation is particulary important for those targeted assays that include vitamin D2 species in the acquisition routine. On the other hand, Liebisch and Matysik utilized the dehydrated product ion 25(OH)D3 at m/z 383 after CID on an Orbitrap instrument, to obtain data with no baseline noise [17]. MS/MS also nicely circumvented potential isobaric problems generated by d 6-25(OH)D2. Liebisch and Matysik used a resolving power of 35,000 for the Orbitrap in MS/MS mode to separate the [M + H – 2H2O]+ interference of d 6-25(OH)D2 from the m/z 383 singly dehydrated ion of 25(OH)D3 [17]. Importantly, the Orbitrap’s ability to separate this particular interference may not necessarily be reproduced on QqTOF instruments because of resolving power limitations of some TOF-based instruments.
To further demonstrate the differences of the two HRMS modes (TOF-MS and TOF-MS/MS) evaluated here, extracted ion chromatograms (EIC) were compared for different widths of the m/z extraction windows. Figure 2a illustrates TOF chromatograms for widths of 0.02, 0.1, and 0.5 u centered on the precursor ion at m/z 401.3414; the same EIC conditions were applied to the TOF-MS/MS data centered on the m/z 383.3308 product ion (Figure 2b). Although narrowing the window size clearly reduced the abundances of isobars in the TOF traces, the problem of isobaric cross-talk persisted, with resulting unsatisfactory signal-to-noise ratios and insufficient precision. Clearly, the higher resolving power obtained from an Orbitrap would be beneficial in this situation [15, 18]. There was little influence on peak areas in the CID-MS/MS traces when the extraction size was reduced, however, as isobaric noise generally was very low in the CID spectra (Figure 1d). The calculated resolution of ~15,000 (FWHM) for m/z 383 provided ample resolution to separate the very low abundant isobars in the CID spectrum.
Extracted ion chromatograms (QqTOF) from a patient serum sample (concentration level of 25(OH)D3 in serum, 31.9 ng/mL) for different TOF extraction window sizes: (a) [M + H]+ at m/z 401.3414 (TOF-MS; isolation width: blue 0.02 u; red 0.1 u; green 0.5 u), (b) [M + H-H2O]+ at m/z 383.3308 (TOF-MS/MS, isolation widths: blue 0.02 u; red 0.1 u; green 0.5 u)
Of note, the abundant chromatographic peak at a retention time of 8.4 min was assigned to a constitutional isomer of 25(OH)D3, namely 7-ketocholesterol (C27H44O2) by comparison to an authentic standard, which was easily separated by LC. This compound was previously described as a potential isobar/isomer in the analysis of 25(OH)D3 [6]; it was detected in human serum before and shown to elute after 25(OH)D3 [6]. Other isobaric interferences of 25(OH)D3 described in the literature are 7α-hydroxy-4-cholestene-3-one (C27H44O2) [9, 22, 23] and 1,2-didecanoyl-sn-glycerol (C23H44O5) [6]. 1,2-Didecanoyl-sn-glycerol exhibited much stronger retention on the reversed-phase column than 25(OH)D3 [6]. 7α-Hydroxy-4-cholestene-3-one is another constitutional isomer of 25(OH)D3 and would therefore likely present a serious tandem mass spectrometric challenge if it co-eluted with 25(OH)D3. It was shown to be easily resolved from 25(OH)D3 by reversed-phase chromatography, however, and eluted much earlier than 25(OH)D3 [22]. Finally, the 3-epimer species of 25(OH)D3, which has been shown to be present in serum samples of adult subjects at considerable levels [8, 22, 24], was also well resolved from its 25(OH)D3 variant [19] and therefore did not constitute an interference in our LRMS and HRMS assays. Figure S1 (Supplementary Material) illustrates the separation of 25(OH)D3 and 3-epi-25(OH)D3 for a CLD patient sample, showing ~5% contribution of the epimer relative to the 25(OH)D3 signal. Note that the peak areas of the two epimers are not directly comparable because of significant response factor differences between the two epimer species, as recently described by van den Ouweland et al. [25] and Flynn et al. [26], requiring a dedicated stable isotope standard for 3-epi-25(OH)D3 [12].
Fit-for-Purpose Method Development
We compared the LRMS (QqQ) and the new HRMS (QqTOF) assay by evaluating selected parameters. Some additional comparison data relating to recovery rates of sample preparation and robustness of chromatography are given in the Supplementary Material (Tables S1 and S2), as they equally applied to both LRMS and HRMS assay.
Method comparison was performed using commercial calibrator solutions at three different 25(OH)D3 serum concentrations (levels 1–3) and for four different QC samples (Table 1; the QC samples were commercial certified reference samples, see Experimental); accuracy and CV values (intra- and inter-day) for the LRMS and HRMS assays are summarized in Table 1. Calibration samples were extracted three times and QC samples at least five times. Every extracted sample was then analyzed in triplicate. Analysis of calibration samples was performed on three different days (inter-day precision).
Linear 3-point calibration (no weighting) yielded good linearity with R 2 of 0.975 (LRMS) and 0.981 (HRMS). The lowest calibration sample (9.61 ng/mL) was set as assay lower limit of quantification (LLOQ) for both LRMS and HRMS, and yielded excellent signal-to-noise ratios (S/N > 65). The limits of detection (LOD) were determined to be 0.1 ng/mL for the LRMS, and 0.3 ng/mL for the HRMS assay at S/N = 3. For accuracy, similarity for both methods was seen in the experiments; accuracies were in the range of 85.7% and 103.3% for LRMS, and 88.6% and 109.2% for the HRMS assay (Table 1). Inter-day precision was also similar for both assays, with CV ranging from 1.6%–3.5% and 1.5%–3.4% for LRMS and HRMS, respectively. Slightly larger deviation was seen for the intra-day CV comparison: CVs using LRMS were lower (between 0.7% and 2.3%) than HRMS, which gave precision data in the range of 3.3%–16.5%.
Overall, both assays were fit-for-purpose with appropriate analytical figures of merit for application to clinical samples.
Clinical Samples from Patient Study
We applied both low and high resolution mass spectrometry assays to the analysis of 97 samples from patients with different chronic liver diseases (CLD) [20]. All samples were previously analyzed by the clinical DiaSorin chemiluminescence immunoassay and concentration values used as reference for accuracy assessments. All samples were extracted in duplicate and analyzed three times with both LC-MS/MS assays. The resulting concentration data were examined by Passing-Bablok regression and Bland-Altman analysis, the outcomes of which are summarized in Figure 3. Both LRMS and HRMS assays exhibited high correlation for serum 25(OH)D3 levels in the Passing-Bablok regression analysis (Figure 3a), yielding an almost linear regression with slope of 1.010 and R 2 value of 0.939. Bland-Altman analysis exhibited normally distributed differences with mean value of –1.132, and marginally larger differences for higher concentrations (Figure 3b). Nevertheless, excellent agreement and no fixed or proportional bias was seen throughout the measurements, with the majority of data points within ±1.96 SD of the mean. The quantitative results from the HRMS assay demonstrated a weaker correlation with measured DiaSorin chemiluminescence levels, with R 2 of 0.500 (Figure 3c); a similar association was seen for the correlation of immunoassay with LRMS (R 2 = 0.580). Nevertheless, Bland-Altman analysis did not show any concentration-dependent or systematic differences between HRMS and DiaSorin (Figure 3d). These results are mostly in agreement with findings from other studies using Orbitraps for HRMS, where generally strong correlations between Orbitrap and QqQ [15], and Orbitrap and IDS-iSYS 25-hydroxyvitamin D immunoassay [18] were shown.
Conclusions
This study has compared the performance of two LC-MS/MS assays for the measurement of 25-hydroxyvitamin D3 in human serum. The two assays differed only in the implemented mass spectrometer, specifically the m/z analyzer used for analyzing the product ions and their different resolving powers (QqQ LRMS versus QqTOF HRMS), with otherwise virtually identical experimental conditions for sample preparation, chromatography, ionization, precursor ion selection, and CID. Particular attention was paid to the role of isobaric interferences and their impact on the specificity of the analysis. Overall, both low and high resolution assays exhibited very similar performances in terms of accuracy and precision of the analysis, which was readily explained by the lack of significant isobaric interferences in the MS/MS domain, thus allowing both quadrupole and TOF to easily resolve the [M + H – H2O]+ product ion used for quantification from isobaric interferences. The QqQ instrument provided slightly lower detection limits, however, which was likely due to the much higher duty cycle of the QqQ mass spectrometer in SRM mode compared with TOF mass analysis. In practice, this was not important, as the LLOQ was set to the same value for both assays, based on the lowest calibrator level (here 9.61 ng/mL), making the assay fit-for-purpose to monitor vitamin D status of individuals (the optimal vitamin D level is often based on the connection of parathyroid hormone (PTH) and 25(OH)D3, with recommendations ranging from 18 to 30 ng/mL [27, 28]; vitamin D insufficiency is often defined as serum 25(OH)D3 levels <20 ng/mL [29]). Other common implementations for LLOQ, for example based on precision [9], would likely yield similar application ranges of LRMS and HRMS assays. The QqTOF assay does offer the advantage of being able to separate potential isobaric interferences. Such interferences might (1) occur in samples other than the patient samples investigated here, (2) originate from fragmentation of other species (e.g., dehydration of an isotope standard of 25-hydroxyvitamin D2 [17, 18]), or (3) result from exogenous isobars introduced by the analytical system, as recently shown for a technical lubricant leaching from the HPLC system [6]. Since one never knows in advance whether additional isobars are present, only high resolution mass spectrometry with sufficient resolving power can ensure analyses free of systematic errors.
References
Couchman, L., Benton, C.M., Moniz, C.F.: Variability in the analysis of 25-hydroxyvitamin D by liquid chromatography-tandem mass spectrometry: the devil is in the detail. Clin. Chim. Acta 413, 1239–1243 (2012)
Volmer, D.A., Mendes, L.R.B.C., Stokes, C.S.: Analysis of vitamin D metabolic markers by mass spectrometry: current techniques, limitations of the “gold standard” method, and anticipated future directions. Mass Spectrom. Rev. 34, 2–23 (2015)
Stokes, C.S., Volmer, D.A., Grünhage, F., Lammert, F.: Vitamin D in chronic liver disease. Liver Int. 33, 338–352 (2013)
de la Hunty, A., Wallace, A.M., Gibson, S., Viljakainen, H., Lamberg-Allardt, C., Ashwell, M.: UK Food Standards Agency Workshop Consensus Report: the choice of method for measuring 25-hydroxy vitamin D to estimate vitamin D status for the UK National Diet and Nutrition Survey. Br. J. Nutr. 104, 612–619 (2010)
Roth, H.J., Schmidt-Gayk, H., Weber, H., Niederau, C.: Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann. Clin. Biochem. 45, 153–159 (2008)
Qi, Y., Geib, T., Schorr, P., Meier, F., Volmer, D.A.: On the isobaric space of 25-hydroxyvitamin D in human serum: potential for interferences in liquid chromatography/tandem mass spectrometry, systematic errors and accuracy issues. Rapid Commun. Mass Spectrom. 29, 1–9 (2015)
Schleicher, R.L., Encisco, S.E., Chaudhary-Webb, M., Paliakov, E., McCoy, L.F., Pfeiffer, C.M.: Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3, and 3-epi-25-hydroxyvitamin D3 in human serum. Clin. Chim. Acta 412, 1594–1599 (2011)
van den Ouweland, J.M.W., Beijers, A.M., van Daal, H.: Fast separation of 25-hydroxyvitamin D3 from 3-epi-25-hydroxyvitamin D3 in human serum by liquid chromatography-tandem mass spectrometry: variable prevalence of 3-epi-25-hydroxyvitamin D3 in infants, children, and adults. Clin. Chem. 57, 1618–1619 (2011)
Carter, G.D.: 25-Hydroxyvitamin D: a difficult analyte. Clin. Chem. 58, 486–488 (2012)
Ding, S., Schoenmakers, I., Jones, K., Koulman, A., Prentice, A., Volmer, D.A.: Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal. Bioanal. Chem. 398, 779–789 (2010)
Higashi, Z., Awada, D., Shimada, K.: Simultaneous determination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human plasma by liquid chromatography-tandem mass spectrometry employing derivatization with a Cookson-type reagent. Biol. Pharm. Bull. 24, 738–743 (2001)
Müller, M.J., Stokes, C.S., Lammert, F., Volmer, D.A.: Chemotyping the distribution of vitamin D metabolites in human serum. Sci. Rep. 6, 21080 (2016)
Ramanathan, R., Jemal, M., Ramagiri, S., Xia, Y.Q., Humpreys, W.G., Olah, T., Korfmacher, W.A.: It is time for a paradigm shift in drug discovery bioanalysis: from SRM to HRMS. J. Mass Spectrom. 46, 595–601 (2011)
Meyer, M.R., Helfer, A.G., Maurer, H.H.: Current position of high-resolution MS for drug quantification in clinical and forensic toxicology. Bioanalysis 6, 2275–2284 (2014)
Bruce, S.J., Rochat, B., Béguin, A., Pesse, B., Guessous, I., Boulat, O., Henry, H.: Analysis and quantification of vitamin D metabolites in serum by ultra-performance liquid chromatography coupled to tandem mass spectrometry and high-resolution mass spectrometry—a method comparison and validation. Rapid Commun. Mass Spectrom. 27, 200–206 (2013)
Abdel-Khalik, J., Crick, P.J., Carter, G.D., Makin, H.L., Wang, Y., Griffiths, W.J.: Studies on the analysis of 25-hydroxyvitamin D3 by isotope-dilution liquid chromatography-tandem mass spectrometry using enzyme-assisted derivatisation. Biochem. Biophys. Res. Commun. 446, 745–750 (2014)
Liebisch, G., Matysik, S.: Accurate and reliable quantification of 25-hydroxy-vitamin D species by liquid chromatography high-resolution tandem mass spectrometry. J. Lipid Res. 56, 1234–1239 (2015)
Raml, R., Ratzer, M., Obermayer-Pietsch, B., Mautner, A., Pieber, T.R., Sinner, F.M., Magnes, C.: Quantifying vitamin D and its metabolites by LC/Orbitrap MS. Anal. Methods 7, 8961–8966 (2015)
Geib, T., Meier, F., Schorr, P., Lammert, F., Stokes, C.S., Volmer, D.A.: A simple micro-extraction plate assay for automated LC-MS/MS analysis of human serum 25-hydroxyvitamin D levels. J. Mass Spectrom. 50, 275–279 (2015)
Stokes, C.S., Grünhage, F., Baus, C., Volmer, D.A., Wagenpfeil, S., Riemschneider, M., Lammert, F.: Vitamin D supplementation reduces depressive symptoms in patients with chronic liver disease. Clin. Nutr. (2015). doi:10.1016/k.lnu.2015.07.004
Nürnberg, B., Gräber, S., Gärtner, B., Geisel, J., Pföhler, C., Schadendorf, D., Tilgen, W., Reichrath, J.: Reduced serum 25-hydroxyvitamin D levels in stage IV melanoma patients. Anticancer Res 29, 3669–3674 (2009)
Shah, I., James, R., Barker, J., Petroczi, A., Naughton, D.P.: Misleading measures in Vitamin D analysis: a novel LC-MS/MS assay to account for epimers and isobars. Nutr. J. 10, 46 (2011)
Maunsell, Z., Wright, D.J., Rainbow, S.J.: Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin. Chem. 51, 1683–1690 (2005)
Lensmeyer, G., Poquette, M., Wiebe, D., Binkley, N.: The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum. J. Clin. Endocrinol. Metab. 97, 163–168 (2012)
van den Ouweland, J.M., Beijers, A.M., van Daal, H.: Overestimation of 25-hydroxyvitamin D3 by increased ionisation efficiency of 3-epi-25-hydroxyvitamin D3 in LC-MS/MS methods not separating both metabolites as determined by an LC-MS/MS method for separate quantification of 25-hydroxyvitamin D3, 3-epi-25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum. J. Chromatogr. B 967, 195–202 (2014)
Flynn, N., Lam, F., Dawnay, A.: Enhanced 3-epi-25-hydroxyvitamin D3 signal leads to overestimation of its concentration and amplifies interference in 25-hydroxyvitamin D LC-MS/MS assays. Ann. Clin. Biochem. 51, 352–359 (2014)
Steingrimsdottir, L., Gunnarsson, O., Indridason, O.S., Franzson, L., Sigurdsson, G.: Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 294, 2336–2341 (2005)
Chapuy, M.C., Preziosi, P., Maamer, M., Arnaud, S., Galan, P., Hercberg, S., Meunier, P.J.: Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 7, 439–443 (1997)
Osteoporosis WSGotPaMo: Prevention and Management of Osteoporosis: Report of a WHO Scientific Group. WHO, Geneva (2003)
Acknowledgments
D.A.V. acknowledges research support by the Alfried Krupp von Bohlen und Halbach-Stiftung and the German Research Foundation (DFG VO 1355/5-1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 104 kb)
Rights and permissions
About this article
Cite this article
Geib, T., Sleno, L., Hall, R.A. et al. Triple Quadrupole Versus High Resolution Quadrupole-Time-of-Flight Mass Spectrometry for Quantitative LC-MS/MS Analysis of 25-Hydroxyvitamin D in Human Serum. J. Am. Soc. Mass Spectrom. 27, 1404–1410 (2016). https://doi.org/10.1007/s13361-016-1412-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-016-1412-2