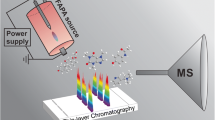
Abstract
Molecular ionization-desorption analysis source (MIDAS), which is a desorption atmospheric pressure chemical ionization (DAPCI) type source, for mass spectrometry has been developed as a multi-functional platform for the direct sampling of surfaces. In this article, its utility for the analysis of thin-layer chromatography (TLC) plates is highlighted. Amino acids, which are difficult to visualize without staining reagents or charring, were detected and identified directly from a TLC plate. To demonstrate the full potential of MIDAS, all active ingredients from an analgesic tablet, separated on a TLC plate, were successfully detected using both positive and negative ion modes. The identity of each of the compounds was confirmed from their mass spectra and compared against standards. Post separation, the chemical signal (blue permanent marker) as reference marks placed at the origin and solvent front were used to calculate retention factor (Rf) values from the resulting ion chromatogram. The quantitative capabilities of the device were exhibited by scanning caffeine spots on a TLC plate of increasing sample amount. A linear curve based on peak are, R2 = 0.994, was generated for seven spots ranging from 50 to 1000 ng of caffeine per spot.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thin-layer chromatography (TLC) can be found in a variety of fields such as agriculture, food, pharmaceutical, clinical, government, and academic research; applications within these and other fields to which TLC is suited are numerous [1]. Detection of compounds on TLC plates comes in many forms, including visual or photometric observations of absorbance or fluorescence as well as through staining using a variety of different chemical reagents [1]. Instrumental identification of compounds can be achieved using ultra-violet/visible, FTIR, and Raman spectroscopy as well as mass spectrometry [1].
Interfacing TLC plate analysis with mass spectrometry has been achieved through direct coupling using sampling probes with solvent elution [2], through direct electrospray ionization from physically modified plates [3], as well as through other commercially available ambient mass spectrometry techniques such as desorption electrospray ionization (DESI) [4–6] and direct analysis in real time (DART) [7–9]. A more complete picture of this landscape can be found in these reviews [10–13].
MIDAS consists of a desorption atmospheric pressure chemical ionization (DAPCI) source, which provides the nitrogen gas and reagent ions required for analyte desorption/ionization, and a sampling platform, which uses a system of interchangeable plates to preform different types of analyses.
DAPCI type sources are based around a high voltage corona discharge electrode [14–16]. Reagent or solvent molecules near the electrode are ionized and directed at the sample surface. A sheath gas can be used to promote ionization through the introduction of solvents/reagents or, if the gas is heated, through thermal desorption [14, 17–19]. Additional DAPCI source descriptions and parameters can be found in a variety of articles [13–27]. Examples of applications where DAPCI type sources have been used include hydrocarbons [28], explosives detection [21, 29, 30], pharmaceuticals [17, 24], imaging [31], and natural products [20, 23].
The development of MIDAS stems from our previous work studying the direct sample analysis (DSA) platform [32]. The DSA is a combined source and sampling platform. The robotic sampler can introduce samples from a 13-spot mesh screen or capillary tube sample holder. The source consists of gas and reagent inlets, a heating element, a wire-in-cylinder corona discharge electrode, and a nozzle. The source is aligned coaxially with the inlet of the mass spectrometer. Schlieren photographs taken of the heated nitrogen gas flow from the DSA revealed that the gas flow profile widened as it left the nozzle [32]. By adapting nozzles of smaller internal diameter, more uniform gas flow was achieved [32]. These observations as well as a desire to make a device capable of analyzing planar and/or irregular surfaces (currently a limitation of the DSA) spurred the development of MIDAS.
Experimental
Materials
Silica gel 60 thin-layer chromatography (TLC) plates (2.5 × 7.5 cm, 250 μm thickness) with F254 fluorescent indicator were used (EMD Millipore, Billerica, MA, USA). Standards of caffeine, acetaminophen, salicylamide, acetylsalicylic acid, arginine, leucine, lysine, methionine, and proline as well as the ninhydrin reagent were obtained from Sigma-Aldrich (St. Louis, MO, USA). Mass spectrometry grade solvents and reagents including ethyl acetate, acetic acid, ethanol, n-hexane, methanol, and water were purchased from Fisher Scientific (Pittsburgh, PA, USA). Analgesic tablets containing caffeine, acetaminophen, salicylamide and acetylsalicylic acid were obtained locally.
TLC Plate Preparation
Individual solutions of five amino acids (arginine, leucine, lysine, methionine, and proline) were prepared in water to a concentration of 5000 ppm. For analysis, 5.0 μL of each solution was spotted onto a TLC plate. The plate was not developed. After analysis with MIDAS, the plate was sprayed with a 0.2% (w:v) ninhydrin solution in ethanol in order to stain the amino acids for visualization.
A standard mixture of caffeine, acetaminophen, salicylamide, and acetylsalicylic acid was prepared at a concentration of 2000 ppm in methanol. One analgesic tablet containing 32 mg of caffeine, 110 mg of acetaminophen, 152 mg of salicylamide, and 162 mg of acetylsalicylic acid, as stated on the packaging, was dissolved in 25.0 mL of methanol. A further 1:1 (v:v) dilution of this sample was made in methanol. For analysis, 1 μL of the final sample and standard were spotted onto a TLC plate and developed in a mixture of acetic acid and ethylacetate, 5:95 (v:v). The plates were dried in an oven at 70°C for 30 min prior to analysis.
TLC Quantitation
Serial dilutions from a 1000 ppm caffeine solution were made in methanol at concentrations of 500, 400, 300, 200, 100, and 50 ppm. One microliter of each solution was manually spotted onto a TLC plate that had been prewashed with methanol. The spots were focused by developing the plate across the short axis with a mixture of methanol, ethylacetate, and n-hexane 10:45:45 (v:v). After focusing, the plate was dried in an oven at 100°C for 30 min.
Mass Spectrometer
All analyses were performed using a Perkin Elmer AxIon Time-of-Flight mass spectrometer running TOF MS Driver software ver. 4.2 (Waltham, MA, USA). The mass spectrometer was tuned and calibrated with Agilent APCI/APPI tuning mix (Santa Clara, CA, USA) prior to use. Spectra from the amino acid TLC plate were acquired at 1.0 spectra·s–1 in positive mode only. Spectra from the analgesic TLC plate were acquired at 5.0 and 2.5 spectra·s–1 in positive and negative mode, respectively. For quantitative TLC plate and rapid qualitative sample analysis, spectra were acquired at 2.0 spectra·s–1. No curtain gas was used at any time. Cylinder, endplate, and capillary entrance voltages were set to 0 V for experiments in each polarity. Additional positive mode instrument parameters include capillary exit, skimmer, radio frequency (rf) and offset voltages of 175, 18, 470, and 12 V, respectively. Additional negative mode instrument parameters include capillary exit, skimmer, rf, and offset voltages of –100, –20, –450, and –10.3 V, respectively.
MIDAS Parameters
The heater was set to supply nitrogen gas at a temperature of 180°C as measured using a thermocouple (Fluke, Wilmington, NC, USA). Incident nozzle angle was set at 50°, nozzle to sample distance was 2.0 mm, and source to inlet distance was 5.0 mm. Inlet nitrogen gas pressure was set at 40 pounds per square inch resulting in a linear velocity of 3.0 m·s–1 exiting the nozzle as measured using an anemometer (Extech, Nashua, NH, USA). Nitrogen gas was supplied from an in-house nitrogen generation system (Parker, Cleveland, OH, USA) at >99% purity. In positive mode, the corona electrode was operated at +3.0 kV and 6.0 μA, in negative mode –2.5 kV and 6.0 μA. No additional reagents or solvents were added to the system during TLC plate analysis. Atmospheric relative humidity during testing was 28%–50% as measured by a digital sling psycrometer (General, New York, NY, USA). For all TLC plate analyses the plate was moved through the sampling region at a rate of 1.6 mm·s–1.
Results and Discussion
MIDAS: Source
The source, a schematic of which can be found in Figure 1, consists of a gas heater, reagent/solvent inlet, and electrode and nozzle assembly. A color photograph can be found in Figure S1 in Electronic Supplementary Material (ESM). Nitrogen gas can be supplied from any desired external source or directly from the mass spectrometer. Gas connection to the heater is achieved by using low pressure PTFE tubing with a 1/4–28 flangeless fitting and a 1/4–28 to 10–32 adapter (IDEX Health and Science, Oak Harbor, WA, USA) to fit the heater inlet. The heater is made of a 190-mm long stainless steel tube with outer and inner diameters (o.d., i.d.) of 6.3 mm and 2.1 mm, respectively. To improve heat transfer to the nitrogen gas, the tube is filled with 1/16′′ stainless steel ball bearings (McMaster-Carr, Robbinsville, NJ, USA). Heating of the source is provided by a 1/2′′ heating tape (HTS/Amptex Co., Stafford, TX, USA), which is wrapped helically around the tube. The tape is connected to a variable power supply (HTS/Amptex Co.), which is used to control the temperature. The heater tube connects to the corona discharge electrode using a 3/8′′ national pipe thread fitting. Nitrogen gas is fed through the heater and over the corona discharge electrode. The electrode is arranged in the wire-in-cylinder configuration. The cylinder has external threads and serves as the base to attach the nozzle.
The nozzle consists of a tapered glass pipette (Fisher Scientific, Pittsburgh, PA, USA) that has been cut and fit through a stainless steel ferrule and secured to the cylinder with a gas fitting. When installed, the glass nozzle is 15 mm long with an i.d at its exit of approximately 1.5 mm. A high voltage power supply (Spellman, Hauppauge, NY, USA) is used to generate the corona discharge. Using this electrode and nozzle system, the corona discharge occurs in an enclosed region, away from the sample surface. Charged reagent gas is directed at the sample surface through the glass nozzle, which is positioned within a few millimeters of the sample surface. If desired, calibrant or other reagents/solvents can be added through a 0.25 mm i.d stainless steel tube (IDEX Health and Science, Oak Harbor, WA, USA), which is connected just after the exit of the heater but before the corona discharge electrode.
MIDAS: Sampler
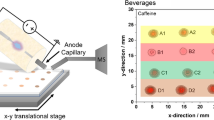
The base for the sampling platform is an aluminum plate supporting a motorized x-y stage. Power for the motor assemblies comes from a 24 V DC power supply. The stage can be moved automatically using two stepper motors, model PK243-01AA (Oriental Motor, Torrance, CA, USA). Each stepper motor has its own SCX11 Universal Stepper Motor Controller and 24 V Microstep Driver, CMD2109P, with computer control provided through the Immediate Motion Creator software ver. 2.11 (Oriental Motor). The stage, which is 127 mm × 85 mm, can travel 70 mm in the x direction and 155 mm in the y direction.
The source is mounted to an adjustment bracket above the sample stage. Adjustment screws on the bracket allow for manual xyz adjustment, up to 15.0 mm of travel on each axis, of the entire source. The pitch of the source is also variable, which enables adjustment of the incident angle to between 40° and 55°. Mounted to the source adjustment bracket, directed at the sampling region, is a small high-definition camera, model uEye XS (IDS Imaging Development Systems Inc., Woburn, MA, USA). The camera allows for remote operation and accurate positioning of the sample under the nozzle. Additionally, using the supplied software, uEye Cockpit (IDS Imaging Development Systems Inc.), still photographs and video of the sample/sampling process can be taken.
Mass Spectrometer Interface
MIDAS requires the use of a stainless steel capillary extension for the mass spectrometer. Desorbed ions are transferred to the mass spectrometer through the extension, which fits over the capillary of the mass spectrometer. The extension measures 160 mm in length with an i.d. of 5 mm, reducing to 2 mm when it reaches the inlet capillary.
TLC Plate Analysis
Compounds separated on a TLC plate may be invisible to the naked eye. The use of TLC plates with a fluorescent indicator often makes the visualization of spots easy; however, not all compounds contain functional groups that can take advantage of this feature. As a result, other methods such as staining or charring must be used in order to detect the presence of a sample. A drawback, however, is that once staining or charring is complete, the sample is destroyed and unusable for further analysis by mass spectrometry. Advantageously, MIDAS can be used to detect compounds on a TLC plate without the need to visualize the spots.
To simulate a separation in which no compounds are visible under white or UV light, five different amino acids were spotted individually on a TLC plate. These amino acids do not contain functional groups amenable to detection using the fluorescent indicator compound on the TLC plate. Figure 2 contains positive mode extracted ion chromatograms (EICs) recorded for the neat amino acids spotted onto a TLC plate. EICs were generated based on the most intense peak observed for each amino acid. The mass spectra, obtained for each amino acid, are available in EMS Figure S2. At the high concentration used, dimerization is readily observed. EMS Figure S3 contains photographs of the plate under white light before and after staining with ninhydrin, a common staining reagent for amino acids [1], as well as under ultraviolet light. Without the stain, the five amino acids analyzed were not visible; however, each one was detected by scanning the plate using MIDAS. The position of each spotted sample was confirmed after staining with ninhydrin. The distorted peak shape for arginine is a result of the initial sample placement under the source before horizontal movement commenced. A brief pause was taken after the leucine spot to ensure proper sample movement.
Analgesic compounds from a tablet were separated on a TLC plate and analyzed in the same manner as the amino acids. EICs were generated using a representative mass for each compound. The displayed baseline subtracted chromatograms in Figure 3 and spectra in Figure 4 were obtained from a single TLC plate.
(a) UV light photograph of TLC plate on MIDAS, separation of analgesic tablet compounds with 1-blue permanent marker, 2-caffeine, 3-acetaminophen, 4-acetylsalicylic acid, 5-salicylamide; (b) EIC positive mode: 1 (m/z 325), 2 (m/z 195) 3 (m/z 303), 5 (m/z 138); (c) EIC negative mode: 1 (m/z 347), 3 (m/z 186), 4 (m/z 275), 5 (m/z 172)
Mass Spectra of compounds obtained directly from the surface of the TLC plate. Positive mode: (a) blue permanent marker (*species not identified, unique to permanent marker), (b) caffeine, (c) acetaminophen, (d) salicylamide. Negative mode: (e) blue permanent marker, (f) acetaminophen, (g) acetylsalicylic acid, (h) salicylamide
Using a blue permanent marker, spots at the origin and solvent front were made after the plated had been developed. When scanned, these chemical markers were used to directly calculate the retention factor (Rf) for each compound using the time point at each peak maximum from the resulting chromatogram. Rf values obtained for caffeine, acetaminophen, acetylsalicylic acid, and salicylamide were 0.18, 0.52, 0.68, and 0.71, respectively. Not all compounds were visible in both polarities; however, all compounds were ultimately detected. No attempt was made to identify peaks observed in the spectrum obtained from the blue permanent marker. In negative mode, acetaminophen and salicylamide were observed as chlorinated adducts, a common ionization pathway in negative ion mode APCI [33]. Under the conditions of this experiment, acetylsalicylic acid was observed as an anion fragment, through the loss of [C2H3O]+.
Under the solvent system used to develop the plate, acetylsalicylic acid and salicylamide were not completely resolved. As a result, they visually appear as one spot. This co-migration makes it difficult to visually identify the spot as containing two unique compounds, and further complicates any additional analysis if the spot were to be removed. Using MIDAS, however, the spot is easily identified as two species with acetylsalicylic acid migrating slower than salicylamide.
TLC Quantitation
The quantitative capabilities of the source were illustrated in the scanning of a TLC plate that had been manually spotted with solutions of caffeine of varying concentrations. The amount of sample remaining after each spot had dried was in the nanogram range. Figure 5 contains the EIC of the representative mass of caffeine during a single pass over the TLC plate as well as the calibration curve constructed using the resulting peak areas. A photograph of the TLC plate under UV light can be found in EMS Figure S4. With a comparable sample concentration range, the linearity of the curve is improved over early DART calibration experiments using caffeine [7] without the need to use a stable isotope dilution analysis.
The results show excellent linearity despite not using an automated TLC spotter as has been used in previous quantitative experiments using a modified, angled DART system [9]. Deviations above are likely the result of manual plate spotting, and the use of an automatic TLC spotter may improve sample positioning and delivery and likely the overall analytical performance of the system.
Conclusion
MIDAS provides direct and rapid analysis of planar samples. MIDAS is a green source in that it uses readily available nitrogen as opposed to DART, which uses less abundant and increasingly expensive helium. It is important to note that this type of APCI-based source is not amenable to large biomolecules where an electrospray-based source would be better suited.
The device inflicted no visible damage to the TLC plates under study. It is therefore still possible to physically remove the spot for further analysis or processing if operating on a preparatory scale. It is important to note that this type of APCI-based source is not amenable to large biomolecules where an electrospray-based source would be better suited.
MIDAS is not limited to only TLC plate analysis; the source is adaptable to the analysis of a variety of different sample types and applications through the use of easily interchangeable sampling plates. Others such as 96-well and 384-spot plates can readily be used with MIDAS to greatly increase sample throughput. The independent control and operation characteristics of MIDAS allow the device to be used on a variety of mass spectrometers with atmospheric inlets from different vendors.
References
Sherma, J., Fried, B.: Handbook of thin-layer chromatography, (3rd ed., revised.). Marcel Dekker, New York (2003)
Morlock, G.E., Brett, N.: Correct assignment of lipophilic dye mixtures? A case study for high-performance thin-layer chromatography–mass spectrometry and performance data for the TLC–MS interface. J. Chromatogr. A 1390, 103–111 (2015)
Hsu, F.L., Chen, C.H., Yuan, C.H., Shiea, J.: Interfaces to connect thin-layer chromatography with electrospray ionization mass spectrometry. Anal. Chem. 75, 2493–2498 (2003)
Van Berkel, G.J., Ford, M.J., Deibel, M.A.: Thin-layer chromatography and mass spectrometry coupled using desorption electrospray ionization. Anal. Chem. 77, 1207–1215 (2005)
Van Berkel, G.J., Kertesz, V.: Automated sampling and imaging of analytes separated on thin-layer chromatography plates using desorption electrospray ionization mass spectrometry. Anal. Chem. 78, 4938–4944 (2006)
Pasilis, S.P., Kertesz, V., Van Berkel, G.J., Schulz, M., Schorcht, S.: Using HPTLC/DESI-MS for peptide identification in 1D separations of tryptic protein digests. Anal. Bioanal. Chem. 391, 317–324 (2008)
Morlock, G., Ueda, Y.: New coupling of planar chromatography with direct analysis in real time mass spectrometry. J. Chromatogr. A 1143, 243–251 (2007)
Chernetsova, E.S., Revelsky, A.I., Morlock, G.E.: Some new features of direct analysis in real time mass spectrometry utilizing the desorption at an angle option. Rapid Commun. Mass Spectrom. 25, 2275–2282 (2011)
Häbe, T.T., Morlock, G.E.: Quantitative surface scanning by direct analysis in real time mass spectrometry: quantitative surface scanning by DART-MS. Rapid Commun. Mass Spectrom. 29, 474–484 (2015)
Pasilis, S.P., Van Berkel, G.J.: Atmospheric pressure surface sampling/ionization techniques for direct coupling of planar separations with mass spectrometry. J. Chromatogr. A 1217, 3955–3965 (2010)
Morlock, G., Schwack, W.: Coupling of planar chromatography to mass spectrometry. Trends Anal. Chem. 29, 1157–1171 (2010)
Cheng, S.C., Huang, M.Z., Shiea, J.: Thin-layer chromatography/mass spectrometry. J. Chromatogr. A 1218, 2700–2711 (2011)
Tuzimski, T.: Application of different modes of thin-layer chromatography and mass spectrometry for the separation and detection of large and small biomolecules. J. Chromatogr. A 1218, 8799–8812 (2011)
Chen, H., Zheng, J., Zhang, X., Luo, M., Wang, Z., Qiao, X.: Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination. J. Mass Spectrom. 42, 1045–1056 (2007)
Chen, H.W., Lai, J.H., Zhou, Y.F., Huan, Y.F., Li, J.Q., Xie, Z., Wang, Z.C., Luo, M.B.: Instrumentation and characterization of surface desorption atmospheric pressure chemical ionization mass spectrometry. Chin. J. Anal. Chem. 35, 1233–1240
Cooks, R.G., Gologan, B., Takáts, Z., Wiseman, J.M., Cotte-Rodriguez, I.: Method and System for Desorption Atmospheric Pressure Chemical Ionization. US 7544933 B2, June 9 (2009)
Williams, J.P., Patel, V.J., Holland, R., Scrivens, J.H.: The use of recently described ionization techniques for the rapid analysis of some common drugs and samples of biological origin. Rapid Commun. Mass Spectrom. 20, 1447–1456 (2006)
Yang, S., Ding, J., Zheng, J., Hu, B., Li, J., Chen, H., Zhou, Z., Qiao, X.: Detection of melamine in milk products by surface desorption atmospheric pressure chemical ionization mass spectrometry. Anal. Chem. 81, 2426–2436 (2009)
Yang, S.P., Chen, H.W., Yang, Y.L., Hu, B., Zhang, X., Zhou, Y.F., Zhang, L.L., Gu, H.W.: Imaging melamine in egg samples by surface desorption atmospheric pressure chemical ionization mass spectrometry. Chin. J. Anal. Chem. 37, 315–318 (2009)
Chen, H., Liang, H., Ding, J., Lai, J., Huan, Y., Qiao, X.: Rapid differentiation of tea products by surface desorption atmospheric pressure chemical ionization mass spectrometry. J. Agric. Food Chem. 55, 10093–10100 (2007)
Cotte-Rodríguez, I., Takáts, Z., Talaty, N., Chen, H., Cooks, R.G.: Desorption electrospray ionization of explosives on surfaces: Sensitivity and selectivity enhancement by reactive desorption electrospray ionization. Anal. Chem. 77, 6755–6764 (2005)
Song, Y., Cooks, R.G.: Atmospheric pressure ion/molecule reactions for the selective detection of nitroaromatic explosives using acetonitrile and air as reagents. Rapid Commun. Mass Spectrom. 20, 3130–3138 (2006)
Wu, Z., Chen, H., Wang, W., Jia, B., Yang, T., Zhao, Z., Ding, J., Xiao, X.: Differentiation of dried sea cucumber products from different geographical areas by surface desorption atmospheric pressure chemical ionization mass spectrometry. J. Agric. Food Chem. 57, 9356–9364 (2006)
Zhang, X., Jia, B., Huang, K., Hu, B., Chen, R., Chen, H.: Tracing origins of complex pharmaceutical preparations using surface desorption atmospheric pressure chemical ionization mass spectrometry. Anal. Chem. 82, 8060–8070 (2010)
Wang, J., Yang, S.P., Yan, F.Y., Liu, Y., Li, M., Song, Y.H., Zhan, Y.B., Chen, H.W.: Rapid determination of dimethoate in nanoliter of juice using surface desorption atmospheric pressure chemical ionization mass spectrometry. Chin. J. Anal. Chem. 38, 453–457 (2010)
Huang, D., Luo, L., Jiang, C., Han, J., Wang, J., Zhang, T., Jiang, J., Zhou, Z., Chen, H.: Sinapine detection in radish taproot using surface desorption atmospheric pressure chemical ionization mass spectrometry. J. Agric. Food Chem. 59, 2148–2156 (2011)
Pi, Z., Yue, H., Ma, L., Ding, L., Liu, Z., Liu, S.: Differentiation of various kinds of fructus schisandrae by surface desorption atmospheric pressure chemical ionization mass spectrometry combined with principal component analysis. Anal. Chim. Acta 706, 285–290 (2011)
Jjunju, F.P.M., Badu-Tawiah, A.K., Li, A., Soparawalla, S., Roqan, I.S., Cooks, R.G.: Hydrocarbon analysis using desorption atmospheric pressure chemical ionization. Int. J. Mass Spectrom. 345/347, 80–88 (2013)
Takáts, Z., Cotte-Rodriguez, I., Talaty, N., Chen, H., Cooks, R.G.: Direct, trace level detection of explosives on ambient surfaces by desorption electrospray ionization mass spectrometry. Chem. Commun. 15, 1950–1952 (2005)
Cotte-Rodríguez, I., Hernández-Soto, H., Chen, H., Cooks, R.G.: In situ trace detection of peroxide explosives by desorption electrospray ionization and desorption atmospheric pressure chemical ionization. Anal. Chem. 80, 1512–1519 (2008)
Li, M., Jia, B., Ding, L., Hong, F., Ouyang, Y., Chen, R., Zhou, S., Chen, H., Fang, X.: Document authentication at molecular levels using desorption atmospheric pressure chemical ionization mass spectrometry imaging: Document authentication by DAPCI-MS. J. Mass Spectrom. 48, 1042–1049 (2013)
Winter, G.T., Wilhide, J.A., LaCourse, W.R.: Characterization of a direct sample analysis (DSA) ambient ionization source. J. Am. Soc. Mass Spectrom. 26, 1502–1507 (2015)
McEwen, C.N., Larsen, B.S.: Ionization mechanisms related to negative Ion APPI, APCI, and DART. J. Am. Soc. Mass Spectrom. 20, 1518–1521 (2009)
Acknowledgments
The authors acknowledge and thank John Cataldi of the UMBC machine shop for his help with the fabrication of source components. The authors acknowledge student funding support provided through The W. R. Grace Foundation Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 703 kb)
Rights and permissions
About this article
Cite this article
Winter, G.T., Wilhide, J.A. & LaCourse, W.R. Molecular Ionization-Desorption Analysis Source (MIDAS) for Mass Spectrometry: Thin-Layer Chromatography. J. Am. Soc. Mass Spectrom. 27, 352–358 (2016). https://doi.org/10.1007/s13361-015-1289-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-015-1289-5