Abstract
The noncovalent complexation of monoamine neurotransmitters and related ammonium and quaternary ammonium ions by a conformationally flexible tetramethoxy glucosylcalix[4]arene was studied by electrospray ionization Fourier transform ion cyclotron resonance (ESI-FTICR) mass spectrometry. The glucosylcalixarene exhibited highest binding affinity towards serotonin, norepinephrine, epinephrine, and dopamine. Structural properties of the guests, such as the number, location, and type of hydrogen bonding groups, length of the alkyl spacer between the ammonium head-group and the aromatic ring structure, and the degree of nitrogen substitution affected the complexation. Competition experiments and guest-exchange reactions indicated that the hydroxyl groups of guests participate in intermolecular hydrogen bonding with the glucocalixarene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The molecular recognition of pharmaceutically interesting compounds is attracting increasing attention and various synthetic materials have been produced for medicinal and biomimetic applications. The ability of synthetic receptors to function as artificial enzymes or ion channels [1–3], to sense biologically important ions [4, 5], to improve chromatographic separation [6–13], and to solubilize, stabilize, and transport drugs is being explored [14, 15]. The design of synthetic receptors for host–guest systems is challenging, however, because of the many factors requiring adjustment for selective complexation. Besides hydrogen bonding and various external factors such as solvent effects, factors such as size, shape, and charge compatibility between the host and guest also have to be considered [16, 17].
A number of synthetic hosts based on macrocyclic compounds able to encapsulate small molecules have been prepared [16, 17]. Calixarenes [18–21], for example, have been designed to recognize a variety of biologically important compounds, including amino acids, peptides, and proteins [22–26].
The recognition of neurotransmitters by synthetic receptors has been studied only sparingly, even though neurotransmitters play major roles in important brain functions, as well as in intra- and intercellular signaling, morphogenesis, and immunoregulation [27–34]. A number of pathologic disorders, including Parkinson’s disease, schizophrenia, and Alzheimer’s disease, are considered to implicate neurotransmitter systems [35–37]. The development of fast, accurate, and cost-effective techniques for the clinical separation and detection of neurotransmitters has thus become of considerable interest [38–49].
Series of thiourea-linked calix[4]arene glycoclusters [50–52] have been synthesized and their interactions with proteins [53–57], anionic species [57], natural amino acids [52], and carboxylates [58] investigated. Possible cooperation between the aromatic cavity, thiourea linkers, and sugar units of glucosylcalixarenes in the guest binding has been indicated in these studies [57]. Clear selectivity towards aromatic amino acids has also been observed, and enhanced by the introduction of an additional hydrogen bonding group to the amino acid side chain [52]. The proximity of the hydrogen bonding groups of the guest also has a major effect on the selectivity of the complexation [58].
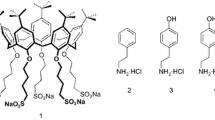
We investigated the noncovalent complexation of monoamine neurotransmitters and related nitrogen compounds by a conformationally flexible tetraglucosylcalix[4]arene 1 (Figure 1). The studies were carried out by using positive ion ESI-FTICR mass spectrometry.
2 Experimental
2.1 Sample Preparation
The glucosylcalixarene was dissolved in methanol and its final concentration in the samples was 4 μM. Host/guest ratios were 1:3, and the final concentration of the guests in samples was 12 μM. Acetic acid (0.05 vol-%) was included in sample solutions to enhance the protonation and decrease the relative intensity of sodium and potassium adducts. Guest compounds were used as hydrochlorides, except for 3,4-dihydroxybenzylamine, which was used as hydrobromide. Carnitine, epinephrine, and norepinephrine were used as racemic mixtures.
2.2 FTICR Mass Spectrometry
All measurements were performed in positive ion mode on a Bruker Daltonics (Billerica, MA, USA) APEX Qe Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer equipped with a 4.7 T superconducting magnet, Infinity ICR cell, Apollo II electrospray ion source, and pre-cell quadrupole interface. The required ~1·10–10 mbar vacuum was obtained by rotary vacuum and turbomolecular pumps supplied by Edwards (West Sussex, UK). The samples were introduced to the ion source through a capillary with a 70o off-axis sprayer at the end. The flow rate of 1.5 μL min–1 was obtained with a Cole-Parmer (Cole-Parmer Instrument Company, Vernon Hills, IL, USA) syringe pump, and Hamilton (Reno, NV, USA) syringes. Nitrogen gas was used for nebulization (1.0 psi) and for drying (4.0 L s–1), and the ion source was heated to 225 oC. The ion source voltages were 3.5 kV to end plate and 4.5 kV to capillary. The capillary exit voltage was 375 V. In most cases, a total of 16 scans were collected, and the whole operation was performed using the Apollo II control ver. 1.3 and XMASS 7.0.8 software, which was also used for data processing.
2.3 Competition Experiments
The competition measurements were performed by mixing two guests and glucosylcalixarene 1 with the host/guest1/guest2 molar ratio of 1:3:3. Each competition experiment was carried out on five parallel samples, and each sample was measured five times. Variances were calculated from the standard deviation of the five samples (s1) and from the standard deviation of the most deviating series of five measurements (s2), with the equation s2 = s 21 + s 22 [59–61]. Values of individual measurements or sample values were discarded if their deviation from the mean was four or more times the average deviation of the retained values. The competition experiments between aromatic amines were performed against dopamine. Competition experiments on aliphatic compounds were performed with competition pairs GABA versus carnitine, carnitine versus carbamylcholine, carbamylcholine versus betaine, and betaine versus acetylcholine.
2.4 Energy-Resolved Collision Induced Dissociation
In the energy-resolved collision induced dissociation (CID) experiments, the precursor ions were isolated in the ICR cell by correlated harmonic excitation fields. After isolation, a short pumping delay (3.0 s) was introduced. Isolated ions were excited by an rf field and allowed to collide with argon gas that was leaked into the ICR cell through a pulsed valve. The E 50%com values were calculated by using the relative intensities of the complexes [r. = ICOMPLEX/(ICOMPLEX+IDISSOCIATION PRODUCTS)]. The E 50%com values represent the center-of-mass collision energy required for dissociation of the complex to its relative half-intensity.
2.5 Ion/Neutral Reactions
In H/D exchange and guest-exchange reactions, deuterated ammonia (ND3) and tripropylamine (Pr3N), respectively, were used as reagents and introduced to the ICR cell from a separate volume through an adjustable needle valve. The reagent gas flow, from the volume to the cell, was set to keep the cell pressure at ~5·10–8 mbar for H/D exchange reactions and ~1·10–7 mbar for guest-exchange reactions. Isolation was performed as with the CID experiments, except that in the H/D exchange reactions single-frequency excitation shots were used to obtain monoisotopic isolation of the precursor ion. Isolated ions were allowed to react with reagent with reaction delay times ranging from 0.01 to 300 s. Sixteen scans were collected when reaction delay was 60 s or less, and six scans when it was more than 60 s. Reaction second-order rate constants (kexp) were obtained from the slopes of the pseudo-first-order rate plots, i.e., ln(Iion/Isum) versus t, where Iion is the intensity of the isolated precursor, Isum is the sum of the intensities of the isolated precursor ion and its exchange product and t is the reaction delay time.
3 Results and Discussion
3.1 Complex Formation
The positive ion ESI-FTICR spectra revealed that, in the gas phase, the glucosylcalixarene 1 existed as singly protonated [1+H]+ ion and formed singly charged 1:1 complexes with all the guest compounds. The complexation ability of the glucosylcalixarene 1 towards the neurotransmitters was evaluated in competition experiments between two guests [62]. Among the aromatic guests (2–9), the binding affinity of glucosylcalixarene toward the compounds followed the order serotonin (9) > norepinephrine (8) ≈ epinephrine (7) ≈ dopamine (6) > 3,4-dihydroxybenzylamine (4) ≈ deoxyepinephrine (5) > histamine (3) ≈ tyramine (2) (Figure 2).
The observed selectivity order is clearly affected by three structural features of the guest. (1) An additional hydrogen bonding group in the guest compound enhances the complexation (6 versus 2 and 5 versus 7). (2) Both the length of the alkyl spacer (between the amine and the aromatic moiety) and the degree of nitrogen substitution affect the complexation. Specifically, ethylene spacer was preferred over methylene (6 versus 4) and primary terminal amine over secondary amine (6 versus 5). (3) The selectivity shown for the serotonin (9) confirmed the preference of glucosylcalixarene for guests bearing indole nuclei. The same was observed earlier in the complexation of tryptophan by 1 [52].
Among the aliphatic guests (10–14), the binding affinity of glucosylcalixarene toward the compounds followed the order GABA (10) > carnitine (14) > carbamylcholine (13) > betaine (11) > acetylcholine (12) (Figure 3). This affinity order further emphasizes the importance of hydrogen bonding group for formation of a stable complex (13 versus 12 and 11 versus 14).
3.2 Relative Kinetic Stability
The relative gas-phase kinetic stabilities of the complexes were evaluated by performing energy-resolved collision induced dissociation (CID) experiments. Upon dissociation of the complexes of primary and secondary ammonium ions (2–10), the proton was left on the calixarene, resulting in increased intensity of the peak corresponding to the [1+H]+ ion. The ion then fragmented further. Dissociation of the complexes of trimethyl ammonium ions (11–14), on the other hand, led to [Guest]+ ions (as expected since there is no mobile proton in the alkyl ammonium ion structure). Except for the relatively small betaine (11), the complexes of GABA and trimethyl ammonium compounds dissociated with relatively low energy, and there were no significant differences between their E 50%com values (Figure 4a). Exceptionally, the E 50%com value for the [1+11]+ ion was notably higher, which indicates higher kinetic stability for the complex with relatively small betaine (11). In fact, the complex of betaine (11) was kinetically more stable than most of the complexes formed with ammonium ions of primary and secondary amines (2–6 and 8, Figure 4b). Of all the guests studied (except betaine), epinephrine (7), serotonin (9), norepinephrine (8), and dopamine (6) exhibited the highest E 50%com values, and thus their kinetic stabilities are in good agreement with the relative binding affinities (obtained from competition experiments).
3.3 Hydrogen Bonding Properties
The H-bonding properties of glucosylcalixarene 1 and selected complexes of 1 were studied by gas-phase hydrogen-deuterium (H/D) exchange reactions (Figure 5). With ND3 as the reagent gas, protonated 1 ([1+H]+) exhibited a bimodal exchange distribution (Figure 5, top), most likely due to co-existing gas-phase conformers of 1 that react with clearly different rates [63–65]. This behavior is consistent with the results of our earlier study [52].
Our previous work suggests that the glucosylthioureido groups of tetraglucosylcalixarenes enable an encircling net of hydrogen bonding around the glucosylcalixarene skeleton and allow free migration of deuterium to glucosylcalixarene [52]. Therefore, the complex formation could disturb the H-bonding and slow down the exchange reactions [66]. Indeed, for the [1+guest]+ complex ions the exchange rates were slower and monomodal exchange distributions were obtained (Figure 5). These data suggest that the complexes are formed with specific conformers of the glucosylcalixarene, either by conformational selection (complexation takes place with certain conformers of the calixarene) or by conformational transformation upon complexation (calixarene organizes its substituents upon complexation). Interestingly, a bimodal exchange distribution was observed for [1+norepinephrine(8)]+ which suggests that the calixarene 1 adopts, at least, two different conformations in this complex.
3.4 Gas-Phase Guest Exchange
Dynamic nature and reversible binding are important properties of host–guest complexes when synthetic hosts are designed for applications related to molecular transport and sensing. In this view, we evaluated guest release by gas-phase ion/neutral reactions, in which the bound guests were displaced by tripropylamine (Pr3N). This reaction results in the formation of [1+Pr3N+H]+, which was detected as the product for all reactive complexes. The fastest ion/neutral reactions were observed with [1+H]+ and [1+GABA(10)]+ ions. In contrast, reactions were not observed (even at the longest reaction time) for histamine (3), deoxyepinephrine (5), epinephrine (7), and serotonin (9) complexes. The guest exchange spectra for [1+GABA(10)]+ ion are presented in Figure 6.
According to the reaction rate constants (Figure 7), the complexes react significantly slower than the protonated glucosylcalixarene ([1+H]+). The complex [1+GABA(10)]+, for example, reacted more than an order of magnitude slower than [1+H]+. The slowest reactions, however, were observed for the complexes of norepinephrine (8) and dopamine (6); their reactions were about three orders of magnitude slower than that of [1+H]+.
ln (Iion/Isum) values versus time in the reactions of complexes of 1 with Pr3N (Iion = intensity of the isolated ion and Isum = the sum intensity of the isolated ion and its exchange product ion). The second-order rate constant (kexp) values (in the units of 10–12 cm3 s–1 mol–1) were obtained from the slopes of the rate plots and are presented in parentheses
Gas-phase guest-exchange reactions with alkyl amines are considered to be proton transfer reactions, where the protonated guest transfers a proton to the reagent via SN2-like transition state [67, 68]. In some cases, however, the proton transfer occurs via protonated host molecule [69]. Thus, the occurrence of guest-exchange reaction is affected by proton affinities (PA) of the participating species, and for efficient proton transfer the PA of the reagent should be higher than the PA of the guest [68].
The tyramine (2) complex was clearly more reactive than the dopamine (6) complex. This finding cannot be explained by differences in their PA values alone. The PA of dopamine (934 ± 6 kJ/mol) [71] is only slightly higher than the PA of tyramine (928 ± 6 kJ/mol) [71] (for comparison, the PA of Pr3N is 991 kJ/mol [70]). Evidently, the additional phenolic hydroxyl group of dopamine participates in the intermolecular H-bonding with glucosylcalixarene 1, increasing the stability of the complex and decreasing the reactivity with Pr3N. This observation is in agreement with the results of the competition and CID experiments, where the dopamine complex exhibited higher relative binding affinity and higher kinetic stability than the tyramine complex.
Interestingly, no guest exchange was observed for deoxyepinephrine (5), epinephrine (7), histamine (3), or serotonin (9) complexes. The PA values of deoxyepinephrine (5) and epinephrine (7) are unknown, but it is reasonable to assume that they are higher than the PA value of dopamine (6) owing to the stronger basicity of secondary amines. Histamine (3), on the other hand, contains imidazole ring, which gives a greater PA value (999.8 kJ/mol) [70] compared with the other guests and makes the proton transfer reaction unfavorable.
4 Conclusions
Glucosylcalixarene 1 binds a variety of monoamine neurotransmitters and related compounds, with a marked preference towards serotonin, norepinephrine, epinephrine, and dopamine. Even though π–π, and especially, cation–π interactions are considered to bring essential contribution to binding affinity for the systems involving aromatic amines [72, 73], the results obtained strongly indicate that the calixarene complexes are mainly stabilized by intermolecular H-bonds. The methoxy groups at the lower rim of the calixarene skeleton give a certain degree of conformational freedom for the calixarene host, enabling multiple conformations in the gas phase. This was indeed evidenced by H/D-exchange reactions in which bimodal exchange distributions were observed. In contrast, for the complexes of 1 monomodal exchange distributions were observed, suggesting that there is conformational selection in the guest binding. The gas-phase guest exchange reactions provided further insights into the binding characteristics. Exchange rates with Pr3N indicated that both the proton affinity as well as the structure of the guest are important parameters providing further evidence on the intrinsic interactions (e.g., H-bonding) between 1 and the studied neurotransmitters.
References
Murakami, Y., Kikuchi, J.-I., Hisaeda, Y., Hayashida, O.: Artificial Enzymes. Chem. Rev. 96, 721–758 (1996)
Motherwell, W.B., Bingham, M.J., Six, Y.: Recent Progress in the Design and Synthesis of Artificial Enzymes. Tetrahedr 57, 4663–4686 (2001)
Sisson, A.L., Shah, M.R., Bhosale, S., Matile, S.: Synthetic Ion Channels and Pores (2004–2005). Chem. Soc. Rev. 35, 1269–1286 (2006)
Valeur, B., Leray, I.: Design Principles of Fluorescent Molecular Sensors for Cation Recognition. Coord. Chem. Rev. 205, 3–40 (2000)
Beer, P.D., Gale, P.A.: Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 40, 486–516 (2001)
Zhang, A., Xiao, C., Xue, W., Chai, Z.: Chromatographic Separation of Cesium by a Macroporous Silica-Based Supramolecular Recognition Agent Impregnated Material. Sep. Pur. Technol. 66, 541–548 (2009)
Xu, W., Li, J.-S., Feng, Y.-Q., Da, S.-L., Chen, Y.-Y., Xiao, X.Z.: Preparation and Characterization of p-tert-Butyl-Calix[6]arene-Bonded Silica Gel Stationary Phase for High Performance Liquid Chromatography. Chromatographia 48, 245–250 (1998)
Peña, M.S., Zhang, Y., Warner, I.M.: Enantiomeric Separation by Use of Calixarene Electrokinetic Chromatography. Anal. Chem. 69, 3239–3242 (1997)
Zhang, X.X., Bradshaw, J.S., Izatt, R.M.: Enantiomeric Recognition of Amine Compounds by Chiral Macrocyclic Receptors. Chem. Rev. 97, 3313–3361 (1997)
Grigorean, G., Lebrilla, C.B.: Enantiomeric Analysis of Pharmaceutical Compounds by Ion/Molecule Reactions. Anal. Chem. 73, 1684–1691 (2001)
Hyyryläinen, A.R.M., Pakarinen, J.M.H., Vainiotalo, P., Stájer, G., Fülöp, F.: Diastereochemical Differentiation of β-Amino Acids Using Host–Guest Complexes Studied by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Am. Soc. Mass Spectrom. 18, 1038–1045 (2007)
Hyyryläinen, A.R.M., Pakarinen, J.M.H., Vainiotalo, P., Fülöp, F.: Differentiation of Diastereomeric Cyclic β-Amino Acids by Varying the Neutral Reagent in Ion/Molecule Reactions Studied by Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Rapid Commun. Mass Spectrom. 22, 337–344 (2008)
Pakarinen, J.M.H., Vainiotalo, P.: Diastereochemical Differentiation of Bicyclic Diols Using Metal Complexation and Collision-Induced Dissociation Mass Spectrometry. Rapid Commun. Mass Spectrom. 23, 1767–1775 (2009)
Del Valle, E.M.M.: Cyclodextrins and Their Uses: A Review. Proc. Biochem. 39, 1033–1046 (2004)
Loftsson, T., Duchêne, D.: Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 329, 1–11 (2007)
Steed, J.W., Atwood, J.L.: Supramolecular Chemistry, 2nd edn. John Wiley and Sons, New York (2009)
Cram, D.J.: The Design of Molecular Hosts, Guests and Their Complexes. J. Incl. Phenom. 6, 397–413 (1988)
Böhmer, V.: Calixarenes, Macrocycles with (Almost) Unlimited Possibilities. Angew. Chem. Int. Ed. Engl. 34, 713–745 (1995)
Ikeda, A., Shinkai, S.: Novel Cavity Design Using Calix[n]arene Skeletons: Towards Molecular Recognition and Metal Binding. Chem. Rev. 97, 1713–1734 (1997)
Casnati, A.: Calixarenes: from Chemical Curiosity to a Rich Source for Molecular Receptors. Gazz. Chim. Ital. 127, 637–649 (1997)
Agrawal, Y.K., Bhatt, H.: Calixarenes and Their Biomimetic Applications. Bioinorg. Chem. Appl. 2, 237–274 (2004)
Ludwig, R.: Calixarenes in Analytical and Separation Chemistry. Fresenius J. Anal. Chem. 367, 103–128 (2000)
Ludwig, R.: Calixarenes for Biochemical Recognition and Separation. Microchim. Acta 152, 1–19 (2005)
Rodik, R.V., Boyko, V.I., Kalchenko, V.I.: Calixarenes in Bio-Medical Researches. Curr. Med. Chem. 16, 1630–1655 (2009)
Sansone, F., Baldini, L., Casnati, A., Ungaro, R.: Calixarenes: From Biomimetic Receptors to Multivalent Ligands for Biomolecular Recognition. New J. Chem. 34, 2715–2728 (2010)
Baldini, L., Sansone, F., Casnati, A., Ungaro, R.: Supramolecular Chemistry: From Molecules to Nanomaterials. Gale, P.A., Steed, J.W. (eds.) John Wiley & Sons, Chichester 3, 863–894 (2012)
Dougherty, D.A.: Is the Brain Ready for Physical Organic Chemistry? J. Phys. Org. Chem. 11, 334–340 (1998)
Lewitt, P., Harvey, J.A., Friedman, E., Simansky, K., Murphy, E.H.: New Evidence for Neurotransmitter Influences on Brain Development. TINS 20, 269–274 (1997)
Blokland, A.: Acetylcholine: A Neurotransmitter for Learning and Memory. Brain Res. Rev. 21, 285–300 (1996)
Berke, J.D., Hyman, S.E.: Addiction, Dopamine, and the Molecular Mechanisms of Memory. Neuron 25, 515–532 (2000)
Wise, R.A., Rompre, P.-P.: Brain Dopamine and Reward. Ann. Rev. Psychol. 40, 191–225 (1989)
Young, S.N., Leyton, M.: The Role of Serotonin in Human Mood and Social Interaction Insight from Altered Tryptophan Levels. Pharmacol. Biochem. Behav. 71, 857–865 (2002)
Lauder, J.M.: Neurotransmitters as Growth Regulatory Signals: Role of Receptors and Second Messengers. TINS 16, 233–240 (1993)
Qiu, Y., Peng, Y., Wang, J.: Immunoregulatory Role of Neurotransmitters. Adv. Neuroimmun. 6, 223–231 (1996)
Gingrich, J.A., Caron, M.G.: Recent Advances in the Molecular Biology of Dopamine Receptors. Ann. Rev. Neurosci. 16, 299–321 (1993)
Muir, J.L.: Acetylcholine, Aging, and Alzheimer’s Disease. Pharmacol. Biochem. Behav. 56, 687–696 (1997)
Meltzer, H.Y., Stahl, S.M.: The Dopamine Hypothesis of Schizophrenia: A Review. Schizophr. Bull. 2, 19–76 (1976)
Chace, D.H.: Mass Spectrometry in the Clinical Laboratory. Chem. Rev. 101, 445–477 (2001)
Tsai, T.-H.: Separation Methods Used in the Determination of Choline and Acetylcholine. J. Chromatogr. 747, 111–122 (2000)
Lin, V.S.-Y., Lai, C.-Y., Huang, J., Song, S.-A., Xu, S.: Molecular Recognition Inside of Multifunctionalized Mesoporous Silicas: Toward Selective Fluorescence Detection of Dopamine and Glucosamine. J. Am. Chem. Soc. 123, 11510–11511 (2001)
Wu, W., Zhu, H., Fan, L., Liu, D., Renneberg, R., Yang, S.: Sensitive Dopamine Recognition by Boronic Acid Functionalized Multiwalled Carbon Nanotubes. Chem. Commun. 2345–2347 (2007)
Makote, R., Collinson, M.M.: Dopamine Recognition in Templated Silicate Films. Chem. Commun. 425–426 (1998)
Zhang, G., Hong, Z., Chai, Y., Zhu, Z., Song, Y., Liu, C., Ji, S., Yin, X., Wu, Y.: A Study on the Chiral Recognition Mechanism of Enantioseparation of Adrenaline and Its Analogues Using Capillary Electrophoresis. Chem. Pharm. Bull. 55, 324–327 (2007)
Secor, K.E., Glass, T.E.: Selective Amine Recognition: Development of a Chemosensor for Dopamine and Norepinephrine. Org. Lett. 6, 3727–3730 (2004)
Ling, T.-R., Syu, Y.Z., Tasi, Y.-C., Chou, T.C., Liu, C.-C.: Size-Selective Recognition of Catecholamines by Molecular Imprinting on Silica-Alumina Gel. Biosen. Bioel. 21, 901–907 (2005)
Maue, M., Schrader, T.: A Color Sensor for Catecholamines. Angew. Chem. 117, 2305–2310 (2005)
Zhu, Y., Wong, P.S.H., Cregor, M., Gitzen, J.F., Coury, L.A., Kissinger, P.T.: In Vivo Microdialysis and Reverse Phase Ion Pair Liquid Chromatography/Tandem Mass Spectrometry for the Determination and Identification of Acetylcholine and Related Compounds in Rat Brain. Rapid Commun. Mass Spectrom. 14, 1695–1700 (2000)
Uutela, P., Reinilä, R., Piipponen, P., Ketola, R.A., Kostiainen, R.: Analysis of Acetylcholine and Choline in Microdialysis Samples by Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 19, 2950–2956 (2005)
Kauppila, T.J., Nikkola, T., Ketola, R.A., Kostiainen, R.: Atmospheric Pressure Photoionization-Mass Spectrometry and Atmospheric Pressure Chemical Ionization-Mass Spectrometry of Neurotransmitters. J. Mass Spectrom. 41, 781–789 (2006)
Baldini, L., Casnati, A., Sansone, F., Ungaro, R.: Calixarene-Based Multivalent Ligands. Chem. Soc. Rev. 36, 254–266 (2007)
Casnati, A., Sansone, F., Ungaro, R.: Peptido- and Glycocalixarenes: Playing with Hydrogen Bonds Around Hydrophobic Cavities. Acc. Chem. Res. 36, 246–254 (2003)
Torvinen, M., Neitola, R., Sansone, F., Baldini, L., Ungaro, R., Casnati, A., Vainiotalo, P., Kalenius, E.: Glucosylthioureidocalix[4]arenes: Synthesis, Conformations and Gas-Phase Recognition of Amino Acids. Org. Biomol. Chem. 8, 906–915 (2010)
André, S., Sansone, F., Kaltner, H., Casnati, A., Kopitz, J., Gabius, H.J., Ungaro, R.: Calix[n]arene-Based Glucoclusters: Bioactivity of Thiourea-Linked Galactose/Lactose Moieties as Inhibitors of Binding of Medically Relevant Lectins to a Glycoprotein and Cell-Surface Glycoconjugates and Selectivity Among Human Adhesion/Growth-Regulatory Galectins. ChemBioChem 9, 1649–1661 (2008)
Arosio, D., Fontanella, M., Baldini, L., Mauri, L., Bernardi, A., Casnati, A., Sansone, F., Ungaro, R.: A Synthetic Divalent Cholera Toxin Glycocalixarene Ligand Having Higher Affinity than Natural GM1 Oligosaccharide. J. Am. Chem. Soc. 127, 3660–3661 (2005)
Sansone, F., Baldini, L., Casnati, A., Ungaro, R.: Conformationally Mobile Glucosylthioureidocalix[6]- and Calix[8]arenes: Synthesis, Aggregation, and Lectin Binding. Supramol. Chem. 20, 161–168 (2008)
André, S., Grandjean, C., Gautier, F.-M., Bernardi, S., Sansone, F., Gabius, H.-J., Ungaro, R.: Combining Carbohydrate Substitutions at Bioinspired Positions with Multivalent Presentation Towards Optimizing Lectin Inhibitors: Case Study with Calixarenes. Chem. Commun. 47, 6126–6128 (2011)
Sansone, F., Chierici, E., Casnati, A., Ungaro, R.: Thiourea-Linked Upper Rim Calix[4]arene Neoglycoconjugates: Synthesis, Conformations, and Binding Properties. Org. Biomol. Chem. 1, 1802–1809 (2003)
Torvinen, M., Kalenius, E., Sansone, F., Casnati, A., Jänis, J.: Noncovalent Complexation of Glucosylthioureidocalix[4]arenes with Carboxylates and Their Gas-Phase Characteristics: An ESI-FTICR Mass Spectrometric Study. J. Mass Spectrom. 46, 787–793 (2011)
Woodget, B.W., Cooper, D.: Samples and Standards, pp. 40–47. John Wiley and Sons Ltd, Chichester (1987)
Hamilton, L.F., Simpson, S.G., Ellis, D.W.: Calculations of Analytical Chemistry, pp. 2–11. McGraw-Hill Inc, New York (1960)
McCormick, D., Roach, A.: Measurements, Statistics, and Computation, pp. 24–31. John Wiley and Sons Ltd, Chichester (1987)
Schalley, C.A.: Supramolecular Chemistry Goes Gas Phase: The Mass Spectrometric Examination of Noncovalent Interactions in Host–Guest Chemistry and Molecular Recognition. Int. J. Mass Spectrom. 194, 11–39 (2000)
Cassady, C.J., Carr, S.R.: Elucidation of Isomeric Structures for Ubiquitin [M+12H]12+ Ions Produced by Electrospray Ionization Mass Spectrometry J. Mass Spectrom. 31, 247–254 (1996)
Freitas, M.A., Hendrickson, C.L., Emmett, M.R., Marshall, A.G.: Gas-Phase Bovine Ubiquitin Cation Conformations Resolved by Gas-Phase Hydrogen/Deuterium Exchange Rate and Extent Int. J. Mass Spectrom. 187, 565–575 (1999)
Lifshitz, C.: A Review of Gas-Phase H/D Exchange Experiments: The Protonated Arginine Dimer and Bradykinin Nonapeptide systems. Int. J. Mass Spectrom. 234, 63–70 (2004)
Winkler, H.D.F., Dzyuba, E.V., Sklorz, J.A.W., Beyeh, N.K., Rissanen, K., Schalley, C.A.: Gas-Phase H/D-Exchange Reactions on Resorcinarene and Pyrogallarene Capsules: Proton Transport Through a One-Dimensional Grotthuss Mechanism. Chem. Sci. 2, 615–624 (2011)
Grigorean, G., Ramirez, J., Ahn, S.H., Lebrilla, C.B.: A Mass Spectrometry Method for the Determination of Enantiomeric Excess in Mixtures of d, l-Amino Acids. Anal. Chem. 72, 4275–4281 (2000)
Blake, R.S., Monks, P.S., Ellis, A.M.: Proton-Transfer Reaction Mass Spectrometry. Chem. Rev. 109, 861–896 (2009)
Kalenius, E., Moiani, D., Dalcanale, E., Vainiotalo, P.: Measuring H-Bonding in Supramolecular Complexes by Gas Phase Ion-Molecule reactions. Chem. Commun. 3865–3867 (2007)
Hunter, E.P., Lias, S.G.: Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update. J. Phys. Chem. Ref. Data 27, 413–656 (1998)
Cardoso, A.M., Alexandre, S.M.G., Barros, C.M.F., Ferrer-Correia, A.J., Nibbering, N.M.M.: Proton Affinities of Phenylalkylamines by the Kinetic Method. Int. J. Mass Spectrom. Ion Process 172, 123–127 (1998)
Zacharias, N., Dougherty, D.A.: Cation-π Interactions in Ligand Recognition and Catalysis. Trends Pharmacol. Sci. 23, 281–287 (2002)
Dougherty, D.A.: Cation-π Interactions Involving Aromatic Amino Acids. J. Nutr. 137, 1504S–1508S (2007)
Acknowledgments
The authors gratefully acknowledge financial support from the National Graduate School of Organic Chemistry and Chemical Biology (MT), the Academy of Finland (EK, project 127941), and MIUR (PRIN project 200858SA98).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Torvinen, M., Kalenius, E., Sansone, F. et al. Noncovalent Complexation of Monoamine Neurotransmitters and Related Ammonium Ions by Tetramethoxy Tetraglucosylcalix[4]arene. J. Am. Soc. Mass Spectrom. 23, 359–365 (2012). https://doi.org/10.1007/s13361-011-0289-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-011-0289-3