Abstract
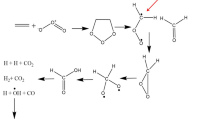
ESI-protonated 1,5-bis-(2-methoxyphenyl)-1,4-pentadien-3-one (1) undergoes a gas-phase Nazarov cyclization and dissociates via expulsions of ketene and anisole. The dissociations of the [M + D]+ ions are accompanied by limited HD scrambling that supports the proposed cyclization. Solution cyclization of 1 was effected to yield the cyclic ketone, 2,3-bis-(2-methoxyphenyl)-cyclopent-2-ene-1-one, (2) on a time scale that is significantly shorter than the time for cyclization of dibenzalacetone. The dissociation characteristics of the ESI-generated [M + H]+ ion of the synthetic cyclic ketone closely resemble those of 1, suggesting that gas-phase and solution cyclization products are the same. Additional mechanistic studies by density functional theory (DFT) methods of the gas-phase reaction reveals that the initial cyclization is followed by two sequential 1,2-aryl migrations that account for the observed structure of the cyclic product in the gas phase and solution. Furthermore, the DFT calculations show that the methoxy group serves as a catalyst for the proton migrations necessary for both cyclization and fragmentation after aryl migration. An isomer formed by moving the 2-methoxy to the 4-position requires relatively higher collision energy for the elimination of anisole, as is consistent with DFT calculations. Replacement of the 2-methoxy group with an OH shows that the cyclization followed by aryl migration and elimination of phenol occurs from the [M + H]+ ion at low energy similar to that for 1.

The role of methoxy group in the Nazarov cyclization of 1,5-bis-(2-methoxyphenyl)-1,4-pentadien-3-one in the gas-phase and condensed phase by June Cyriac, Justin Paulose, M. George, Department of Chemistry, Sacred Heart College, Thevara, Cochin, Kerala, India-682013., M. Ramesh, R. Srinivas, National center for Mass Spectrometry, IICT, Hyderabad, India. Daryl Giblin and Michael L. Gross, Department of Chemistry, Washington University in St.Louis, St.Louis, USA, MO 63130.















Similar content being viewed by others
References
Pellissier, H.: Recent developments in the Nazarov process. Tetrahedron 61, 6479–6517 (2005)
Tius, M.: Some new Nazarov Chemistry. Eur. J. Org. Chem. 2193–2206 (2005)
Frontier, A.J., Collison, C.: The Nazarov cyclization in organic synthesis. Recent advances. Tetrahedron 61, 7577–7606 (2005)
Yaji, K., Shindo, M.: Construction of a fully substituted cyclopentenone as the core skeleton of stemonamide via a Nazarov cyclization. Tetrahedron Lett. 51, 5469–5472 (2010)
Malona, J.A., Colbourne, J.M., Frontier, A.J.: A general method for the catalytic nazarov cyclization of heteroaromatic compounds. Org. Lett. 8, 5661–5664 (2006)
Banaag, A.R., Tius, M.A.: Design of chiral auxiliaries for the allene ether Nazarov Cyclization. J. Am. Chem. Soc. 129, 5328–5329 (2007)
Polo, V., Andres, J.: Lewis acid and substituent effects on the molecular mechanism for the Nazarov reaction of Penta-1,4-dien-3-one and derivatives. A topological analysis based on the combined use of electron localization function and catastrophe theory. J. Chem. Theory Comput. 3, 816–823 (2007)
Smith, D.A., Ulmer, C.W.: Effects of substituents in the 3-position on the [2 + 2] pentadienyl cation electrocyclization. J. Org. Chem. 62, 5110–5115 (1997)
Shi, F.Q., Li, X., Xia, Y., Zhang, L., Yu, Z.X.: DFT study of the mechanisms of in water Au(I)-catalyzed tandem [3,3]-rearrangement/Nazarov reaction/[1,2]-hydrogen shift of enynyl acetates: a proton-transport catalysis strategy in the water-catalyzed [1,2]-hydrogen shift. J. Am. Chem. Soc. 129, 15503–15513 (2007)
Wang, X., Holmes, J.L.: A study of the isomerization and dissociation of formal [acetone-methanol]+. ion–molecule complexes. Can. J. Chem 83, 1903–1912 (2005)
Trikoupis, M.A., Burgers, P.C., Ruttink, P.J.A., Terlouw, J.K.: Benzonitrile assisted enolization of the acetone and acetamide radical cations: proton-transport catalysis versus an intermolecular H+/D+ transfer mechanism. Int. J. Mass Spectrom 210/211, 489–502 (2001)
O’Hair, R.A.J.: The 3D quadrupole ion trap mass spectrometer as a complete chemical laboratory for fundamental gas-phase studies of metal mediated chemistry. Chem. Commun. 1469–1481 (2006)
Kingston, E.E., Beynon, J.H., Liehr, J.G., Meyrant, P., Flammang, R., Maquestiau, A.: The Claisen rearrangement of protonated allyl phenyl ether. Org. Mass Spectrom. 20, 351–359 (1985)
Moolayil, J.T., George, M., Srinivas, R., Russell, A., Giblin, D., Gross, M.L.: Protonated nitro group as a gas-phase electrophile: experimental and theoretical study of the cyclization of o-nitrodiphenyl ethers, amines, and sulfides. J. Am. Soc. Mass Spectrom. 18, 2204–2217 (2007)
Moolayil, J.T., George, M., Srinivas, R., Swamy, N.S., Russell, A., Giblin, D., Gross, M.L.: he mass spectrometry-induced cyclization of protonated N-[2-(benzoyloxy)phenyl]-benzamide: a gas-phase analog of a solution reaction. Int. J. Mass Spectrom 249/250, 21–30 (2006)
Zhou, Y., Pan, Y., Cao, X., Wu, J., Jiang, K.: Gas-phase Smiles rearrangement reactions of deprotonated 2-(4,6-dimethoxypyrimidin-2-ylsulfanyl)-N-phenylbenzamide and its derivatives in electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 1813–1820 (2007)
Wang, F.: Collision-induced gas-phase smiles rearrangement in phenoxy-N-phenylacetamide derivatives. Rapid Commun. Mass Spectrom. 20, 1820–1821 (2006)
Howell, J.A.S., O’Leary, P.J., Yates, P.C.: Acyclic O– and N-substituted pentadienyl cation: structural characterization, cyclisation, and computational results. Tetrahedron 51(26), 7231–7246 (1995)
Huang, J., Leboeuf, D., Frontier, A.J.: Understanding the fate of the oxyallyl cation following Nazarov electrocyclization: sequential Wagner Meerwein migrations and the synthesis of spirocyclic cyclopentenones. J. Am. Chem. Soc. 133, 6307–6317 (2011)
George, M., Sebastian, V.S., Nagi Reddy, P., Srinivas, R., Giblin, D., Gross, M.L.: Gas-phase Nazarov cyclization of protonated 2-methoxy and 2-hydroxychalcone: an example of intramolecular proton-transport catalysis. J. Am. Soc. Mass Spectrom. 20, 805–818 (2009)
Lawrence, N.J., Simon, E., Armitage, M., Greedy, B.: Cook. D., Ducki, S., McGown, A.T.: The synthesis of indanones related to combretastatin A-4 via microwave-assisted Nazarov cyclization of chalcones. Tetrahedron Lett. 47, 1637–1640 (2006)
Adams, B.K., Ferst, E.M., Davis, M.C., Herold, M., Kurtkaya, S., Camalier, R.F., Hollingshead, M.G., Kaur, G., Sausville, E.A., Rickles, F.R., Snyder, J.P., Liotta, D.C., Shojia, M.: Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem 12, 3871–3883 (2004). Tetrahedron Lett. 47, 1637–1640 (2006)
Weber, W.M., Hunsaker, L.A., Abcouwer, S.F., Decka, L.M., Vander Jagt, D.L.: Antioxidant activities of curcumin and related enones. Bioorganic. Med. Chem. 13, 3811–3820 (2005)
Weber, W.M., Hunsaker, L.A., Roybal, C.N., Bobrovnikova-Marjon, E.V., Abcouwer, S.F., Royer, R.E., Decka, L.M., Vander Jagt, D.L.: Activation of NFκB is inhibited by curcumin and related enones. Bioorg. Med. Chem. 14, 2450–2461 (2006)
Stewart, J.J.P.: Optimization of parameters for semiempirical methods. I. Method. J. Comp. Chem. 10, 209–220 (1989)
Stewart, J.J.P.: Optimization of parameters for semiempirical methods. II. Applications. J. Comp. Chem. 10, 221–264 (1989)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Vakrzewski, V.G., Montgomery Jr., J.A., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al- Laham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Andres, J.L., Gonzalez, C., Head-Gordon, M., Replogle, E.S., Pople, J.A.: Gaussian 98, Revision A.6. Gaussian, Inc, Pittsburgh (1998)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery Jr., J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision C.02. Gaussian, Inc, Wallingford CT (2004)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09, Revision A.02. Gaussian, Inc, Wallingford CT (2009)
Zhao, Y., Truhlar, D.G.: The M06 suite of density functionals for main-group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account 120, 215–241 (2008)
Zhao, Y., Truhlar, D.G.: Density functionals with broad applicability in chemistry. Acct. Chem. Res. 41(2), 157–167 (2008)
Scott, A.P., Radom, L.: Harmonic Vibrational Frequencies: An Evaluation of Hartree-Fock, MØller-Plesset, Quadratic Configuration Interaction, Density Functional Theory, and Semiempirical Scale Factors. J. Phys. Chem. 100, 16502–16513 (1996)
Shephard, M.J., Paddon-Row, M.N.: Gas phase structure of the bicyclo[2.2.1]heptane (norbornane) cation radical: a combined ab initio MO and density functional study. J. Phys. Chem 99, 3101–3108 (1995)
Tureček, F.: Proton affinity of dimethyl sulfoxide and relative stabilities of C2H6OS molecules and C2H7OS+ ions. A comparative G2(MP2) ab initio and density functional theory study. Phys Chem. A 102, 4703–4713 (1998)
Bohme, D.K.: Proton transport in the catalyzed gas-phase isomerization of protonated molecules. Int. J. Mass Spectrom. Ion Processes 115, 95–110 (1992)
Chalk, A.J., Radom, L.: Proton-transport catalysis: a systematic study of the rearrangement of the isoformyl cation to the formyl cation. J. Am. Chem. Soc. 119, 7573–7578 (1997)
Chalk, A.J., Radom, L.: Ion-transport catalysis: catalyzed isomerizations of NNH+ and NNCH3 +. J. Am. Chem. Soc. 121, 1574–1581 (1999)
Ruttink, P.J.A., Burgers, P.C., Fell, L.M., Terlouw, J.K.: Dissociation of Ionized 1,2-Ethanediol and 1,2-Propanediol: Proton-Transport Catalysis with Electron Transfer. J. Phys. Chem. 102, 2976–2980 (1998)
Franchetti, V., Solka, B.H., Baitinger, W.E., Amy, J.W., Cooks, R.G.: Soft landing of ions as a means of surface modification. Int. J. Mass Spectrom. Ion Processes 23, 29–35 (1977)
Verbeck, G., Hoffmann, W., Walton, B.: Soft-landing preparative mass spectrometry. Analyst 137, 4393–4407 (2012)
Acknowledgments
The authors J.C., J.P., and M.G. thank the principal, Sacred Heart College, Thevara, for providing infrastructure. R.S. and V.R. thank Dr. J. S. Yadav, Director, IICT, Hyderabad, for facilities. Research at WU was supported by the National Centers for Research Resources of the NIH, NIGMS grant P41 GM103422-35, and by the Washington University Computational Chemistry Facility, supported by NSF grant CHE-0443501.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cyriac, J., Paulose, J., George, M. et al. The Role of Methoxy Group in the Nazarov Cyclization of 1,5-bis-(2-Methoxyphenyl)-1,4-Pentadien-3-one in the Gas Phase and Condensed Phase. J. Am. Soc. Mass Spectrom. 25, 398–409 (2014). https://doi.org/10.1007/s13361-013-0785-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-013-0785-8