Abstract
We describe direct analysis of self-assembled monolayers (SAMs) on copper surfaces by low temperature plasma (LTP) mass spectroscopy (MS). Two kinds of SAMs formed from n-dodecylmercaptan (NDM) and l-phenyl-5-mercaptotetrazole (PMTA) were prepared on copper by spontaneous chemisorption. With the LTP probe, desorption and ionization of the SAMs was easily achieved, and the ions produced were introduced into MS for analysis. Characteristic fragment ions from NDM SAMs, mainly [M + M – H]+ (M is the NDM molecule) and from PMTA SAMs, mainly [M + H – S]+ (M is the PMTA molecule), were both absent in the MS spectra of neat NDM and PMTA samples. This provided evidence of the formation of SAMs on copper. As a supplementary method, LTP-MS is helpful in obtaining information on the barrier properties of SAMs on copper, such as inhibitor efficiency (IE) and the surface adsorption concentration of corrosive electrolyte (Γ*) surrounding copper. Aiming for an evaluation of the reliability of LTP-MS, a comparative study of our method and the traditional method of cyclic voltammetry (CV) showed a correlation coefficient higher than 0.97. In addition, a rough, simple procedure for imaging of the distribution of the molecules adsorbed on copper surface was presented. The study supplied a rapid and simple method for direct investigation of SAMs on copper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Self-assembled monolayers (SAMs) are commonly used as corrosion inhibitors for copper, which may replace expensive gold in many fields such as small integration circuit assemblies [1, 2]. The characterizations of SAMs on copper, such as surface morphology, defects in the film, adhesion, wettability, and molecular identification [1–4], are very important. Many complementary methods have been utilized for the characterization of SAMs on copper, including (but are not limited to) reflectance infrared spectroscopy (IR) [3, 5], X-ray photoelectron spectroscopy (XPS) [6, 7], scanning electronic microscopy (SEM) [8, 9], ellipsometry, contact angles (CA) [3, 9], as well as electrochemical analysis, including cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) [10–13]. IR and XPS identified specific functional groups and oxidation states. SEM supplied the information about surface topography. Ellipsometry and CA measured surface hydrophobicity and molecular angle relative to the surface. Among these techniques, electrochemical analysis method is the most widely used, which is efficient for evaluating the barrier properties of SAMs on copper. For example, it can evaluate the defects in the film that allow electrons to penetrate through SAM to copper [3], via inhibitor efficiency (IE) [14, 15] and the surface adsorption concentration of corrosive electrolyte (Γ*) [16] and so on. Nevertheless, it is less powerful in qualitative analysis such as chemical specificity of the SAMs on copper. In addition, none of these methods mentioned above can provide definitive structure information about the SAMs on copper.
Several mass spectroscopy (MS) techniques are used to provide direct molecular identification of SAMs on metal, such as secondary ion mass spectrometry (SIMS) [17], laser desorption/ionization mass spectrometry (LDI-MS) [18], matrix assisted laser desorption/ionization and time of flight mass spectrometry (MALDI-TOF MS) [19, 20], and direct analysis in real time (DART) mass spectrometry [4]. However, each of them possesses certain limitations; for example, they may need energetic ion beams, laser irradiation, vacuum, higher temperatures, complicated chambers, or high-speed airflow. The LTP source utilizes a dielectric barrier discharge to produce plasma, with helium as discharge gas, which is capable of desorbing and ionizing samples at ambient conditions [21–24]. The simple configuration and small size of LTP source makes it convenient enough to analyze samples on various substrates and simple to use. LTP-MS has many advantages such as low temperature, easy to operate, in-situ analysis in real time and ambient conditions without any sample preparation [25–27]. These may make LTP-MS appropriate for the detection of the SAMs on copper.
In this assay, LTP-MS has been utilized for the analysis of two typical kinds of SAMs on copper formed from n-dodecylmercaptan (NDM) and l-phenyl-5-mercaptotetrazole (PMTA). Through the characteristic ions of SAMs on copper surfaces, the molecular structure information was achieved. According to the relative MS intensities, the variation trend of characteristic ions with immersed time of copper in NDM was acquired, which was in accordance with that of electrochemical parameters (IE versus time and Γ* versus time). A coarse MS imaging of the SAMs on copper by LTP-MS was also presented. Compared with the traditional methods, the feasibility of LTP-MS for the characterization of SAMs on copper has been confirmed. The LTP-MS method will have a great potential in the field of self-assembly monolayers research.
2 Experimental
2.1 Materials
All the reagents used were of analytical grade. Copper foil (99.9999%, 1 cm × 1 cm), l-phenyl-5-mercaptotetrazole (PMTA) and n-dodecylmercaptan (NDM) were purchased from Alfa Aesar, a Johnson Matthey Company (Ward Hill, MA, USA). Nitric acid (HNO3), sodium hydroxide (NaOH), absolute alcohol, and acetone were bought from Beijing Chemical Works. Nitrogen (N2, 99.999%) and helium (He, 99.999%) were bought from Beijing Millennium Gas Marketing Center. Doubly deionized water was used throughout the experiments and was obtained by passing house-distilled water through a Millipore Simplicity 185 water purification system (Millipore, Bedford, MA, USA).
2.2 Apparatus
The commercial CTP-2000K discharge power source was purchased from Tianjin Ganze Industry and Trade Co. Ltd. (Tianjin, China). 3D automatic moving stage was purchased from Beijing Winner Optics Instruments Co., Ltd. Thermo LTQ linear ion trap mass spectrometer was from Thermo Fisher Scientific, San José, CA, USA. Cyclic voltammetric experiments were performed on a CHI650 electrochemical workstation (CH Instrument Inc., Shanghai, China).
Scanning electron microscope (SEM) was bought from Hitachi high-technologies Co., Ltd. Japan (Hitachi S-4800). SEM was performed with a scanning electron microscope with an acceleration voltage of 10 kV. Before SEM imaging, a thin layer of Pt was sprayed on copper surface by an ion sputter (Hitachi E-1045, Hitachi high-technologies Co., Ltd. Japan) at current 10 mA for 120 s.
2.3 Preparation of NDM and PMTA SAMs on Copper
Copper foil (1 cm × 1 cm) was sonicated in water, absolute alcohol, and acetone for 15 min, in that order. Then the cleaned copper was dried with nitrogen gas for 2 min. The copper surface was sonicated in diluted nitric acid (1.6 M) for 1 min to remove the oxide films and the top layer of copper atoms, which may have adsorbed contaminants, then rinsed with water and dried by a stream of dry nitrogen gas. NDM SAMs on copper was synthesized by immersing copper in an alcohol solution of NDM (4.09 M, room temperature) for a set period of time. The copper was last rinsed by alcohol and dried by nitrogen gas. PMTA SAMs on copper was synthesized by immersing copper in an aqueous solution of PMTA (0.5 g L–1, 50 °C) [28] for 1 h. The copper was finally rinsed by distilled water and dried by nitrogen gas. The modified copper was stored in desiccators at room temperature.
2.4 Cyclic Voltammetry (CV)
The electrochemical measurements were carried out in a single-compartment three-electrode glass cell with copper modified by SAMs as the working electrode, Ag/AgCl as the reference electrode and a Pt-wire as the counter electrode. NaOH (0.1 M) serves as electrolyte at a potential scan rate of 0.1 v/s.
Inhibitor efficiency (IE) and surface adsorption concentration of the electrolyte (Γ*) surrounding copper can be calculated by equation (1) [14, 15] and equation (2) [16], respectively.
Where i corr refers to corrosion current of copper with SAMs, \( i_{{corr}}^{\prime } \) refers to corrosion current of bare copper.
Where i p is redox peak current, R is gas constant, T is Kelvin temperature, n is the number of moles of the electrons transferred, F is the Faraday constant, ν is scan rate, A is the geometric area of copper.
2.5 Low-Temperature Plasma Mass Spectrometry (LTP-MS)
LTP (Figure S-1, see Supplementary Information) consists of a quartz tube (i.d. 3.91 mm and o.d. 5.60 mm) with copper rod (1.10 mm o.d.) as an internal electrode centered axially inside the quartz tube, and a piece of copper sheet (10.5 × 21.3 mm) surrounding the outside of the quartz tube is used as the counter electrode. The wall of quartz tube serves as the dielectric barrier. A high-voltage alternating current (AC) was applied to both electrodes. Helium gas was utilized as discharge gas. In this study, the LTP probe was coupled to mass spectrometry and was applied in the detection of SAMs on copper. The LTP probe was placed with its end 1 cm away from the surface with an angle of about 45° from the sample surface. For the detection of SAMs on copper, the copper was placed on the 3D automated moving stage under LTP probe.
Experiments were carried out on a Thermo LTQ linear ion trap mass spectrometer (Thermo Fisher Scientific, San José, CA, USA). Data acquisition was via the Xcalibur software. Positive-ion detection was used for all the ink-jet experiments and helium was used as the discharge gas at a pressure of 0.6 MPa, excited by 10 kV. The main experimental parameters used were as followed: m/z range 20–500; capillary temperature 150 °C; tube lens 65 V; capillary voltage 15 V. Tandem mass spectrometry experiments were performed with collision-induced dissociation (CID) experiments.
Low resolution MS imaging was achieved by the scan of the copper with NDM SAMs line by line at a speed of 700 μm/s; then the desorbed ions were introduced into mass spectrometry for analysis. From the intensity of the characteristic ion monitored in the obtained spectra, a density image can be created. A self-written program was used for providing bmp images of the rough MS imaging.
3 Results and Discussion
3.1 Morphologic Characterization of SAMs on Copper
The microstructures of bare copper, NDM SAMs on copper and PMTA SAMs on copper are observed by SEM (Fig. 1). Figure 1A is the SEM micrograph of bare copper surface after nitric acid treatment at room temperature for 1 min, which shows uneven surface of copper. Figure 1B shows the surface morphology of NDM SAMs on copper; it can be seen that a layer of a regular cobblestone-shaped structure is formed on the copper surface. A compact, club-shaped layer is formed on the surface of copper by immersing treated copper in PMTA solution (Fig. 1C), and the pits on the copper surface are well covered. The SEM images demonstrate that NDM and PMTA SAMs are well formed on copper surface.
3.2 Detection of NDM SAMs on Copper and Neat NDM by LTP-MS
Mass spectra obtained from NDM SAMs on copper and neat NDM in the positive mode are shown in Fig. 2. Figure 2A shows the spectra of NDM SAMs on the copper surface, with the primary characteristic ions of [M – H]+ (m/z 201), [M – H – H2]+ (m/z 199), [M + M – H]+ (m/z 403), and some oxidation products such as [M + O – H]+ (m/z 217) and [M + M + O – H]+ (m/z 419), where M is the NDM molecule. Mass spectra obtained from neat NDM (Fig. 2B) contains characteristic ions of [M – H]+ (m/z 201), [M – H – H2]+ (m/z 199) as well as some oxidation products including [M + O – H]+ (m/z 217), [M + O – 3 H]+ (m/z 215), and [M + M + O – H]+ (m/z 419). The observation of dimer ion [M + M – H]+ (m/z 403) in the spectra of NDM SAMs, which was absent in the spectra of neat NDM, can provide evidence indicating the formation of SAMs on copper. Dimers from NDM SAMs have also been found with other mass spectrometric methods such as direct analysis in real time (DART) mass spectrometry [4] and two-laser mass spectrometry (L2MS) [29].
Scheme S-1 (in Supplementary Information) shows the proposed mechanism of the main produced ions based on Penning mechanism [30–32]. The formation of [M – H]+ (m/z 201) from NDM SAMs and neat NDM is attributed to charge transfer [30, 31], and the possible mechanism of the formation of [M + M – H]+ (m/z 403) from NDM SAMs is attributed to proton transfer and charge transfer [30, 31]. More details can be seen in the Supplementary Information. The generation of dimer requires that adjacent S atoms must be within some minimum distance of each other [29], which may explain why no dimer is observed in the spectra of neat NDM.
It is less likely that the dimers are from the disulfide impurities of the excessive NDM. First, disulfide was reported to be poor in the adsorption onto copper surface because copper was easily oxidized in the air, whereas disulfide had low affinity for oxidized copper and the excessive disulfide could be rinsed off easily [2, 33]. Second, it was reported that disulfide actually adsorbed onto the oxide-free copper surface as two separate alkanethiolate species, indicating that there was no difference between the bonding of thiols and disulfides onto copper [29]. Third, hardly any dimer is observed in the spectra of neat NDM, which provides evidence that the dimers are not generated from the disulfide impurities of excessive NDM.
3.3 Detection of PMTA SAMs on Copper and Neat PMTA by LTP-MS
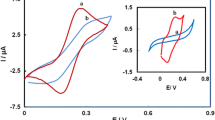
Apart from NDM SAMs, the investigation of l-phenyl-5-mercaptotetrazole (PMTA) SAMs on copper has been carried out. Figure 3A shows the mass spectra of PMTA SAMs on copper in positive mode. The primary characteristic ions occur at m/z 147 and 119, corresponding to [M + H–S]+ (Fig. 3A, m/z 147) and [M + H–S–N2]+ (Fig. 3A, m/z 119), respectively, where M is the PMTA molecule. Figure 3B shows the mass spectra of neat PMTA in positive mode. The primary characteristic ion is m/z 149, corresponding to [M–HN2]+ (Fig. 3B, m/z 149).
The difference of primary characteristic ions between PMTA SAMs on copper and neat PMTA indicates the formation of PMTA SAMs, rather than physical adsorbed PMTA molecules. And the preferred elimination of S atom from PMTA SAMs demonstrates that S atom plays an important role in the coordination of PMTA with copper, which is accordant with previous reports [34–37]. In neat PMTA the elimination of S atom first is less likely because breaking C = S is very difficult. The formation of [M + H – S]+ ion (m/z 147) is also attributed to proton transfer and charge transfer [30, 31]; and then [M + H – S]+ can lose N2 to produce [M + H – S – N2]+ (m/z 119), which matches well with the results of collision induced dissociation (CID) (the insert of Fig. 3A).
3.4 Surface Properties Analysis of SAMs by LTP-MS in Comparison with Electrochemical Analysis
As described above, LTP-MS can provide direct molecular information about SAMs on copper. Moreover, in our experiment, since the data from LTP-MS was in accordance with that from inhibitor efficiency (IE) and surface adsorption concentration of corrosive electrolyte surrounding copper (Γ*), LTP-MS can be a supplementary method for electrochemical techniques. In this work, NDM SAMs on copper were illustrated as an example.
Figure 4 shows the SEM images of NDM SAMs on copper with different immersed time of copper in NDM: 1, 2, 3, 4, 6, and 8 h. It can be seen that when the immersed time is lower that 3 h, the copper surface is apparently uneven, indicating a low coverage rate of copper surface by NDM. When the immersed time reaches 6 h, the rough surface of copper was almost completely covered, demonstrating a well-formed layer. It is clearly seen that the density of NDM SAMs adsorbed on the copper surface gradually increases as the immersed time increases. Thus, it is hypothesized that the amount of NDM molecules adsorbed on copper surface will also increase. The NDM SAMs on copper with different immersed time (1 , 2, 3, 4, 6, and 8 h) of copper in NDM were detected by LTP-MS in the experiment. It is observed that the relative MS signal intensities of characteristic ion [M + M – H]+ (m/z 403) from copper surface (1 cm × 1 cm) gradually increases with the increasing immersed time (Fig. 5A); which is consistent with the inference mentioned above.
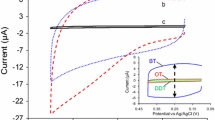
The variation trend of barrier properties of NDM SAMs on copper, with different immersed time of copper in NDM, was determined by cyclic voltammetry (CV) in NaOH solution (0.1 M) with the scan rate at 0.1 V s–1. As can be seen in Fig. 5B, the CV peak currents decrease gradually with the immersed time increasing from 0 to 8 h, indicating an increase of the inhibiting capacity of NDM SAMs. According to the equations given in Experimental Section, IE of NDM SAMs and Γ* can be calculated; it is obtained that with the decrease of the peak current, IE will increase and Γ* will decrease. The relationship between the immersed time and IE as well as that between the immersed time and Γ* is shown in Fig. 5C. It can be seen that IE has a positive correlation with the immersed time while Γ* has a negative one with it.
The correlations between the relative MS intensities of [M + M – H]+ and IE and that between the relative intensities and Γ* are shown in Fig. 6. The correlation coefficients are 0.97359 (relative intensities versus IE) and –0.97358 (relative intensities versus Γ*), suggesting a good correlation between two techniques. Hence, LTP-MS can be an auxiliary tool for electrochemical method, providing information on IE as well as Γ* indirectly. Although LTP-MS cannot supply the value of IE and Γ*, and the analysis of them by relative MS intensities can only be qualitative; LTP-MS can indicate the variation trends of the two parameters with time indirectly.
In addition, through the scan of the copper surface, LTP-MS can also present us a simplified form of ion distribution on the SAM with a self-written program, supplying information about the distribution of the NDM molecules adsorbed on copper (Supplementary Information).
4 Conclusions
The LTP-MS provides us with an efficient means of molecular identification of SAMs on copper, complemented for electrochemical analysis. At the same time, it can assist electrochemical techniques in the detection of inhibitor efficiency (IE) of SAMs on copper and surface adsorption concentration of the corrosive electrolyte (Γ*) surrounding copper. Moreover, it supplies the distribution information about the molecules adsorbed on copper by a simple MS imaging. LTP-MS will have a great potential in the application of analytical chemistry.
References
Campos, M.A.C., Trilling, A.K., Yang, M.L., Giesbergs, M., Beekwilder, J., Paulusse, J.M.J., Zuihof, H.: Self-assembled functional organic monolayers on oxide-free copper. Langmuir 27, 8126–8133 (2011)
Dilimon, V.S., Fonder, G., Delhalle, J., Mekhalif, Z.: Self-assembled monolayer formation on vopper: a real time electrochemical impedance study. J. Phys. Chem. C 115, 18202–18207 (2011)
Rao, B.V.A., Iqbai, M.Y., Sreedhar, B.: Self-assembled monolayer of 2-(octadecylthio)benzothiazole for corrosion protection of copper. Corros. Sci. 51, 1441–1452 (2009)
Kpegba, K., Spadaro, T., Cody, R.B., Nesnas, N., Olson, J.A.: Analysis of self-assembled monolayers on gold surfaces using direct analysis in real time mass spectrometry. Anal. Chem 79, 5479–5483 (2007)
Hosseinpour, S., Hedberg, J., Baldelli, S., Leygraf, C., Johnson, M.: Initial oxidation of alkanethiol-covered copper studied by vibrational sum frequency spectroscopy. J. Phys. Chem. C 115, 23871–23879 (2011)
Dubowski, J.J., Voznyy, O., Marshall, G.M.: Molecular self-assembly and passivation of GaAs (001) with alkanethiol monolayers: a view towards bio-functionalization. Appl. Surf. Sci. 256, 5714–5721 (2010)
Marshall, G.M., Lopinski, G.P., Bensebaa, F., Dubowski, J.J.: Surface dipole layer potential induced IR absorption enhancement in n-alkanethiol SAMs on GaAs (001). Langmuir 25, 13561–13568 (2009)
Tan, G.Q., Zheng, Y.Q., Miao, H., Bo, H.Y., Xia, A.: Preparation of BiFeO3 thin film by the liquid phase deposition (LPD) method on functionalized organic self-assembled monolayers (SAMs). Mater. Lett. 64, 657–660 (2010)
Yuan, S.J., Pehkonen, S.O., Liang, B., Ting, Y.P., Neoh, K.G., Kang, E.T.: Superhydrophobic fluoropolymer-modified copper surface via surface graft polymerization for corrosion protection. Corros. Sci. 53, 2738–2747 (2011)
Telegdi, J., Szabó, T., Al-Taher, F., Pfeifer, É., Kuzmann, E., Vértes, A.: Coatings against corrosion and microbial adhesion. Mater. Corros. 61, 1000–1007 (2010)
Blanchard, P.Y., Alévéque, O., Boisard, S., Gautier, C., El-Ghayoury, A., Derf, F.L., Breton, T., Levillain, E.: Intermolecular interactions in self-assembled monolayers of tetrathiafulvalene derivatives. Phys. Chem., Chem. Phys. 13, 2118–2120 (2011)
Rao, B.V.A., Iqbai, M.Y., Sreedhar, B.: Electrochemical and surface analytical studies of the self-assembled monolayer of 5-methoxy-2-(octadecylthio)benzimidazole in corrosion protection of copper. Electrochim. Acta 55, 620–631 (2010)
Alagta, A., Felhösi, I., Bertoti, I., Kálmán, E.: Corrosion protection properties of hydroxamic acid self-assembled monolayer on carbon steel. Corros. Sci. 50, 1644–1649 (2008)
Ma, H.Y., Yang, C., Yin, B.S., Li, G.Y., Chen, S.H., Luo, J.L.: Electrochemical characterization of copper surface modified by n-alkanethiols in chloride-containing solutions. Appl. Surf. Sci. 218, 143–153 (2003)
Ma, H.Y., Chen, S.H., Niu, L., Shang, S.X., Li, S.L., Zhao, S.Y., Quan, Z.L.: Studies on electrochemical behavior of copper in aerated NaBr solutions with Schiff base, N, N'-o-phenylen-bis (3-methoxysalicylidenimine). J. Electrochem. Soc. 148, B208–B206 (2001)
Li, Q.L. Electroanalytical Chemistry, Beijing Normal University Press (Beijing), pp. 240 (1995)
Tencer, M., Nie, H.Y., Berini, P.: Formation and electrochemical desorption of self-assembled monolayers as studied by TOF-SIMS. Surf. Interface Anal. 43, 993–997 (2011)
Ha, T.K., Lee, T.G., Song, N.W., Moon, D.W., Han, S.Y.: Cation-assisted laser desorption/ionization for matrix-free surface mass spectrometry of alkanethiolate self-assembled monolayers on gold substrates and nanoparticles. Anal. Chem. 80, 8526–8531 (2008)
Quiñones, R., Gawalt, E.S.: Study of the formation of self-assembled monolayers on nitinol. Langmuir 23, 10123–10130 (2007)
Su, J., Mrksich, M.: Using MALDI-TOF Mass spectrometry to characterize interfacial reactions on self-assembled monolayers. Langmuir 19, 4867–4870 (2003)
Na, N., Zhao, M.X., Zhang, S.C., Yang, C.D., Zhang, X.R.: Development of a dielectric barrier discharge ion source for ambient mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 1859–1862 (2007)
Garcia-Reyes, J.F., Harper, J.D., Salazar, G.A., Charipar, N.A., Ouyang, Z., Cooks, R.G.: Detection of explosives and related compounds by low-temperature plasma ambient ionization mass spectrometry. Anal. Chem. 83, 1084–1092 (2011)
Venter, A., Nefliu, M., Cooks, R.G.: Ambient desorption ionization mass spectrometry. Trends Anal. Chem. 27, 284–290 (2008)
Ma, X.X., Zhang, S.C., Lin, Z.Q., Liu, Y.Y., Xing, Z., Yang, C.D., Zhang, X.R.: Real-time monitoring of chemical reactions by mass spectrometry utilizing a low-temperature plasma probe. Analyst 134, 1863–1867 (2009)
Na, N., Xia, Y., Zhu, Z.L., Zhang, X.R., Cooks, R.G.: Birch reduction of benzene in a low-temperature plasma. Angew. Chem. Int. Ed. 48, 2017–2019 (2009)
Huang, G.M., Ouyang, Z., Cooks, R.G.: High-throughput trace melamine analysis in complex mixtures. Chem. Commun. 5, 556–558 (2009)
Zhang, J.I., Tao, W.A., Cooks, R.G.: Facile determination of double bond position in unsaturated fatty acids and esters by low temperature plasma ionization mass spectrometry. Anal. Chem. 83, 4738–4744 (2011)
Wang, P., Liang, C.H., Zhang, J.: Anti-tarnish treatment of brass for coinage. Mater. Corros. 58, 604–608 (2007)
Trevor, J.L., Lykke, K.R., Pellin, M.J., Hanley, L.: Two-laser mass spectrometry of thiolate, disulfide, and sulfide self-assembled monolayers. Langmuir 14, 1664–1673 (1998)
Cody, R.B.: Observation of molecular ions and analysis of nonpolar compounds with the direct analysis in real time ion source. Anal. Chem. 81, 1101–1107 (2009)
Chan, G.C.Y., Shelley, J.T., Wiley, J.S., Engelhard, C., Jackson, A.U., Cooks, R.G., Hieftje, G.M.: Elucidation of reaction mechanisms responsible for afterglow and reagent-ion formation in the low-temperature plasma probe ambient ionization source. Anal. Chem. 83, 3675–3686 (2011)
Kauppila, T.J., Kuuranne, T., Meurer, E.C., Eberlin, M.N., Kotiaho, T., Kostiainen, R.: Atmospheric pressure photoionization mass spectrometry. Ionization mechanism and the effect of solvent on the ionization of naphthalenes. Anal. Chem. 74, 5470–5479 (2002)
Mekhalif, Z., Fonder, G., Auguste, D., Laffineur, F., Delhalle, J.J.: Impact of the anchoring groups X (–SH, –S–S–, –SeH, and –Se–Se–) of CF3(CF2)3(CH2)11X molecules self-assembled on oxidized electroplated copper. Electroanal. Chem. 618, 24–32 (2008)
Ye, X.R., Xin, X.Q., Zhu, J.J., Xue, Z.L.: Coordination compound films of 1-phenyl-5-mercaptotetrazole on copper surface. Appl. Surf. Sci. 135, 307–317 (1998)
Jiménez-Sandoval, O., Cea-Olivares, R., Hernández-Ortega, S., Silaghi-Dumitrescu, I.: Structural studies of tetrazoles. Crystal and molecular structure and ab initio calculations of 1-phenyl-1H-tetrazole-5-thiolate, as its [diaqua(18-crown-6)sodium] salt: an anionic tetrazole free of direct metal interactions. Heteroatom Chem. 8, 351–359 (1997)
Liang, C., Wang, P., Wu, B., Huang, N.: Anti-tarnish treatment of brass for coinage (II) color analysis, adsorption model, and quantum chemical calculations. Mater. Corros. 62, 53–60 (2011)
Han, K.P., Liu, Q., Ye, X.R., Fang, J.L.: An anti-tarnish film on brass. Surf. Coat. Tech. 99, 326–329 (1998)
Acknowledgments
The authors gratefully acknowledge the support from the National Nature Science Foundation of China (91027034, 20975016, 21005007), National Grant of Basic Research Program of China (no. 2011CB915504), and Specialized Research Fund for the Doctoral Program of Higher Education (200800270006, 20100003120014).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 492 kb)
Rights and permissions
About this article
Cite this article
Ma, L., Jia, M., Hu, J. et al. The Characterization of Self-Assembled Monolayers on Copper Surfaces by Low-Temperature Plasma Mass Spectrometry. J. Am. Soc. Mass Spectrom. 23, 1271–1278 (2012). https://doi.org/10.1007/s13361-012-0378-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-012-0378-y