Abstract
The efficiencies of charge exchange reaction in dopant-assisted atmospheric pressure chemical ionization (DA-APCI) and dopant-assisted atmospheric pressure photoionization (DA-APPI) mass spectrometry (MS) were compared by flow injection analysis. Fourteen individual compounds and a commercial mixture of 16 polycyclic aromatic hydrocarbons were chosen as model analytes to cover a wide range of polarities, gas-phase ionization energies, and proton affinities. Chlorobenzene was used as the dopant, and methanol/water (80/20) as the solvent. In both techniques, analytes formed the same ions (radical cations, protonated molecules, and/or fragments). However, in DA-APCI, the relative efficiency of charge exchange versus proton transfer was lower than in DA-APPI. This is suggested to be because in DA-APCI both dopant and solvent clusters can be ionized, and the formed reagent ions can react with the analytes via competing charge exchange and proton transfer reactions. In DA-APPI, on the other hand, the main reagents are dopant-derived radical cations, which favor ionization of analytes via charge exchange. The efficiency of charge exchange in both DA-APPI and DA-APCI was shown to depend heavily on the solvent flow rate, with best efficiency seen at lowest flow rates studied (0.05 and 0.1 mL/min). Both DA-APCI and DA-APPI showed the radical cation of chlorobenzene at 0.05–0.1 mL/min flow rate, but at increasing flow rate, the abundance of chlorobenzene M+. decreased and reagent ion populations deriving from different gas-phase chemistry were recorded. The formation of these reagent ions explains the decreasing ionization efficiency and the differences in charge exchange between the techniques.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atmospheric pressure chemical ionization (APCI) [1–3] and atmospheric pressure photoionization (APPI) [4] are widely used for interfacing liquid chromatography (LC) with mass spectrometry (MS). They are typically used for small (<1000 u) molecules that are not sufficiently polar for efficient ionization by electrospray ionization (ESI). However, APPI is generally perceived to be more efficient for low polarity compounds than APCI, as has been shown for polycyclic aromatic hydrocarbons (PAHs) [5], triterpenes [6], certain steroids [7], and pharmaceuticals [8].
The ionization mechanisms in APCI [9, 10] (Scheme 1) and APPI [4] (Scheme 2) are very similar. The most notable difference between the techniques is the initial method of ionization: corona discharge in the kV range is used in APCI (Scheme 1, Reaction 1), whereas APPI-MS ion sources use 10.0 and 10.6 eV photons emitted by a krypton discharge lamp (Scheme 2, Reaction 1). In both methods, gas-phase reactions tend to lead to formation of protonated water and/or solvent clusters (Scheme 1, Reactions 2–6, Scheme 2, Reactions 2, 3). The protonated solvent (cluster) subsequently reacts with the analytes by proton transfer provided the proton affinity (PA) of the analyte is higher than that of the solvent (cluster) (Scheme 1 Reaction 7, and Scheme 2, Reaction 4).
Ionization reactions in APCI [9]. S = solvent, D = dopant, M = analyte, IE = ionization energy, PA = proton affinity, RE = recombination energy
Ionization reactions in DA-APPI [4]. S = solvent, D = dopant, M = analyte, IE = ionization energy, PA = proton affinity, RE = recombination energy
In APPI, a lot of effort has been invested into tailoring the gas-phase chemistry to favor charge exchange reaction (Scheme 2, Reaction 6) in reversed-phase liquid chromatography (RPLC) conditions [11, 12] because this reaction route makes possible the ionization of nonpolar compounds that are difficult to ionize by proton transfer reactions. One of the most successful charge exchange dopants has been chlorobenzene [12, 13], which has IE of 9.1 eV [14]. The chlorobenzene radical cation does not go through proton transfer reaction with solvent clusters (Scheme 2, Reaction 3) in RPLC-APPI-MS conditions [12], and is therefore not depleted in the ion source in the presence of LC solvents but is, instead, available for charge exchange with the analytes. In the case of APCI, only a few literature reports have shown that dopants (i.e., benzene and chlorobenzene) can also be used in APCI to enhance the ionization of analytes through charge exchange [15, 16]. However, dopant-assisted (DA-)APCI has not been widely explored. Moreover, to our best knowledge, a direct comparison of dopant-assisted (DA-)APPI and DA-APCI has not been reported in literature, although accurate characterization of the differences of charge exchange reaction in APPI and APCI would be important for their applications in the study of neutral and nonpolar compounds.
To fill this gap, the efficiency of charge exchange reaction in DA-APPI and DA-APCI is compared in this contribution in RPLC-MS conditions with chlorobenzene as the dopant. A representative set of 14 compounds with different polarities, sizes, and functional groups resulting in different gas-phase IEs and PAs, and a commercial mixture of polycyclic aromatic hydrocarbons were chosen as model analytes. Structures of the compounds can be found in Supplementary Figure S1 in the Electronic Supporting Material. Methanol/water (80:20) was used as the solvent because methanol has lower PA compared with acetonitrile, and it therefore shows better ionization efficiency for low PA compounds [17]. First of all, the feasibility of charge exchange reaction for the ionization of the model compounds by APCI, DA-APCI and DA-APPI was determined. Next, comparisons between DA-APCI and DA-APPI were made in solvent flow rate range 0.05–0.8 mL/min in more detail. Solvent reagent ions formed in DA-APCI and DA-APPI were compared in order to explain the differences of the two techniques. Finally, the mechanisms behind the observed differences in the charge exchange efficiencies between DA-APCI and DA-APPI are discussed.
Experimental
Chemicals
Apomorphine hydrochloride hemihydrate (98%), benzo[a]pyrene (B[a]P, ≥97%), bisphenol A (BPA, ≥99%), cholesterol (≥99%), dihydrotestosterone (DT, 5α-androstan-17β-ol-3-one, ≥97.5%), β-estradiol (E2, ≥98%), 4-nonylphenol (99.8%), squalene (≥98%), sulindac sulfide (≥98%), testosterone (≥98%), tonalide (6-acetyl-1,1,2,4,4,7-hexanemethyltetralin, ≥98%), verapamil hydrochloride (98%), anisole (99.7%), chlorobenzene (99.9%), toluene (≥99.9%), dichloromethane (≥99.8%), and methanol (≥99.9%) were from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Luteolin was from Extrasynthese (≥99%; Genay, France), and ceramide C12 (ceramide C12:ON-lauroyl-D-erythro-sphingosine) from Genzyme Pharmaceuticals (Liestal, Switzerland). TCL PAH mix (certified reference material 48905) was from Supelco (Bellefonte, PA, USA) and it contained 2 000 μg/mL of acenaphthene, acenaphthylene, anthracene, benz[a]anthracene, benzo[a]pyrene, benzo[b]fluoranthene benzo[g,h,i]perylene, benzo[k]fluoranthene, chrysene, dibenz[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-cd]pyrene, naphthalene, phenanthrene, and pyrene in benzene/dichloromethane. Water was purified by MilliQ water purifying system (Millipore, Molsheim, France).
Stock solutions of the standards were prepared in toluene (B[a]P, squalene), dichloromethane (ceramide C12), or methanol (rest of the analytes). Concentrations of the stock solutions were 10 mM except for luteolin, which was prepared at 1 mM, and BPA, which was prepared at 20 mM. All analyzed solutions were prepared in methanol/water (80/20 v/v). The composition of the in-house prepared mixtures and gas-phase properties of the compounds can be found in Table 1. For the analyses, the PAH mix was diluted to a concentration corresponding to 1 μg/mL of each analyte. The gas-phase properties of the PAH mix compounds can be found in Table 2.
Flow Injection Mass Spectrometry
Flow injection was performed using an Acquity UPLC (Waters, Milford, MA, USA) with methanol/water (80/20) as the solvent. The injection volume was 10 μL. Dopant (chlorobenzene at 10% of solvent flow rate) was mixed with the solvent flow via a PEEK t-piece prior to ion source. Agilent 1100 series capillary LC (Agilent Technologies Inc., Santa Clara, CA, USA) was used to degas and pump the dopant at flow rates ≤20 μL/min and an Agilent 1100 series HPLC degasser and pump at flow rates greater than 20 μL/min. The analytes were ionized in an orthogonal commercial APCI source (Agilent), and the ions were detected by Agilent 6410 QQQ. For APPI, the APCI needle was removed and replaced by a krypton discharge vacuum UV lamp and a lamp power source from a commercial APPI ion source (Agilent). All the experiments were conducted in positive ion mode. The ion source heater temperature was set at 450 °C and the nebulizer gas at 20 psi. Flow rate and temperature of the counter gas were 5 L/min and 350 °C, respectively. Capillary voltage was 3500 V and corona current was 4 μA in APCI and 0 μA in APPI. Fragmentor voltage was set to 113 V for studying the analyte ions. To ensure more efficient transmission of low m/z ions, the solvent ions were recorded using fragmentor voltage of 30 V. Scanned m/z range was 120–600 for analyte ions and 10–200 for solvent ions. Each analyte mixture was injected four times at all studied experimental conditions. The solvent ion spectra were recorded for 1 min.
Results
Observed Ions
First, APCI, DA-APCI, and DA-APPI were compared in typical APCI conditions at 0.8 mL/min solvent flow rate. A major difference between the techniques was observed in background ion signals, as an order of magnitude lower total ion count was achieved in (dopant-free) APCI compared with the dopant-assisted techniques at m/z range 120–600. The high background signals in DA-APCI and DA-APPI were partly due to a few abundant ions possibly deriving from the dopant, e.g., at m/z 128 (with 37Cl isotope peak at m/z 130) and at m/z 124 in DA-APCI and DA-APPI, respectively, but also due to increased chemical noise that was especially high below m/z 300. After determining the background signal, the ionization of the model analytes was studied by APCI, DA-APCI, and DA-APPI. The results are given in Table 3.
In APCI without a dopant, the main species for most analytes were [M + H]+ or [MH-H2O]+ ions. In addition, other minor fragment ions were observed. Nonylphenol and PAHs with less than 14 carbons were not detected, and BPA showed a fragment at m/z 135 ([MH-C6H5OH]+). Radical cations of the analytes were not observed. In DA-APCI, [M + H]+, M+., and fragment ions were detected depending on the analyte. Ceramide C12, cholesterol, DT, and testosterone showed signals only for the protonated species with or without water or hydrogen loss. For apomorphine, B[a]P, BPA, E2, sulindac sulfide, squalene, and the C20H12 and C22H12 compounds of the PAH mixture, [M + H]+ was the main species, but it was accompanied by a small amount of radical cations. Nonylphenol, fluorene (PAH mix), the C14-C18 PAHs, and dibenz[a,h]anthracene (C22H14) in the PAH mix showed mainly radical cations in DA-APCI. For BPA, the radical cation was observed, but the main ion was a fragment at m/z 213. Deducing from the structure of BPA, the fragment is most likely a resonance stabilized carbocation, which is formed by the loss of a methyl group from the radical cation (Supplementary Figure S5). Tonalide showed the [M + H]+ ion and a fragment at m/z 243. The detection of naphthalene was hindered by an abundant background ion at the same mass with the analyte M+. at m/z 128. DA-APPI showed mainly the same [M + H]+, M+., and fragment ions as DA-APCI. In DA-APPI, a background ion at m/z 154 disturbed the detection of acenaphthene.
Effect of Solvent Flow Rate
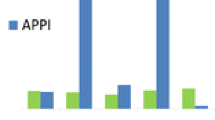
Since DA-APPI has been reported to give the best performance at lower solvent flow rates [8, 28–30] than those typically used in APCI, we tested if this is the case also in DA-APCI. The flow rate of the dopant was kept at 10% of the solvent flow rate. Flow rate dependence of APCI and DA-APPI was measured as a reference. Examples of the results can be found in Figure 1, and data for other studied analytes is given in Supplementary Figure S2. In DA-APCI, the trend for peak areas of analyte radical cations (e.g., B[a]P, nonylphenol, and PAH-mix C16H10 in Figure 1) was clear: 0.1 mL/min flow rate gave the largest peak areas, and decreasing or increasing the flow rate resulted in decreasing peak areas. Also in DA-APPI, the largest peak areas were obtained at the lowest studied flow rates (0.05–0.1 mL/min), but the peak areas decreased less steeply than in DA-APCI when the flow rate of the solvent was increased. In cases of [M + H]+ and [MH-H2O]+ ions, there were no uniform trends, but the flow rate dependence of the peak area varied from analyte to analyte and between the three techniques. APCI without a dopant gave an unstable signal at 0.2 mL/min flow rate resulting in low peak areas for the analytes. At other studied flow rates, the signals were stable and the peak areas for PAHs, BPA, luteolin, squalene, sulindac sulfide, tonalide, and DT were largest at 0.05 and 0.1 mL/min, and the rest of the studied analytes gave somewhat similar peak areas at low (0.05 and 0.1 mL/min) and high (0.4–0.8 mL/min) flow rates.
Peak areas for selected analyte ions as a function of flow rate in APCI (without dopant), dopant-assisted APCI (DA-APCI), and dopant-assisted APPI (DA-APPI). Dopant flow rate was 10% of the solvent flow rate in dopant-assisted APCI and APPI. The areas for [M + H]+ ion of B[a]P and C16H10 PAH were obtained by deducing the natural abundance of the 13C isotope of the respective M+. ion
Next, the effect of the dopant flow rate in DA-APCI was measured. The flow rate of the solvent was kept constant at 0.8 mL/min, and the flow rate of the dopant was varied between 0 and 20% of the solvent flow rate. Representative examples of the results are given in Supplementary Figure S3: the peak areas of analyte radical cations increased somewhat linearly as the flow rate of the dopant increased, while the peak areas for [M + H]+ ions were somewhat constant or decreased mildly as the flow rate was increased.
Reagent Ions
To better understand the effect of the flow rate on the ionization of the analytes, the formation of reagent ions from solvent and dopant was investigated (Figure 2). Since the experiments were conducted on a MS instrument with an atmospheric pressure interface that was not specifically designed for measuring clusters, we were not able to measure the true solvent cluster ion distribution in the source [31]. This is because the clusters dissociate due to high electric fields in the ion optics before detection. Furthermore, atmospheric pressure ion sources discriminate the transmission of smaller ions (m/z < approximately 40–80). However, the study design allowed monitoring changes in the dopant-derived reagent ions that are especially interesting when trying to understand the charge exchange reaction between the dopant and the analytes.
Abundance of the main reagent ions as function of solvent [MeOH:H2O (80:20)] flow rate in APCI (without dopant), dopant-assisted APCI (DA-APCI), and dopant-assisted APPI (DA-APPI). The isotope patterns of solvent ions at m/z 112 and 128 indicate that they contain a chlorine atom. Note that the results for APCI and the dopant-assisted techniques are presented on different scales
The reagent ion populations with APCI, DA-APCI, and DA-APPI were very different. In APCI, methanol [M + H]+ and [2M + H]+ ions were observed together with unidentified ions that most likely originate from impurities or residues in used gases, solvents, laboratory air, and equipment. In DA-APCI, the radical cation of chlorobenzene (m/z 112 and 37Cl isotope at m/z 114) was observed at all studied flow rates, but its absolute abundance decreased dramatically as the flow rate increased. On the other hand, the relative abundances of ions at m/z 108 and 128 (37Cl isotope peak at m/z 130) grew as the flow rate increased. MS/MS-measurement of the m/z 108 ion and anisole standard showed that in DA-APCI the ion at m/z 108 is a mixture: M+. of anisole was the main species and an unidentified component gave a minor signal (see Supplementary Figure S4). In DA-APPI, the chlorobenzene radical cation appeared as the most abundant solvent ion at 0.05 and 0.1 mL/min flow rate, but an ion at m/z 108 was the most prominent at higher flow rates. Robb et al. have also reported the m/z 108 reagent ion for chlorobenzene in DA-APPI-MS under slightly different experimental conditions [12]. Here, in DA-APPI, the MS/MS measurement showed that the fragmentation of the m/z 108 ion is identical to that of M+. ion of anisole (Supplementary Figure S4). The abundance of reagent ions at 0.2–0.6 mL/min flow rates was higher in DA-APPI compared with DA-APCI, and this was mainly due to the m/z 108 ion.
Discussion
Ionization of Model Compounds in APCI
Fourteen individual compounds and a commercial mixture of PAHs were chosen as model analytes for the study. The analytes included mid- to nonpolar compounds with a heterogenic set of functional groups. Their reported or estimated IEs range from 6.1 to 10.2 eV and PAs from 803 to 980 kJ/mol (Tables 1, 2). APCI (without a dopant) was suited for the ionization of most of the model compounds selected for the study: only nonylphenol and C10H8, C12H10, and C13H10 PAHs gave extremely low signals at most of the used experimental conditions. Proton transfer was clearly the main ionization route in APCI, and in the case of ceramide C12, cholesterol, DT, and E2, it was associated with the loss of water from the protonated analyte, whereas BPA formed an ion at m/z 135. The protonation of the tertiary amines (apomorphine, verapamil), conjugated carbonyl-containing molecules (tonalide, T, luteolin), and compounds containing large aromatic moieties are explained by proton attachment to the electronegative atoms (N in amine, O in carbonyls and C in π-systems). The loss of water from protonated molecules may be related to protonation of the hydroxyl group, subsequent weakening of the C–O bond and formation of a stable carbenium ion. As an example of more specific fragmentation, the suggested structure of the m/z 135 ion of BPA is presented in Supplementary Figure S5. The protonation of analytes and the absence of analyte radical cations in the spectra show that our measurements are in agreement with the generally accepted ionization mechanism of APCI [9], where the corona discharge ionizes nitrogen, leading to the formation of protonated solvent clusters that protonate the analytes (Scheme 1, Reactions 1-7).
Ionization of Model Compounds in DA-APCI
Based on previous results from Song et al. [16] and Perazzolli et al. [15], it was expected that analyte radical cations could be formed in DA-APCI with chlorobenzene as the dopant. If the ionization mechanism is charge exchange between the dopant and the analyte as suggested for benzene dopant by Perazzolli et al. [15] (Scheme 1, Reaction 8), the compounds that have lower IEs than the chlorobenzene dopant (9.1 eV [14]) should be ionized by charge exchange in DA-APCI. Indeed, ceramide C12, DT, T, tonalide, and verapamil, which have reported or estimated IEs above the IE of chlorobenzene (Table 1) were protonated, but did not show radical cations in DA-APCI. On the other hand, all compounds with IEs below the IE of chlorobenzene, except cholesterol, were ionized at least to some extent by charge exchange (Table 3, Figure 1 and Supplementary Figure S2). Charge exchange was the main ionization reaction in DA-APCI in the case of nonylphenol, BPA (assuming [M-CH3]+ ion at m/z 213 derives from the radical cation and has the structure depicted in Supplementary Figure S5), and fluorene in the PAH mix, and somewhat equally efficient with proton transfer for the other compounds of the PAH mix. The aromatic rings of these compounds are only weak gas-phase bases, but can easily lose an electron due to low ionization energy of conjugated π-bonds. For the rest of the analytes (apomorphine, B[a]P, E2, luteolin, sulindac sulfide, squalene), the efficiency of charge exchange was low compared with the efficiency of proton transfer at typical APCI flow rates (0.2–0.8 mL/min). Thus at DA-APCI, there are two main reaction pathways, namely charge exchange and proton transfer, and their relative efficiency depends on the flow rate of the solvent as shown in Figure 1 and Supplementary Figure S2, and discussed below. The measurement of the solvent reagent ions shows that analyte radical cations may be formed in charge exchange with dopant radical cations, but additionally, anisole radical cation and an unidentified reagent ion at m/z 128 may also be involved in the ionization reactions. The IE of anisole is 8.2 eV [14], so it is able to react by charge exchange with most of the analytes showing radical cations in this study (Tables 1, 2, and 3).
The addition of chlorobenzene dopant to the solvent flow in APCI had a significant effect on the ionization of several of the analytes. Most importantly, nonylphenol and fluorene (PAH mix) showed extremely small signals in APCI, but were efficiently ionized in DA-APCI via charge exchange. As can be seen from Tables 1 and 2, the estimated or previously reported PAs for nonylphenol and fluorene are amongst the lowest for the chosen model analytes. They lack functional groups with significant gas-phase basicity, but the aromatic rings make them susceptible to charge exchange. Thus, it can be concluded that the use of a dopant extends the application range of APCI toward low PA compounds. Secondly, C14-C18 PAHs in the PAH mix were observed as protonated molecules in APCI without a dopant, but showed radical cations as main ions in DA-APCI. This resulted in better sensitivity, especially at flow rate 0.2 mL/min and above (Figure 1 and Supplementary Figure S2). Again deriving from the gas-phase properties of the compounds, this shows that DA-APCI can provide better ionization efficiency for low PA, low IE compounds by the alternative charge exchange ionization mechanism.
Ionization of Model Compounds in DA-APPI
The analyte ions observed with DA-APPI were very similar to those in DA-APCI (Table 3). Radical cation formation in DA-APPI is typically explained by photon induced ionization of the dopant (Scheme 2, Reaction 1), and subsequent charge exchange reaction between the dopant radical cation and the analytes (Scheme 2, Reaction 6). As expected, ionization by charge exchange in DA-APPI occurred to compounds with IEs (Table 1, 2) below the IE of the dopant (9.1 eV) with the exception of cholesterol, which showed the [MH-H2O]+ ion. The high abundance of cholesterol [MH-H2O]+ ion may be due to a thermodynamically favorable conjugated structure, which can form by rearrangement after the protonation and water loss (Supplementary Figure S6). Nevertheless, when the solvent ions are considered, the absence of chlorobenzene radical cation at solvent flow rate 0.4 mL/min and higher, and the high abundance of the radical cation of anisole at m/z 108 in the DA-APPI solvent spectra, indicate that besides direct dopant radical cation–analyte neutral interactions, more complicated reaction routes are likely to be involved in the charge exchange reactions. Since anisole has IE of 8.2 eV, its radical cation may also react with the analytes and, therefore, contribute to the ionization process in DA-APPI.
Proton transfer was observed also in DA-APPI, although the radical cation of the chlorobenzene dopant is not likely to transfer a proton to analyte molecules, and the photon energy in APPI (10.0 and 10.6 eV) is not sufficient to ionize water (IE = 12.6 eV [14]) or methanol (IE = 10.8 eV [14]) molecules. As discussed earlier in literature [31, 32], water and solvent clusters must thus take part in the ionization process. Water and methanol clusters have sufficiently low IEs [33, 34] to be ionized by the 10.0 and 10.6 eV photons, and subsequent self-protonation can lead to generation of protonated solvent clusters. Alternatively, neutral solvent molecules can associate with dopant radical cations, and this also leads to generation of protonated solvent clusters [31]. The protonated solvent clusters can thereby protonate the analytes (Scheme 2, Reaction 4), possibly by ligand-switching as suggested by Klee et al. [31].
Effect of Flow Rate
In DA-APCI, the most favorable experimental conditions for charge exchange were reached at 0.1 mL/min flow rate (Figure 1 and Supplementary Figure S2). Both the absolute peak areas of the radical cations and the relative abundance compared with [M + H]+ or [MH-H2O]+ ions reached maxima, and decreased as the flow rate was increased. Also the abundance of the radical cation of chlorobenzene had a maximum at 0.1 mL/min flow rate, and it decreased as the flow rate was increased (Figure 2). Overall, the abundance of the dopant radical cation followed closely the peak area trend of the analyte radical cations. This is similar to results obtained for DA-APPI here and in literature [28]. It has been suggested that impurities in the solvent start to dominate the ionization in APPI when the flow rate is increased, and this would lead to suppression of ionization of analytes [28, 35]. The solvent spectra in DA-APCI show the appearance of reagent ions at m/z 108 and 128 with increasing flow rate (Figure 2), but besides impurities, the ions could also derive from gas-phase reactions of the dopant. Another possible explanation for the decreased signals at increased flow rate could be a change in cluster size distribution: the PAs of water and methanol clusters increase with increasing cluster size [36], and IEs of methanol clusters are also smaller than for the free molecule [33, 34]. Because the clusters shift to larger sizes with increasing flow rate, the clusters will effectively neutralize the dopant radical cations, and disappearance of the dopant radical cation thus prevents the charge exchange between the dopant and the analytes. Reactions of dopant radical cations via charge exchange with solvent clusters are unlikely, since the IEs of methanol clusters (n < 9) are above the IE of chlorobenzene. Additionally, it is possible that the increased density of solvent in the source at high flow rates increases the probability of depletion of both dopant and analyte radical cations by neutralization and other reactions before they are admitted to the MS. This is supported by a study of Lorenz et al. [37], which showed that in atmospheric pressure source, radical cations are depleted quicker than protonated molecules, and the survival of radical cations from the site of ionization to the MS depends, e.g., on the amount of oxygen in the ion source.
Interestingly, low flow rates (0.05–0.1 mL/min) were also extremely feasible for proton transfer in APCI in the case of C14-C22 PAHs of the PAH mix (Supplementary Figure S2). These analytes formed mainly radical cations in DA-APCI, but protonated molecules in APCI. The result is in agreement with previous reports using APCI, which have shown signal decrease at high flow rates, especially for lower polarity compounds [38, 39]. The flow rate dependence in APCI could be explained by the growth of solvent cluster size, which reduces proton transfer between protonated solvent clusters and analytes with low PA. Also, an increased amount of solvent impurities in the source may lead to competition for protons between analytes and the impurities, and decrease the ionization efficiency of the analytes.
Comparison of Charge Exchange Efficiency in DA-APCI and DA-APPI
Relative efficiency of charge exchange compared with proton transfer was greater in DA-APPI than in DA-APCI at all studied flow rates (A(M+.)/A([M + H]+) or A(M+.)/A([MH-H2O]+) values being 1.1-fold to 84-fold (median 4.0) in APPI compared with APCI, see Figure 1 and Supplementary Figure S2). Comparison of peak areas in DA-APCI and DA-APPI shows that the absolute charge exchange efficiency was similar or larger in DA-APPI compared with DA-APCI at 0.05 and 0.2–0.6 mL/min, whereas DA-APCI was slightly better or somewhat equal to DA-APPI at 0.1 mL/min flow rate depending on the analyte. The difference between the techniques was generally small, but at 0.2–0.6 mL/min flow rate DA-APPI was more efficient for low IE, low PA compounds that are poorly ionized by proton transfer, as peak areas for radical cations were even 3-fold compared with those in DA-APCI (Figure 1 and Supplementary Figure S2).
The higher efficiency of charge exchange in comparison to proton transfer in DA-APPI is expected to derive from the equilibrium of the two competing reaction pathways. The restricted photon energy in DA-APPI does not allow the efficient ionization of solvent molecules and small solvent clusters mainly responsible for protonation of analytes, and favors the formation of dopant M+. ion, which is the main charge exchange reagent in the solvent system. Also, self-protonation of chlorobenzene or generation of protonated solvent clusters via interaction between chlorobenzene radical cation and neutral solvent clusters [31] are not able to efficiently generate protonated reagents, and proton transfer between chlorobenzene radical cation and analytes is expected to be negligible. On the other hand, in DA-APCI the generation of both protonated solvent clusters and dopant radical cations is feasible. This rationale would indicate higher relative amount of the protonated solvent clusters in DA-APCI compared with DA-APPI, which would in turn favor proton transfer of the analytes as observed in our measurements. This would also mean that charge exchange efficiency in DA-APCI could be increased by increasing the flow rate of the dopant, since it would increase the amount of charge exchange reagent ions compared with the protonating species favoring charge exchange ionization of analytes. This is supported by measurement of DA-APCI spectra at constant solvent flow rate of 0.8 mL/min and varying the dopant flow rate (0–20% of the flow rate of the solvent, Supplementary Figure S3) as peak areas of radical cations increased somewhat linearly as the flow rate of the dopant was increased, while peak areas for analyte [M + H]+ remained almost constant.
Comparison of the solvent ions in DA-APPI and DA-APCI suggests that there are also differences between the reaction routes leading to charge exchange (Figure 2). In the DA-APPI spectra, the signals of chlorobenzene radical cations decreased rapidly when the flow rate was increased, and were completely absent at high flow rates (0.4–0.8 mL/min), where m/z 108 ion (anisole M+.) showed a high signal, roughly an order of magnitude higher than in DA-APCI. In DA-APCI, low, somewhat equal abundances were observed for chlorobenzene and m/z 108 ion, and the unidentified ion at m/z 128. This may be important for the charge exchange ionization of the analytes in DA-APPI since the higher production of the anisole radical cation in DA-APPI coincides with higher analyte radical cation production compared to DA-APCI (Figure 2). Since anisole has IE of 8.2 eV, it is possible that it reacts with the analytes.
Robb et al. [12] previously suggested that in DA-APPI the m/z 108 ion (anisole) is formed in a substitution reaction between neutral methanol and chlorobenzene radical cation following a general reaction scheme for several halobenzenes:
where P is the product (here anisole) and X the halogen. If the reaction occurred according to Reaction 1 in our experiments, we would expect observing similar relative abundance of the chlorobenzene radical cation (m/z 112) and the anisole radical cation (m/z 108) in both DA-APCI and DA-APPI at the same experimental conditions. However, in DA-APCI the relative abundance of anisole radical cation was much lower than in DA-APPI. To explain the observed results, we hypothesize that the m/z 108 ion derives from a reaction between neutral methanol radical and the dopant:
Since the UV radiation in APPI is not sufficiently energetic to ionize methanol molecules, but the methanol OH bond dissociation energy is lower, 4.5 eV [40], photolysis is a likely result of photon absorption by methanol. Formation of neutral radicals in APPI has been shown previously for O2 and H2O [41]. Different solvent ion compositions in DA-APPI and DA-APCI are thus suggested to reflect also neutral radical compositions of the ion sources and derive from the different energy distributions of primary ionizing species (photons versus corona discharge).
Conclusions
In this work, we showed that both proton transfer and charge exchange occur in dopant-assisted APCI and APPI, and the studied analytes form mainly the same ions in both techniques. Overall, in both techniques several ions were observed for most analytes studied, which may complicate the identification of unknowns. The charge exchange efficiencies of the techniques were found somewhat similar, although DA-APPI was slightly more efficient at typical LC flow rates (0.2–0.6 mL/min) compared with DA-APCI. Thus, both techniques can be used for compounds that cannot be ionized by proton transfer in APCI without a dopant, and the results encourage the use of dopant-assisted APCI instead of APPI if both charge exchange and proton transfer chemistry are needed for ionization of target analytes. We can also recommend the use of low flow rates (0.1 mL/min or lower) to favor ionization by charge exchange. Although the results for the analytes were closely similar, the two techniques showed different solvent ions indicating different side reactions affecting the pathway of analyte radical cation formation. While here the gas-phase reactions of the dopant with the solvent were found useful, they also mean that the experimental conditions need to be carefully optimized in the dopant-assisted techniques.
References
Carroll, D.I., Dzidic, I., Stillwell, R.N., Haegele, K.D., Horning, E.C.: Atmospheric pressure ionization mass spectrometry. Corona discharge ion source for use in a liquid chromatograph-mass spectrometer-computer analytical system. Anal. Chem. 47, 2369–2373 (1975)
Horning, E.C., Horning, M.G., Carroll, D.I., Dzidic, I., Stillwell, R.N.: New picogram detection system based on a mass spectrometer with an external ionization source at atmospheric pressure. Anal. Chem. 45, 936–943 (1973)
Hunt, D.F., McEwen, C.N., Harvey, T.M.: Positive and negative chemical ionization mass spectrometry using a Townsend discharge ion source. Anal. Chem. 47, 1730–1734 (1975)
Kauppila, T.J., Syage, J.A., Benter, T.: Recent developments in atmospheric pressure photoionization-mass spectrometry. Mass Spectrom. Rev. (2015). doi:10.1002/mas.21477
Ghislain, T., Faure, P., Michels, R.: Detection and monitoring of PAH and Oxy-PAHs by high resolution mass spectrometry: comparison of ESI, APCI and APPI source detection. J. Am. Soc. Mass Spectrom. 23, 530–536 (2012)
Rhourri-Frih, B., Chaimbault, P., Claude, B., Lamy, C., André, P., Lafosse, M.: Analysis of pentacyclic triterpenes by LC–MS. A comparative study between APCI and APPI. J. Mass Spectrom. 44, 71–80 (2009)
Wang, C., Gardinali, P.R.: Comparison of multiple API techniques for the simultaneous detection of microconstituents in water by on-line SPE-LC-MS/MS. J. Mass Spectrom. 47, 1255–1268 (2012)
Yang, C., Henion, J.: Atmospheric pressure photoionization liquid chromatographic–mass spectrometric determination of idoxifene and its metabolites in human plasma. J. Chromatogr. A 970, 155–165 (2002)
Carroll, D.I., Dzidic, I., Horning, E.C., Stillwell, R.N.: Atmospheric pressure ionization mass spectrometry. Appl. Spectrosc. Rev. 17, 337–406 (1981)
Good, A., Durden, D.A., Kebarle, P.: Ion-molecule reactions in pure nitrogen and nitrogen containing traces of water at total pressures 0.5–4 Torr. Kinetics of clustering reactions forming H+(H2O)n. J. Chem. Phys. 52, 212–221 (1970)
Kauppila, T.J., Kostiainen, R., Bruins, A.P.: Anisole, a new dopant for atmospheric pressure photoionization mass spectrometry of low proton affinity, low ionization energy compounds. Rapid Commun. Mass Spectrom. 18, 808–815 (2004)
Robb, D.B., Smith, D.R., Blades, M.W.: Investigation of substituted-benzene dopants for charge exchange ionization of nonpolar compounds by atmospheric pressure photoionization. J. Am. Soc. Mass Spectrom. 19, 955–963 (2008)
Smith, D.R., Robb, D.B., Blades, M.W.: Comparison of dopants for charge exchange ionization of nonpolar polycyclic aromatic hydrocarbons with reversed-phase LC-APPI-MS. J. Am. Soc. Mass Spectrom. 20, 73–79 (2011)
NIST Chemistry WebBook, NIST Standard Reference Database Number 69. In: Linstrom, P.J., Mallard, W.G. (eds.) National Institute of Standards and Technology, Gaithersburg MD, 20899. available at: http://webbook.nist.gov. Accessed 20 Oct 2015
Perazzolli, C., Mancini, I., Guella, G.: Benzene-assisted atmospheric-pressure chemical ionization: a new liquid chromatography/mass spectrometry approach to the analysis of selected hydrophobic compounds. Rapid Commun. Mass Spectrom. 19, 461–469 (2005)
Song, L., Cho, D.S., Bhandari, D., Gibson, S.C., McNally, M.E., Hoffman, R.M., Cook, K.D.: Liquid chromatography/dopant-assisted atmospheric pressure chemical ionization mass spectrometry for the analysis of nonpolar compounds. Int. J. Mass Spectrom. 303, 173–180 (2011)
Kostiainen, R., Kauppila, T.J.: Effect of eluent on the ionization process in liquid chromatography–mass spectrometry. J. Chromatogr. A 1216, 685–699 (2009)
Chiaia-Hernandez, A.C., Krauss, M., Hollender, J.: Screening of lake sediments for emerging contaminants by liquid chromatography atmospheric pressure photoionization and electrospray ionization coupled to high resolution mass spectrometry. Environ. Sci. Technol. 47, 976–986 (2013)
Vendrame, R., Ferreira, M.M.C., Collins, C.H., Takahata, Y.: Structure–activity relationships (SAR) of contraceptive progestogens studied with four different methods using calculated physicochemical parameters. J. Mol. Graph. Model. 20, 345–358 (2002)
Novak, I., Kovač, B.: Electronic structure and biological activity of steroids. Biophys. Chem. 78, 233–240 (1999)
Lei, H., Snyder, S.A.: 3D QSPR models for the removal of trace organic contaminants by ozone and free chlorine. Water Res. 41, 4051–4060 (2007)
Aue, D.H., Guidoni, M., Betowski, L.D.: Ab initio calculated gas-phase basicities of polynuclear aromatic hydrocarbons. Int. J. Mass Spectrom. 201, 283–295 (2000)
Östman, P., Pakarinen, J.M.H., Vainiotalo, P., Franssila, S., Kostiainen, R., Kotiaho, T.: Minimum proton affinity for efficient ionization with atmospheric pressure desorption/ionization on silicon mass spectrometry. Rapid Commun. Mass Spectrom. 20, 3669–3673 (2006)
Anstead, G.M., Kym, P.R.: Benz[a]anthracene diols: Predicted carcinogenicity and structure-estrogen receptor binding affinity relationships. Steroids 60, 383–394 (1995)
Leopoldini, M., Pitarch, I.P., Russo, N., Toscano, M.: Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J. Phys. Chem. A 108, 92–96 (2004)
Zhang, D., Liu, Y., Chu, L., Wei, Y., Wang, D., Cai, S., Zhou, F., Ji, B.: Relationship between the structures of flavonoids and oxygen radical absorbance capacity values: a quantum chemical analysis. J. Phys. Chem. A 117, 1784–1794 (2013)
Simonsick, W.J., Hites, R.A.: Characterization of high molecular weight polycyclic aromatic hydrocarbons by charge exchange chemical ionization mass spectrometry. Anal. Chem. 58, 2114–2121 (1986)
Kauppila, T.J., Bruins, A.P., Kostiainen, R.: Effect of the solvent flow rate on the ionization efficiency in atmospheric pressure photoionization-mass spectrometry. J. Am. Soc. Mass Spectrom. 16, 1399–1407 (2005)
Short, L.C., Cai, S.-S., Syage, J.A.: APPI-MS: effects of mobile phases and VUV lamps on the detection of PAH compounds. J. Am. Soc. Mass Spectrom. 18, 589–599 (2007)
Robb, D.B., Blades, M.W.: Effects of solvent flow, dopant flow, and lamp current on dopant-assisted atmospheric pressure photoionization (DA-APPI) for LC-MS. Ionization via Proton transfer. J. Am. Soc. Mass Spectrom. 16, 1275–1290 (2005)
Klee, S., Albrecht, S., Derpmann, V., Kersten, H., Benter, T.: Generation of ion-bound solvent clusters as reactant ions in dopant-assisted APPI and APLI. Anal. Bioanal. Chem. 405, 6933–6951 (2013)
Kauppila, T.J., Kuuranne, T., Meurer, E.C., Eberlin, M.N., Kotiaho, T., Kostiainen, R.: Atmospheric pressure photoionization mass spectrometry. Ionization mechanism and the effect of solvent on the ionization of naphthalenes. Anal. Chem. 74, 5470–5479 (2002)
Kostko, O., Belau, L., Wilson, K.R., Ahmed, M.: Vacuum-ultraviolet (VUV) photoionization of small methanol and methanol − water clusters. J. Phys. Chem. A 112, 9555–9562 (2008)
Martrenchard, S., Grégoire, G., Dedonder-Lardeux, C., Jouvet, C., Solgadi, D.: Proton transfer mechanism in the ionic methanol dimer. PhysChemCommun 4, 15–19 (1999)
Robb, D.B., Blades, M.W.: State-of-the-art in atmospheric pressure photoionization for LC/MS. Anal. Chim. Acta 627, 34–49 (2008)
Knochenmuss, R., Cheshnovsky, O., Leutwyler, S.: Proton transfer reactions in neutral gas-phase clusters: 1-naphthol with H2O, D2O, CH3OH, NH3, and piperidine. Chem. Phys. Lett. 144, 317–323 (1988)
Lorenz, M., Schiewek, R., Brockmann, K.J., Schmitz, O.J., Gäb, S., Benter, T.: The distribution of ion acceptance in atmospheric pressure ion sources: spatially resolved APLI measurements. J. Am. Soc. Mass Spectrom. 19, 400–410 (2008)
Asperger, A., Efer, J., Koal, T., Engewald, W.: On the signal response of various pesticides in electrospray and atmospheric pressure chemical ionization depending on the flow-rate of eluent applied in liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 937, 65–72 (2001)
Garcia, D.M., Huang, S.K., Stansbury, W.F.: Optimization of the atmospheric pressure chemical ionization liquid chromatography mass spectrometry interface. J. Am. Soc. Mass Spectrom. 7, 59–65 (1996)
Blanksby, S.J., Ellison, G.B.: Bond dissociation energies of organic molecules. Acc. Chem. Res. 36, 255–263 (2003)
Kersten, H., Funcke, V., Lorenz, M., Brockmann, K.J., Benter, T., O’Brien, R.: Evidence of neutral radical induced analyte ion transformations in APPI and Near-VUV APLI. J. Am. Soc. Mass Spectrom. 20, 1868–1880 (2009)
Acknowledgments
The authors acknowledge Academy of Finland for financial support (grant numbers 218150, 125758, 255559, 275089, and 251575).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1147 kb)
Rights and permissions
About this article
Cite this article
Vaikkinen, A., Kauppila, T.J. & Kostiainen, R. Charge Exchange Reaction in Dopant-Assisted Atmospheric Pressure Chemical Ionization and Atmospheric Pressure Photoionization. J. Am. Soc. Mass Spectrom. 27, 1291–1300 (2016). https://doi.org/10.1007/s13361-016-1399-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-016-1399-8